Abstract
Antral gastrin is the hormone known to stimulate acid secretion and proliferation of the gastric corpus epithelium. Patients with mutations in the multiple endocrine neoplasia type 1 (MEN1) locus, which encodes the protein menin, develop pituitary hyperplasia, insulinomas, and gastrinomas in the duodenum. We previously hypothesized that loss of menin leads to derepression of the gastrin gene and hypergastrinemia. Indeed, we show that menin represses JunD induction of gastrin in vitro. Therefore, we examined whether conditional deletion of Men1 (Villin-Cre and Lgr5-EGFP-IRES-CreERT2), with subsequent loss of menin from the gastrointestinal epithelium, increases gastrin expression. We found that epithelium-specific deletion of Men1 using Villin-Cre increased plasma gastrin, antral G cell numbers, and gastrin expression in the antrum, but not the duodenum. Moreover, the mice were hypochlorhydric by 12 mo of age, and gastric somatostatin mRNA levels were reduced. However, duodenal mRNA levels of the cyclin-dependent kinase inhibitor p27Kip1 were decreased, and cell proliferation determined by Ki67 staining was increased. About 11% of the menin-deficient mice developed antral tumors that were negative for gastrin; however, gastrinomas were not observed, even at 12 mo of age. No gastrinomas were observed with conditional deletion of Men1 in the Lgr5 stem cells 5 mo after Cre induction. In summary, epithelium-specific deletion of the Men1 locus resulted in hypergastrinemia due to antral G cell hyperplasia and a hyperproliferative epithelium, but no gastrinomas. This result suggests that additional mutations in gene targets other than the Men1 locus are required to produce gastrin-secreting tumors.
Keywords: somatostatin, p27Kip1, tumor, Villin-Cre, Lgr5
menin is a ubiquitously expressed tumor suppressor protein encoded by the multiple endocrine neoplasia type 1 (MEN1) gene (8). Mutations in this locus are responsible for endocrine-secreting tumors in the pituitary, parathyroid, pancreas, and duodenum (7, 9, 21). Gastrinomas are the most malignant tumor induced by autosomal-dominant mutations in the MEN1 locus, and these tumors are more common in the duodenum than the pancreas (1, 13, 17). Important features of sporadic and MEN1-driven gastrinomas include hypergastrinemia and hypersecretion of gastric acid, resulting in diarrhea and extensive duodenal peptic ulcers, collectively known as the Zollinger-Ellison syndrome (ZES) (17, 30, 37). Although not attributed to a specific genetic defect, pseudo-ZES was characterized in 1972 as hypergastrinemia due to antral G cell hyperplasia without tumors (12, 27). Described prior to the positional cloning of MEN1, antral G cell hyperplasia is considered a cause of ZES that can exist in the absence of an actual tumor (4, 27).
Since the initial cloning of MEN1, there has been heightened interest in understanding why endocrine organs are especially susceptible to hypersecretion and tumor formation. To dissect its function in vivo, the Men1 locus was deleted in mice by homologous recombination (5, 10). Complete deletion of the Men1 locus (Men1−/−) in mice causes embryonic lethality at embryonic day 11.5–13.5 due to multiple developmental defects. However, mice heterozygous for Men1 (Men1+/−) are viable, appear normal, and do not show abnormalities before 9 mo of age (5, 10). By contrast, older Men1+/− mice develop a variety of endocrine tumors consistent with those observed in the human MEN1 syndrome (5, 10). The one exception is a gastrinoma that does not develop in the Men1+/− mice, despite endocrine tumor development in the pituitary and pancreas (10). Although Bertolino et al. (5) reported an extrapancreatic gastrinoma in their Men1+/− mice, the histological evidence consisted of an adenomatous tumor with patches of G cell hyperplasia. More importantly, neither study measured the levels of plasma gastrin or determined gastrin mRNA levels in the stomach or duodenum. Thus, surprisingly, there is little evidence that deletion of Men1 alone is sufficient to induce generation of a gastrinoma as well as induce hypergastrinemia.
We previously showed that menin suppresses gastrin gene expression and that reducing menin expression is sufficient to induce gastrin expression (23). Therefore, we hypothesize in this study that loss of menin expression will be sufficient to induce gastrin expression. Since prior studies of heterozygous Men1 deletion did not reveal gastrinomas, we also examined whether tissue-specific homozygous Men1 deletion is sufficient for gastrinoma development. To test these hypotheses, we analyzed mice in which the Men1 locus was conditionally deleted simultaneously in the antral and intestinal epithelium (Men1FL/FL:Villin-Cre and Lgr5-CreERT2). We quantified the levels of plasma and tissue gastrin at different time points and found that deletion of Men1 was indeed sufficient to generate hypergastrinemia due to antral G cell hyperplasia but was not sufficient to induce gastrin-secreting tumors.
MATERIALS AND METHODS
Animals and genotyping.
Mice with the floxed Men1 locus in which LoxP sites flank exons 3–8 of the Men1 gene (10) were purchased (stock number 005109, Jackson Laboratory) and then bred to a Villin-Cre mouse line (20) (a gift from D. Gumucio) to generate Villin-Cre × Men1FL/FL (designated VC:Men1FL/FL) mice or to a Lgr5-EGFP-IRES-CreERT2 mouse line (stock number 008875, Jackson Laboratory) to generate Lgr5-EGFP-IRES-CreERT2 × Men1FL/FL (designated Lgr5:Men1FL/FL) mice. In the Lgr5-EGFP-IRES-CreERT2 mice, the Cre recombinase was activated by an intraperitoneal injection of tamoxifen (1 mg/mouse; Sigma-Aldrich, St. Louis, MO) once a day for 5 consecutive days. All mice were on a C57BL/6J × FVB;129S mixed background, with an average of 85.33% C57BL/6 determined by single nucleotide polymorphism analysis (Charles River Laboratories, Troy, NY). The mice were housed in a facility with a 12:12-h light-dark cycle and allowed access to food and water ad libitum. The mice were genotyped using the following primers: exon 8 [5′-ATTGATGAGACCGCAAGGAC-3′ (forward) and 5′-GTCCTGGAGAGCAGAACCTTG-3′ (reverse)] and exon 2 [5′-CCACATCCAGTCCCTCTTCAGCT-3′ (forward) to detect tissue-specific Men1 ablation] (31). PCR analysis was performed using GoTaq DNA polymerase (Promega, Madison, WI) in 20 μl of reaction mix for 35 cycles according to standard procedures. Each cycle consisted of denaturation at 94°C for 1 min, annealing at 58°C for 1 min, and extension at 72°C for 1 min followed by a final single 5-min extension at 72°C. For genotyping, DNA was isolated using the HotSHOT protocol (33). For the tissue-specific Men1 deletion analysis, the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) was used. PCR products were analyzed in 1% agarose gels. Animal experiments were conducted according to protocols approved by the University of Michigan Committee on the Use and Care of Animals.
RT-quantitative PCR.
After removal, fresh tissues were homogenized in TRIzol reagent (GIBCO/Invitrogen, Carlsbad, CA). RNA was isolated according to the manufacturer's instructions using the RNeasy Mini kit (Qiagen) after DNase A treatment. The first-strand cDNA was synthesized using 1 μg of total RNA and the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Quantitative PCRs (qPCRs) were carried out using a thermal cycler (model C1000, Bio-Rad) with SYBR Green dye (Molecular Probes, Carlsbad, CA) and Platinum Taq DNA polymerase (Invitrogen). Each reaction was performed three times in triplicate as follows: 3 min at 95°C, 40 cycles of 9 s at 95°C, 1 min at 60°C, and 1 min at 55°C. The mouse primers were as follows: hypoxanthine-guanine phosphoribosyltransferase (Hprt) [5′-AGGACCTCTCGAAGTGTTGGATAC-3′ (forward) and 5′-AACTTGCGCTCATCTTAGGCTTTG-3′ (reverse)], Men1 [5′-CCAGTCCCTCTTCAGCTTCA-3′ (forward) and 5′-GATGGACATCTCTGAGACCCA-3′ (reverse)], gastrin [5′-ACACAACAGCCAACTATTC-3′ (forward) and 5′-CAAAGTCCATCCATCCGTAG-3′ (reverse)], somatostatin [5′-TCTGCATCGTCCTGGCTTTGG-3′ (forward) and 5′-TCATTCTCTGTCTGGTTGGGCTC-3′ (reverse)], p27Kip1 [5′-GCGGTGCCTTTAATTGGGTCTC-3′ (forward) and 5′-GTTCTGTTGGCCCTTTTGTTTTGC-3′ (reverse)], H+-K+-ATPase-α [5′-TGTACACATGAGAGTCCCCTTG-3′ (forward) and 5′-GAGTCTTCTCGTTTTCCACACC-3′ (reverse)], H+-K+-ATPase-β [5′-AACAGAATTGTCAAGTTCCTC-3′ (forward) and 5′-AGACTGAAGGTGCCATTG-3′ (reverse)], sonic hedgehog [Shh, 5′ ATGTTTTCTGGTGATCCTTGCT 3′ (forward) and 5′ ATCGTTCGGAGTTTCTTGTGAT 3′ (reverse)], and glioma-associated oncogene homolog 1 [Gli1, 5′ TTGGGATGAAGAAGCAGTTG 3′ (forward) and 5′ GGAGACAGCATGGCTCACTA 3′ (reverse)].
Western blots.
Tissues were homogenized in RIPA lysis buffer (Sigma-Aldrich) supplemented with Complete protease inhibitor cocktail (Roche, Indianapolis, IN). Protein concentration in the lysates was measured using the Bradford colorimetric [bicinchoninic acid (BCA)] protein assay kit (Thermo Scientific, Waltham, MA) after centrifugation at 12,000 g for 15 min. The lysates were loaded on 10% SDS-polyacrylamide minigels. After transfer and blocking, polyvinylidene difluoride membranes were incubated overnight at 4°C in a 1:1,000 dilution of rabbit polyclonal anti-menin antibody (Bethyl Labs, Montgomery, TX) in 5% nonfat dry milk dissolved in 1× Tris-buffered saline and 0.1% Triton X-100 and then for 1 h at room temperature with a 1:2,000 dilution of mouse monoclonal anti-GAPDH (Millipore, Temecula, CA) antibody. After the sample was washed, horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) were used to visualize the proteins.
Immunohistochemical staining.
After the animals were euthanized, the stomach and duodenum were removed, opened along the greater curvature, immobilized on bite wax, and fixed in place overnight at 4°C in 4% formaldehyde solution (Sigma-Aldrich) diluted in 1× PBS. Paraffin sections (5 μm) were coimmunostained with goat anti-gastrin antibody (1:200 dilution; Santa Cruz Biotechnology) and then incubated with rabbit anti-menin antibody (1:2,000 dilution; Bethyl Labs). In separate experiments, specific gastric cell types were identified by immunostaining for rabbit anti-gastrin (1:1,000 dilution; Dako, Carpinteria, CA), rabbit anti-Ki67 (1:200 dilution; Thermo Scientific), rabbit anti-phosphorylated (Ser10) histone H3 (1:500 dilution; Millipore), and mouse anti-proton pump/H+-K+-ATPase-α (1:500 dilution; MLB, Woburn, MA). A rabbit anti-somatostatin antibody (1:200 dilution; Sigma-Aldrich) was used to stain D cells. Griffonia simplicifolia II (GSII) lectin (1:1,000 dilution of Alexa 488-conjugated GSII; Vector Laboratories, Olean, NY) was used for mucous neck cells. For immunofluorescence analysis, Cy3-conjugated donkey anti-rabbit (1:500 dilution) or Alexa Fluor 488-conjugated donkey anti-rabbit or anti-goat (1:500) secondary antibody or TSA Plus Cy3 System (Perkin Elmer, Waltham, MA) was used to detect primary antibodies. ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) was used for nuclear counterstaining and as a mounting medium. In separate experiments, Mouse-on-Mouse HRP Polymer Kit for mouse antibodies on mouse tissues (Biocare Medical, Concord, CA) or rabbit-specific HRP/diaminobenzidine detection immunohistochemistry kit (Abcam, Cambridge, MA) for rabbit antibodies was used.
Plasma gastrin determination.
Mice were deprived of food for 16 h before they were euthanized. Blood was collected by cardiac puncture in lithium-heparin-coated tubes (Sarstedt, Aktiengesellschaft, Numbrecht, Germany). After centrifugation, the plasma was separated and kept at −80°C until it was assayed. Gastrin levels in plasma were measured by the Gastrin I (human) enzyme immunoassay kit (Enzo Life Sciences, Farmingdale, NY) or RIA. RIA was performed using [125I][15Met]-human G-17 and antiserum 1296 (a gift from the Center for Ulcer Research and Education, University of California Los Angeles), as described previously (36).
Basal gastric acid content.
After fasted mice were euthanized, the stomachs were removed and opened along the greater curvature. The content was flushed with 2 ml of 0.9% NaCl (pH 7.0). H+ concentration of the samples was measured by base titration using 0.005 N NaOH and a titration system (PHM290 pH-Stat Controller, Radiometer Analytical, Westlake, OH). Data were normalized to mouse body weight.
Statistical analysis.
Values are means ± SE. Statistical analysis was performed using Student's t-test (GraphPad Prism5, GraphPad Software, San Diego, CA). P < 0.05 was considered to be significant.
RESULTS
Gastrointestinal-specific deletion of Men1.
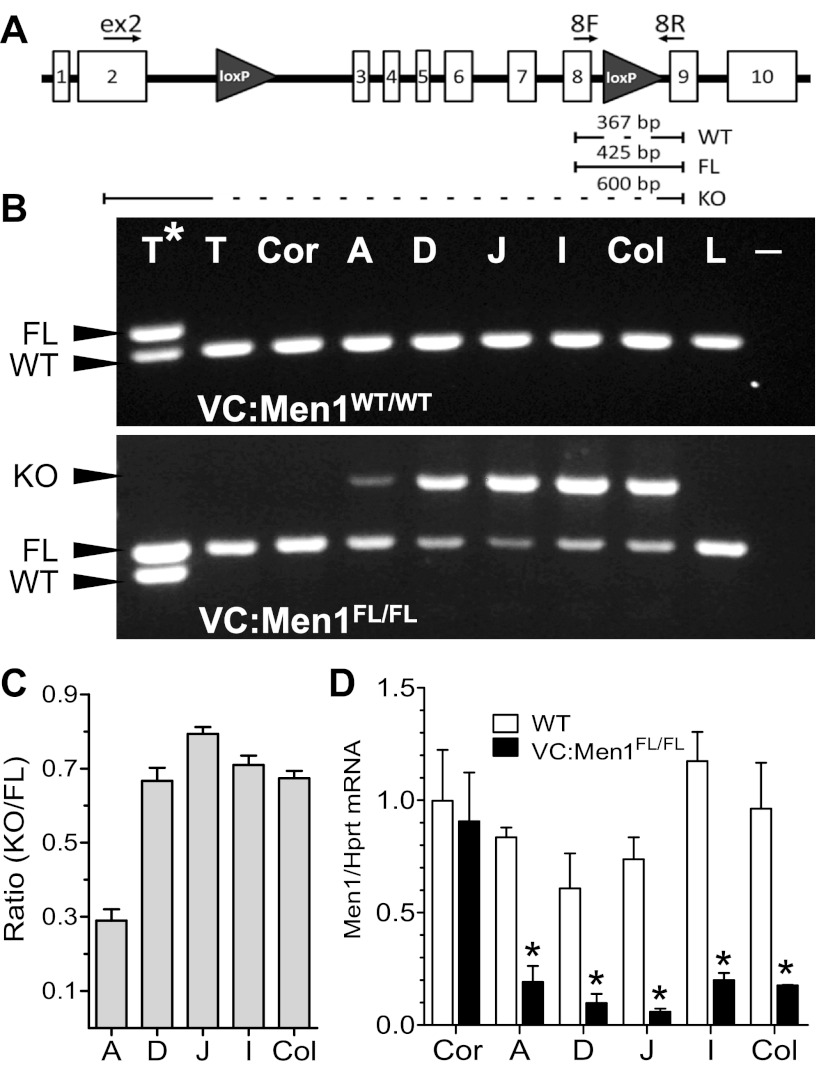
We previously showed that menin inhibits gastrin gene expression in vitro and that reduced levels of menin increase gastrin expression (23). Therefore, to test whether deletion of the menin locus increased gastrin expression in vivo, mice expressing Cre recombinase from a modified villin transgene (Villin-Cre) were bred to the MenFL/FL line (VC:Men1FL/FL). Prior expression analysis using lineage-tracing studies showed that Villin-Cre is expressed in all epithelial cell lineages of the small and large intestines, as well as all gastric epithelial cell lineages of the antral glands (20, 28). To demonstrate the efficiency of Villin-Cre recombinase at the Men1 locus, we isolated DNA from tissues expected to express the transgene (duodenum, jejunum, ileum, colon, and stomach antrum) and compared these tissues with those not expected to express Cre recombinase on the basis of the expression pattern of the modified villin promoter used to generate the transgene (tail, stomach corpus, and liver). PCR analysis using primers that distinguish wild-type (WT), floxed, and Cre-mediated deletion of the Men1 gene was performed (31). The location of the LoxP sites within the Men1 gene and the primer locations are shown in Fig. 1A. As expected, a single band, corresponding to the WT Men1 allele, was detected in all tissues examined in the VC:Men1+/+ (WT) mice (Fig. 1B, top). PCR using tissues from mice carrying floxed alleles resulted in amplification of additional PCR products in the antrum, duodenum, jejunum, ileum, and colon, corresponding to the deleted Men1 allele (Fig. 1B, bottom). In tissues where knockout of the Men1 gene was detected, we analyzed the ratio of the knockout to the floxed bands to compare the efficiency of Men1 recombination (Fig. 1C). We found ratios of 0.23–0.33 in the antrum and 0.6–0.83 in the duodenum, jejunum, ileum, and colon of VC:Men1FL/FL mice. Our analysis confirmed that the Cre recombinase was ∼50% less active in the antrum than in the remainder of the GI tract, consistent with the Villin-Cre promoter expression. Specifically, the expression of Cre in the antrum was as previously reported (19, 28). In addition, we performed RT-qPCR analysis of Men1 mRNA expression in the same tissues of the luminal GI tract and found that Men1 mRNA levels were significantly (up to 95%) reduced in the antrum, duodenum, jejunum, ileum, and colon, but not the corpus, of VC:Men1FL/FL compared with WT mice (Fig. 1D). Thus the PCR analysis confirmed that Cre-mediated Men1 deletion occurred in the antrum, duodenum, jejunum, ileum, and colon, but not in other tissues, including the stomach corpus. Interestingly, even a low level of Cre was sufficient to significantly reduce Men1 RNA levels in the antrum.
Fig. 1.
Analysis of Villin-Cre-mediated Men1 excision. A: schematic view of the mouse Men1 gene from the Men1FL/FL mouse line. Clear rectangles with numbers indicate exons; gray triangles indicate the LoxP site; arrows indicate location of forward and reverse primers for genotyping. Sizes indicate PCR products, amplified from wild-type (WT), floxed (FL), or knockout (KO) alleles. B: analysis of PCR products detecting Cre recombinase-mediated deletion of the Men1 allele in various tissues. PCR bands, corresponding to the WT, FL, and KO alleles, are indicated with arrowheads. T*, tail VC:Men1WT/FL mouse; T, tail; Cor, corpus; A, antrum; D, duodenum; J, jejunum; I, ileum; Col, colon; L, liver; −, without DNA. DNA was isolated from tissues from VC:Men1WT/WT and VC:Men1FL/FL mice. C: analysis of Villin-Cre recombination efficiency, shown as ratio of KO to FL PCR products. Only Villin-Cre-expressing tissues were used for analysis. Values are means ± SE; n = 3. D: Men1 mRNA expression determined by RT-quantitative PCR (qPCR) analysis of tissues from VC:Men1FL/FL mice. Values (means ± SE; n = 3) are shown relative to hypoxanthine-guanine phosphoribosyltransferase (Hprt) mRNA expression measured in the same samples. *P < 0.05.
Effect of Men1 deletion in the duodenum.
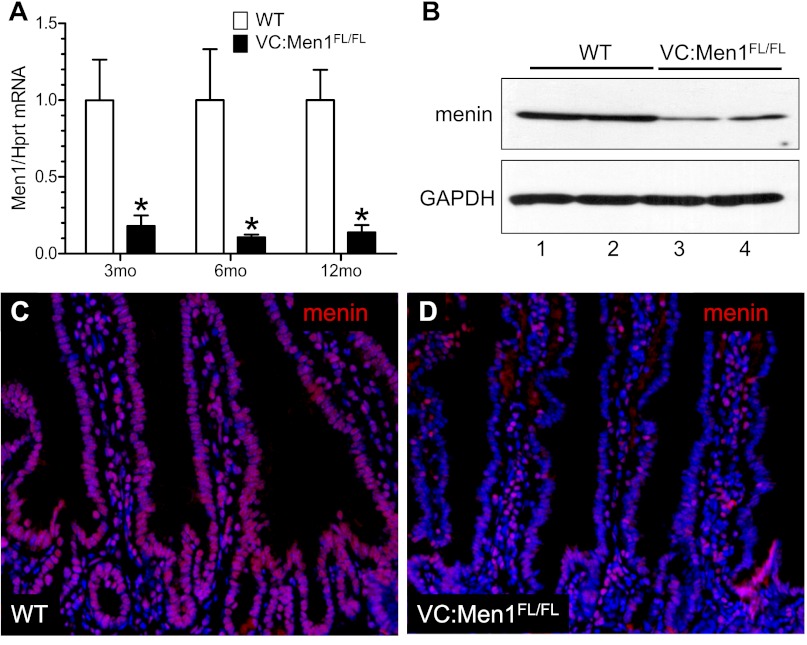
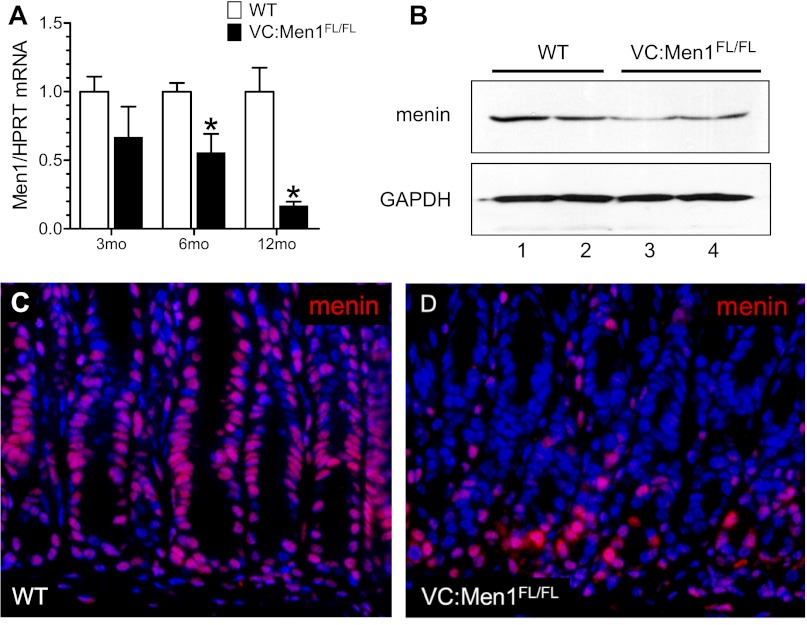
Since Men1-related gastrinomas frequently arise in the duodenum (1), we first analyzed the effect of menin deletion on the duodenal epithelium. Men1 mRNA expression was significantly reduced (up to 90%) in the duodenal mucosa from VC:Men1FL/FL mice compared with WT (control) mice as early as 3 mo of age (Fig. 2A). In addition, reduced levels of menin protein at 12 mo of age were detected by Western blot (Fig. 2B). Menin is ubiquitously expressed and is primarily a nuclear protein (8). Indeed, in the duodenum of a WT mouse, nuclear localization of menin was confirmed by immunofluorescence (Fig. 2C). In contrast, we observed reduced menin protein levels in the epithelial cells of VC:Men1FL/FL duodenum, but not lamina propria (Fig. 2D). This result confirms faithful epithelium-specific expression of the Villin-Cre transgene in the duodenum and correlates with the genotyping in Fig. 1B (20).
Fig. 2.
Epithelium-specific Men1 deletion in the duodenum. A: Men1 mRNA determined by RT-qPCR analysis of duodenal mucosa from 3-, 6-, and 12 mo-old VC:Men1FL/FL and WT mice. Values (means ± SE; n = 3–4) are shown relative to Hprt mRNA expression measured in the same samples. *P < 0.05. B: Western blot analysis of menin protein levels performed with duodenal mucosal scrapings from 2 WT (lanes 1 and 2) and 2 VC:Men1FL/FL (lanes 3 and 4) mice at 12 mo of age. C and D: representative images of menin (red) immunofluorescent staining using duodenum tissues from 12-mo-old WT and VC:Men1FL/FL mice. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, blue). Original magnification: ×200.
Men1 deletion in the duodenum did not affect gastrin expression but increased proliferation.
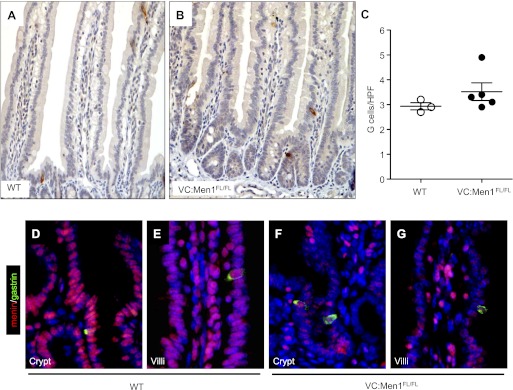
Menin is a negative regulator of gastrin gene expression (23), prompting us to examine the effect of epithelium-specific menin deletion on gastrin expression in the duodenum. Since the levels of gastrin mRNA were not detectable in the duodenum by RT-qPCR, we performed immunohistochemical staining for gastrin. There was no difference in the number of gastrin-expressing (G) cells in the duodenums of the VC:Men1FL/FL compared with WT mice (Fig. 3, A–C). We observed an average of one to two G cells per approximately three crypts or villi of the duodenum. Double immunofluorescent staining revealed that menin is highly expressed in duodenal gastrin-producing cells of the crypts (Fig. 3D) and villi (Fig. 3E) of the WT mice. However, in the VC:Men1FL/FL mice, menin protein was immunodetected in duodenal G cells, but the levels appeared significantly reduced (Fig. 3, F and G).
Fig. 3.
Conditional Men1 deletion in the duodenum does not alter G cell number. A and B: gastrin immunostaining in duodenum of 12-mo-old WT and VC:Men1FL/FL mice. Original magnification: ×200. C: G cell quantification in duodenum of 12-mo-old VC:Men1FL/FL and WT mice. Values are numbers of gastrin-immunostained cells per high-power field (HPF). D–G: coimmunostaining of paraffin sections from duodenum for gastrin (green) and menin (red) with DAPI nuclear stain (blue) to identify double-positive cells in WT and VC:Men1FL/FL mice within crypt (D and F) and villi (E and G). Original magnification: ×600.
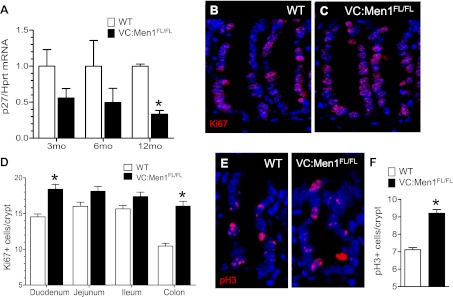
The cyclin-dependent kinase inhibitor p27Kip1 regulates the transition of cells from the G1 to the S phase and is an important readout of the menin signaling pathway (15, 16, 24). Therefore, we quantified the levels of p27Kip1 mRNA in the duodenum of VC:Men1FL/FL mice at 3, 6, and 12 mo of age. A decrease in p27Kip1 mRNA in the duodenal mucosa of VC:Men1FL/FL mice was detected at 3 mo of age and achieved significance by 12 mo (Fig. 4A). The reduced levels of p27Kip1 correlated with an increase in the proliferation marker Ki67 (Fig. 4C vs. 4B), as well as the decrease in menin expression (Fig. 2A). Additionally, we observed a significant increase in the mitosis marker phosphorylated histone H3 in the duodenal crypts (Fig. 4, E and F). Thus, in the duodenum, reduced Men1 mRNA and protein levels correlated with a decrease in p27Kip1 mRNA expression and an increase in epithelial proliferation.
Fig. 4.
Conditional Men1 deletion in the duodenum modulates proliferation. A: expression of p27Kip1 determined by RT-qPCR analysis of duodenal RNA in 3-, 6-, and 12-mo-old WT and VC:Men1FL/FL mice. Values are means ± SE; n = 3–4. *P < 0.05. B and C: immunofluorescent staining for the proliferative marker Ki67 (red) in duodenal sections from 12-mo-old WT and VC:Men1FL/FL. Blue, DAPI. Original magnification: ×600. D: quantification of Ki67-positive cells in tissues from 12-mo-old WT and VC:Men1FL/FL mice. Values are means ± SE; n = 3. *P < 0.05. E: immunofluorescent staining for the mitosis marker phosphorylated (Ser10) histone H3 (pH3, red) in duodenal sections from 12-mo-old WT and VC:Men1FL/FL mice. Blue, DAPI. Original magnification: ×600. F: quantification of pH3-positive cells in the duodenum of 12-mo-old WT and VC:Men1FL/FL mice. Values are means ± SE; n = 3. *P < 0.05.
Antral Men1 deletion leads to G cell hyperplasia.
We examined the levels of Men1 mRNA in the stomach of the VC:Men1FL/FL and WT mice at 3, 6, and 12 mo of age. We observed a reduction in Men1 mRNA in 6- and 12-mo-old VC:Men1FL/FL mice (68% and 90%, respectively; Fig. 5A). Consistent with reduced Men1 mRNA, the levels of menin protein, determined by Western blotting (Fig. 5B, lanes 3 and 4 vs. lanes 1 and 2) or immunofluorescent staining (Fig. 5D vs. 5C), were also reduced in the antrum of 12-mo-old VC:Men1FL/FL mice.
Fig. 5.
Menin deletion in the antrum of VC:Men1FL/FL mice. A: RT-qPCR analysis of Men1 mRNA in the stomach of 3-, 6-, and 12-mo-old WT or VC:Men1FL/FL mice. Values are means ± SE; n = 3–4. *P < 0.05. B: Western blot analysis of menin protein in antrum of 2 WT (lanes 1 and 2) and 2 VC:Men1FL/FL (lanes 3 and 4) mice at 12 mo of age. C and D: menin (red) immunofluorescence in the antrum of 12-mo-old WT and VC:Men1FL/FL mice. Blue, DAPI. Original magnification: ×400.
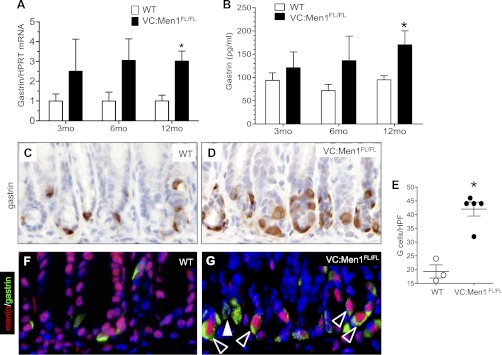
The major source of circulating gastrin is the G cells in the stomach antrum (6, 34). Gastrin mRNA levels in the antrum were significantly increased in 12-mo-old VC:Men1FL/FL compared with WT mice. In addition, one of four (25%) and two of four (50%) mutant mice at 3 and 6 mo of age, respectively, showed elevated (in some cases up to 7-fold) gastrin mRNA levels (Fig. 6A). RIA revealed a significant 1.7-fold increase in the plasma gastrin levels of VC:Men1FL/FL mice by 12 mo of age (Fig. 6B). This increase in gastrin mRNA and protein correlated with a twofold increase in the number of antral G cells (Fig. 6, C–E), suggesting that these cells are the main source of circulating gastrin in the VC:Men1FL/FL mice. We previously reported that menin colocalizes with gastrin in G cells of the mouse antrum (23). Surprisingly, double immunostaining of menin and gastrin revealed residual menin protein expression in some antral G cells (Fig. 6, F and G). Nevertheless, the significant increase in gastrin mRNA, plasma gastrin, and G cell numbers demonstrated that a sufficient number of G cells exhibited reduced menin levels to permit derepression of gastrin gene expression.
Fig. 6.
Menin deficiency in the antrum leads to G cell hyperplasia and hypergastrinemia. A: RT-qPCR measurement of gastrin mRNA normalized to Hprt mRNA levels in stomach of VC:Men1FL/FL and age-matched WT mice. Values are means ± SE; n = 3–4. *P < 0.05. B: RIA and enzyme immunoassay measurement of gastrin concentration in circulating plasma from mice fasted for 16 h. Values are means ± SE; n = 4–13. *P < 0.05. C and D: paraffin sections of stomach antrum from 12-mo-old WT and VC:Men1FL/FL immunostained for gastrin. Original magnification: ×400. E: morphometric analysis of G cell number in 12-mo-old WT and VC:Men1FL/FL mice. Values are numbers of gastrin-immunostained cells per HPF (×400 magnification). *P < 0.05. F and G: coimmunostaining of paraffin sections for menin (red) and gastrin (green) revealing colocalization of these proteins to antral G cells of WT and VC:Men1FL/FL mice. Nuclei were stained with DAPI (blue). Original magnification: ×400. Open arrowheads indicate menin-expressing G cells; closed arrowhead indicates a menin-negative G cell.
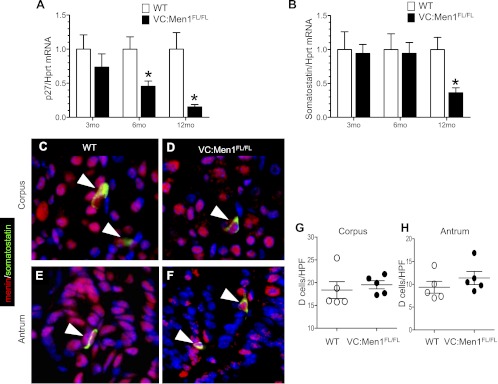
Men1 deletion in the antrum correlates with decreased somatostatin.
As observed in the duodenum, p27Kip1 mRNA, an important menin target, was depressed in the antrum of ∼90% of the VC:Men1FL/FL mice by 12 mo of age (Fig. 7A). Interestingly, somatostatin, a paracrine inhibitor of gastrin, was also downregulated (Fig. 7B). Somatostatin mRNA levels were reduced ∼80% in the VC:Men1FL/FL mice at 12 mo of age, consistent with reciprocal changes in the levels of this paracrine inhibitor of gastrin (Fig. 7B). Double staining of menin and somatostatin in the corpus and antrum of WT mice revealed colocalization of menin to somatostatin-producing D cells (Fig. 7, C and E); even in the VC:Men1FL/FL mice, menin was detected in the D cells, suggesting an indirect effect of menin ablation on the regulation of somatostatin mRNA levels (Fig. 7, D and F). Despite changes in somatostatin RNA, there was no effect on D cell number in the corpus and antrum (Fig. 7, G and H), indicating that menin regulates a change in somatostatin mRNA expression per cell.
Fig. 7.
Somatostatin expression decreased in stomachs of Men1-deficient mice. A and B: RT-qPCR measurement of p27Kip1 and somatostatin mRNA expression in the stomach of 3-, 6-, and 12-mo-old WT and VC:Men1FL/FL mice. Results were normalized to Hprt mRNA expression in the same sample. Values are means ± SE; n = 3–4. *P < 0.05. C–F: coimmunostaining of paraffin sections for menin (red) and somatostatin (green) revealing colocalization of these 2 proteins in the corpus and antrum of WT and VC:Men1FL/FL mice. White arrowheads indicate menin colocalized to D cell. G and H: morphometric analysis of D (somatostatin) cell number in the corpus and antrum of 12-mo-old WT and VC:Men1FL/FL mice. Values (means ± SE) represent number of somatostatin-immunostained cells in well-oriented tissues per HPF (×400 magnification).
Menin deficiency rarely leads to adenoma development in the antrum.
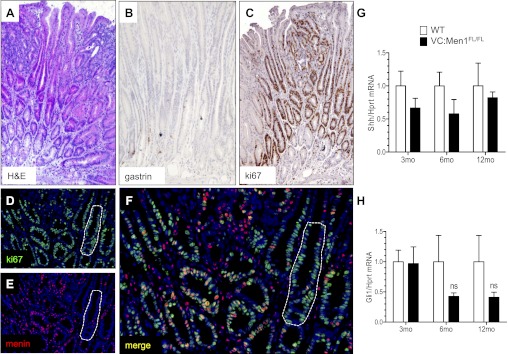
Although gastrin-expressing tumors were not observed in any of the mice, 2 of 18 (11.1%) 12-mo-old VC:Men1FL/FL mice developed adenomas in the antrum, but not the duodenum. The histology of the adenomas is shown in Fig. 8A, and staining for gastrin peptide confirmed that G cells were essentially absent in these hyperplastic regions (Fig. 8B). By contrast, most of the cells in the hyperplastic antrums were highly proliferative and positive for Ki67 (Fig. 8C). Costaining for menin and Ki67 revealed that menin expression was dramatically reduced in the proliferating cells (Fig. 8E), with patches of cells completely negative for menin (Fig. 8, D–F). Since previous studies showed that the Shh pathway is altered in the hypergastrinemic mice (11), we investigated the effects of menin loss on the Shh pathway. We found that mRNA levels of Shh ligand and the Gli1 transcription factor trended downward, but the change was not statistically significant (Figs. 8, G and H). Therefore, canonical Shh signaling does not appear to be playing a role in the hyperplastic changes of the antrum after Men1 deletion.
Fig. 8.
Menin deletion in the antrum promotes adenoma formation. A: hematoxylin-eosin (H&E)-stained paraffin section from the hyperplastic antrum of 12-mo-old VC:Men1FL/FL mice. B: diaminobenzidine immunostaining with hematoxylin counterstaining showing localization of G cells in the hyperplastic tissues by gastrin. C: proliferative cells in the hyperplastic antrum visualized by immunostaining for Ki67. Original magnification: ×200 (A–C). D and E: coimmunostaining for Ki67 (green) and menin (red) with DAPI nuclear counterstain (blue). F: higher-power magnification (×400) and merge of D and E. Patches of hyperplastic (Ki67-positive) cells negative for menin are outlined with a dotted line (D–F). G and H: RT-qPCR measurement of sonic hedgehog (Shh) mRNA and glioma-associated oncogene homolog 1 (Gli1) mRNA normalized to Hprt mRNA levels in stomachs of VC:Men1FL/FL and age-matched WT mice. Values are means ± SE; n = 3–4. ns, Not significant.
Menin expression in the corpus of VC:Men1FL/FL mice is not affected.
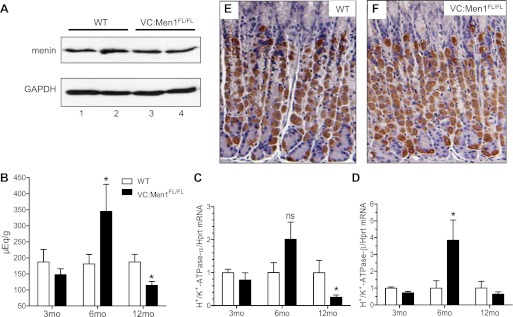
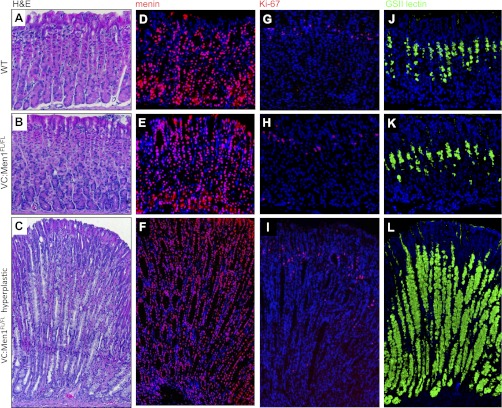
Western blotting revealed no difference in menin expression in the corpus of 12-mo-old VC:Men1FL/FL and WT mice (Fig. 9A). We measured the basal acid content and mRNA expression of H+-K+-ATPase-α- and -β from 3-, 6-, and 12-mo-old VC:Men1FL/FL and WT mice (Fig. 9, B–D). There were no changes in gastric acid level at 3 mo of age in the menin-deficient mice (Fig. 9B), despite some increase in gastrin mRNA and plasma levels (Fig. 6, A and B). Therefore, gastrin expression and peptide increased before changes in gastric acid levels were detected in the menin-deficient mice. By 6 mo of age, gastrin acid levels peaked, but by 12 mo of age they were suppressed, in menin-deficient mice (Fig. 9B). These changes in gastric acid levels were mirrored by changes in H+-K+-ATPase-α- and -β mRNA expression. (Fig. 9, C and D). By contrast, the menin-deficient mice remained hypergastrinemic (Figs. 6, A and B). Therefore, our data suggest that conditional deletion of menin in the antral epithelial cells increases gastrin expression in the antrum first, then as a secondary effect, the stomach acidity changes. Hypergastrinemic VC:Men1FL/FL mice showed initial increases followed by sustained decreases in acid secretion, which is consistent with the hypergastrinemic insulin-gastrin (InsGas) mouse model (35). Analysis of parietal cells by immunostaining for H+-K+-ATPase-α revealed no significant changes in the number of acid-secreting cells at 12 mo of age (Fig. 9, E and F). In addition, VC:Men1FL/FL mice rarely developed hyperplastic lesions in the corpus (Fig. 10, A–C). In the corpus of WT, as well as VC:Men1FL/FL, mice, menin was ubiquitously expressed as a nuclear protein (Fig. 10, D and E). The hyperplastic lesions in the corpus did not show a decrease in menin expression (Fig. 10F), were negative for an increase in Ki67 (Fig. 10I), and primarily showed expansion of cells staining for the mucous neck cell marker GSII (Fig. 10L). We attributed the changes in the corpus to the indirect effects of hypergastrinemia as observed in the InsGas mouse model (35).
Fig. 9.
Menin expression is not altered in the corpus of VC:Men1FL/FL mice. A: Western blot analysis of menin protein in the corpus of 2 WT (lanes 1 and 2) and 2 VC:Men1FL/FL (lanes 3 and 4) mice at 12 mo of age. B: basal gastric acid levels in 3-, 6-, and 12-mo-old fasted WT and VC:Men1FL/FL mice. Values are means ± SE; n = 4–13. *P < 0.05. C and D: relative H+-K+-ATPase-α and -β mRNA expression measured by RT-qPCR from the gastric mucosa of WT and VC:Men1FL/FL mice and normalized to Hprt mRNA. Values are means ± SE; n = 3–4. *P < 0.05. E and F: paraffin sections immunostained with H+-K+-ATPase-α antibodies showing parietal cells in the corpus of WT and VC:Men1FL/FL mice.
Fig. 10.
Characteristics of corpus hyperplasia in 12-mo-old VC:Men1FL/FL mice. A–C: representative hematoxylin-eosin-stained sections of the corpus from a WT and a VC:Men1FL/FL mouse and hyperplastic corpus from a VC:Men1FL/FL mouse. D and E: immunofluorescent staining of sections of corpus from a WT and a VC:Men1FL/FL mouse and hyperplastic corpus from a VC:Men1FL/FL mouse. Nuclear staining of menin (red) and DAPI (blue). G–I: proliferative cells in sections of corpus from a WT and a VC:Men1FL/FL mouse and hyperplastic corpus from a VC:Men1FL/FL mouse visualized by immunostaining for Ki67. J–L: mucous neck cells visualized by staining with Griffonia simplicifolia II (GSII) lectin in sections of corpus from a WT and a VC:Men1FL/FL mouse and a hyperplastic corpus from a VC:Men1FL/FL mouse. Original magnification: ×200.
Men1 deletion in Lgr5-positive stomach antral stem cells does not affect menin expression in G cells.
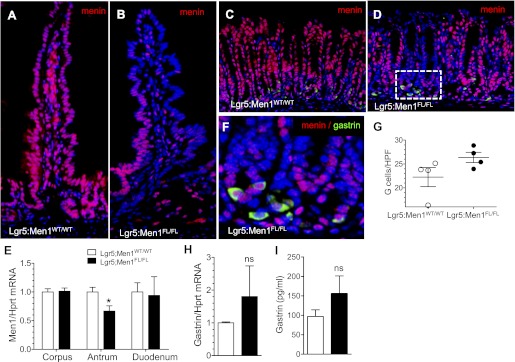
An orphan G protein-coupled receptor, Lgr5, has been shown to mark crypt base columnar stem cells in the intestine and stomach antrum (2, 3). To further characterize the role of menin in the GI epithelium, we bred Men1FL/FL mice to the Lgr5-EGFP-IRES-CreERT2 mouse line to generate Lgr5:Men1FL/FL mice. At 5 mo after tamoxifen-induced Cre activation, we observed loss of menin expression cells in stem cell progeny in the duodenum (Fig. 11B) and whole glands in the antrum (Fig. 11D). RT-qPCR revealed a significant decrease in Men1 mRNA expression in the antrum, but not the corpus and duodenum, of the Lgr5:Men1FL/FL vs. WT mice (Fig. 11E). The low efficiency of Men1 deletion in the duodenum is consistent with variegated expression of the Lgr5 promoter in the small intestine, which limits the use of these mice in Lgr5-Cre-driven knockout strategies. Interestingly, double immunostaining of menin and gastrin revealed residual menin protein expression in some antral G cells, even in the glands that had undergone successful menin deletion (Fig. 11F). Surprisingly, we observed a pattern of deletion in the epithelial vs. gastrin-expressing cells that was the same as that observed with Villin-Cre (Fig. 6G). In addition, G cell numbers (Fig. 11G), gastrin mRNA expression (Fig. 11H), and circulating plasma gastrin levels (Fig. 11I) tended to be higher in these Lgr5:Men1FL/FL mice than in control mice, although the numbers did not reach statistical significance. These results were consistent with data we obtained using the epithelium-specific Cre driver Villin-Cre (Fig. 6, A, B, and E).
Fig. 11.
Epithelium-specific Men1 deletion does not lead to complete menin deletion in G cells. A and B: representative images of menin (red) immunofluorescent staining in sections of duodenum from Lgr5:Men1WT/WT and Lgr5:Men1FL/FL mice 5 mo after tamoxifen administration. Nuclei were stained with DAPI (blue). Original magnification: ×200. C and D: coimmunostaining of paraffin sections for menin (red) and gastrin (green) confirms colocalization of these proteins to antral G cells of Lgr5:Men1WT/WT and Lgr5:Men1FL/FL mice. Nuclei were stained with DAPI (blue). Original magnification: ×200. E: RT-qPCR measurement of Men1 mRNA normalized to Hprt mRNA levels in the corpus, antrum, and duodenum of Lgr5:Men1FL/FL and Lgr5:Men1WT/WT mice. Values are means ± SE; n = 3. *P < 0.05. F: higher-power magnification (×600) of D. G: morphometric analysis of G cell number in Lgr5:Men1WT/WT and Lgr5:Men1FL/FL mice 5 mo after tamoxifen administration. Values are numbers of gastrin-immunostained cells per HPF (×400 magnification). H: RT-qPCR measurement of gastrin mRNA normalized to Hprt mRNA levels in the stomach of Lgr5:Men1FL/FL and Lgr5:Men1WT/WT mice. Values are means ± SE; n = 3. I: enzyme immunoassay measurement of gastrin concentration in circulating plasma of mice fasted for 16 h. Values are means ± SE; n = 3.
DISCUSSION
It is well established that MEN1-driven tumor development follows Knudson's “two-hit” gene model for transformation (18). Because complete Men1-null mice are not viable, analysis of the Men1 locus requires analysis of heterozygous mice or a conditional null allele. All prior studies describing the heterozygous Men1-related phenotype reported tumors in a variety of endocrine organs except the GI tract (5, 10). We used the Villin-Cre mouse line to perform tissue-specific Men1 deletion in the epithelial cells of the duodenum and antrum (19, 20, 28). Deletion of Men1 in the antral epithelial cells induced a nearly twofold increase in gastrin mRNA expression in antral G cell numbers and levels of plasma gastrin. Nearly 50% (6 of 13) of the 12-mo-old VC:Men1FL/FL mice were hypergastrinemic without evidence of gastrin-secreting tumors. These changes in the antrum, in ∼10% of the mice, affected the corpus by inducing the expansion of the GSII-positive mucous neck cell lineage, typically called spasmolytic polypeptide-expressing metaplasia (26). However, in the absence of a robust increase in corpus proliferation and no changes in menin expression, the expansion of these mucous neck cells likely represents a compensatory change in cellular differentiation due to increased gastrin (25).
The effect of Men1 deletion on somatostatin gene expression and subsequent hypergastrinemia was less clear. Although somatostatin mRNA expression was downregulated in the stomach of menin-deficient mice, we did not observe changes in the number of D cells and, specifically, the number of D cells that express menin. We previously showed that somatostatin increases menin protein levels in the gastrin-expressing human gastric adenocarcinoma (AGS) cell line and that the induction requires inhibition of protein kinase A (22). Thus we expected that a deletion of Men1 would prevent somatostatin-mediated induction of menin protein and subsequent suppression of gastrin gene expression by menin in the G cell. Indeed, our results are consistent with this mechanism in the G cell but do not explain why somatostatin levels were also suppressed by 12 mo of age. We considered the possibility that menin might also regulate somatostatin gene expression, but this has not been directly tested. In fact, little is known regarding the upstream regulators of somatostatin gene expression, with the exception that hypergastrinemia correlates with reduced somatostatin levels (36). Since menin levels in the corpus did not change, our results support the notion that reduced menin protein levels prevent suppression of gastrin gene expression by somatostatin, at least in some G cells. Moreover, it raises the possibility that chronic hypergastrinemia, which apparently is an early change after menin deletion, is sufficient to suppress somatostatin.
Consistent with a prior study (29), we observed an increase in proliferation in the duodenal mucosa of 12-mo-old VC:Men1FL/FL mice that correlated with epithelium-specific Men1 deletion. p27Kip1 is a well-known target of somatostatin and menin signaling (14–16, 24, 32). Therefore, the mRNA levels of this cyclin-dependent kinase inhibitor were suppressed in the VC:Men1FL/FL mice as expected and were sufficient to explain the hyperplastic changes in the antral and duodenal mucosa. However, adenomas were observed only in antrums of VC:Men1FL/FL mice, albeit rarely. The duodenal hyperplasia was restricted to the crypt proliferative zone and, surprisingly, did not develop into tumors. More interestingly, no hyperplastic changes occurred in gastrin-expressing cell types in the stomach or duodenum, suggesting that epithelial deletion of menin alone was not a sufficient catalysis for transformation, despite a hyperplastic epithelium. A corollary to this observation was the fact that several months were required before phenotypic changes, such as hypergastrinemia, emerged in the VC:Men1FL/FL mice, suggesting that other genetic defects are likely necessary for development of gastrinomas.
Despite one report suggesting that Men1 deletion alone is sufficient to produce gastrinomas (5), our study and the original report on the conditional deletion of this locus (10) agree that loss of menin alone is insufficient to produce gastrinomas. Moreover, Crabtree et al. (10) examined Men1+/− mice that developed loss of heterozygosity in the pituitary and adrenal glands and pancreatic islets, where hormone-producing tumors eventually developed. More importantly, neither study measured plasma gastrin levels to demonstrate that loss of menin derepresses gastrin gene expression. While the number of antral G cells that remained positive for menin was surprising, we concluded that a sufficient number of these neuroendocrine cells exhibited reduced menin levels, permitting derepression of the gastrin gene. Even deletion of the Men1 gene in the Lgr5-positive stem cells mimicked our findings with respect to gastrin in 6-mo-old VC:Men1FL/FL mice.
In conclusion, tissue-specific deletion of Men1 in the mucosa of the luminal GI tract is sufficient to induce hypergastrinemia and epithelial hyperplasia, but not gastrinomas. This result raises the possibility that gastrin-expressing tumors exhibit differential sensitivity to regulation by menin. In particular, transformation of the gastrin-expressing cell type to a bona fide malignancy appears to require additional gene mutations that have yet to be determined.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R37 DK-45729 (to J. L. Merchant) and the use of core facilities of the University of Michigan Digestive Disease Research Center (Peptide Center) supported by Grant P30 DK-34933.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.A.V. and J.L.M. are responsible for conception and design of the research; N.A.V., M.M.H., and J.M.V. performed the experiments; N.A.V. and J.L.M. analyzed the data; N.A.V. and J.L.M. interpreted the results of the experiments; N.A.V. prepared the figures; N.A.V. and J.L.M. drafted the manuscript; N.A.V., M.M.H., J.M.V., and J.L.M. edited and revised the manuscript; N.A.V., M.M.H., J.M.V., and J.L.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank M. Van Dort for use of the gamma counter.
REFERENCES
- 1. Anlauf M, Perren A, Meyer CL, Schmid S, Saremaslani P, Kruse ML, Weihe E, Komminoth P, Heitz PU, Kloppel G. Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology 128: 1187–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6: 25–36, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome. I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2,229 cases from the literature. Medicine (Baltimore) 85: 295–330, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol 17: 1880–1892, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Brand SJ, Fuller PJ. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem 263: 5341–5347, 1988 [PubMed] [Google Scholar]
- 7. Brandi ML. Multiple endocrine neoplasia type 1. Rev Endocr Metab Disord 1: 275–282, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Crabtree JS, Wang Y, Roe BA, Weisemann J, Boguski MS, Agarwal SK, Kester MB, Kim YS, Heppner C, Dong Q, Spiegel AM, Burns AL, Marx SJ. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276: 404–407, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Chandrasekharappa SC, Teh BT. Functional studies of the MEN1 gene. J Intern Med 253: 606–615, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, Lorang D, Libutti SK, Chandrasekharappa SC, Marx SJ, Spiegel AM, Collins FS. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci USA 98: 1118–1123, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El-Zaatari M, Tobias A, Grabowska AM, Kumari R, Scotting PJ, Kaye P, Atherton J, Clarke PA, Powe DG, Watson SA. De-regulation of the sonic hedgehog pathway in the InsGas mouse model of gastric carcinogenesis. Br J Cancer 96: 1855–1861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friesen SR, Tomita T. Pseudo-Zollinger-Ellison syndrome: hypergastrinemia, hyperchlorhydria without tumor. Ann Surg 194: 481–493, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gibril F, Venzon DJ, Ojeaburu JV, Bashir S, Jensen RT. Prospective study of the natural history of gastrinoma in patients with MEN1: definition of an aggressive and a nonaggressive form. J Clin Endocrinol Metab 86: 5282–5293, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Horiguchi K, Yamada M, Satoh T, Hashimoto K, Hirato J, Tosaka M, Yamada S, Mori M. Transcriptional activation of the mixed lineage leukemia-p27Kip1 pathway by a somatostatin analogue. Clin Cancer Res 15: 2620–2629, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, Hayes DN, Shanmugam KS, Bhattacharjee A, Biondi CA, Kay GF, Hayward NK, Hess JL, Meyerson M. Menin associates with a trithorax family histone methyltransferase complex and with the HoxC8 locus. Mol Cell 13: 587–597, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Karnik SK, Hughes CM, Gu X, Rozenblatt-Rosen O, McLean GW, Xiong Y, Meyerson M, Kim SK. Menin regulates pancreatic islet growth by promoting histone methylation and expression of genes encoding p27Kip1 and p18INK4c. Proc Natl Acad Sci USA 102: 14659–14664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kloppel G, Anlauf M. Gastrinoma—morphological aspects. Wien Klin Wochenschr 119: 579–584, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Knudson AG. Antioncogenes and human cancer. Proc Natl Acad Sci USA 90: 10914–10921, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Q, Jia Z, Wang L, Kong X, Guo K, Tan D, Le X, Wei D, Huang S, Mishra L, Xie K. Disruption of Klf4 in villin-positive gastric progenitor cells promotes formation and progression of tumors of the antrum in mice. Gastroenterology 142: 531–542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem 277: 33275–33283, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Marx SJ, Stratakis CA. Multiple endocrine neoplasia—introduction. J Intern Med 257: 2–5, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Mensah-Osman E, Zavros Y, Merchant JL. Somatostatin stimulates menin gene expression by inhibiting protein kinase A. Am J Physiol Gastrointest Liver Physiol 295: G843–G854, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mensah-Osman EJ, Veniaminova NA, Merchant JL. Menin and JunD regulate gastrin gene expression through proximal DNA elements. Am J Physiol Gastrointest Liver Physiol 301: G783–G790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, Schnepp RW, Krankel C, Livolsi VA, Gibbs D, Hua X, Roeder RG, Meyerson M, Hess JL. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci USA 102: 749–754, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM, Jr, Konieczny SF, Goldenring JR. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139: 2028–2037; e2029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nomura S, Baxter T, Yamaguchi H, Leys C, Vartapetian AB, Fox JG, Lee JR, Wang TC, Goldenring JR. Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology 127: 582–594, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Polak JM, Stagg B, Pearse AG. Two types of Zollinger-Ellison syndrome: immunofluorescent, cytochemical and ultrastructural studies of the antral and pancreatic gastrin cells in different clinical states. Gut 13: 501–512, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiao XT, Ziel JW, McKimpson W, Madison BB, Todisco A, Merchant JL, Samuelson LC, Gumucio DL. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology 133: 1989–1998, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ratineau C, Bernard C, Poncet G, Blanc M, Josso C, Fontaniere S, Calender A, Chayvialle JA, Zhang CX, Roche C. Reduction of menin expression enhances cell proliferation and is tumorigenic in intestinal epithelial cells. J Biol Chem 279: 24477–24484, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Roy PK, Venzon DJ, Feigenbaum KM, Koviack PD, Bashir S, Ojeaburu JV, Gibril F, Jensen RT. Gastric secretion in Zollinger-Ellison syndrome. Correlation with clinical expression, tumor extent and role in diagnosis—a prospective NIH study of 235 patients and a review of 984 cases in the literature. Medicine (Baltimore) 80: 189–222, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Scacheri PC, Crabtree JS, Kennedy AL, Swain GP, Ward JM, Marx SJ, Spiegel AM, Collins FS. Homozygous loss of menin is well tolerated in liver, a tissue not affected in MEN1. Mamm Genome 15: 872–877, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Taguchi R, Yamada M, Horiguchi K, Tomaru T, Ozawa A, Shibusawa N, Hashimoto K, Okada S, Satoh T, Mori M. Haploinsufficient and predominant expression of multiple endocrine neoplasia type 1 (MEN1)-related genes, MLL, p27Kip1 and p18Ink4C in endocrine organs. Biochem Biophys Res Commun 415: 378–383, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and Tris (HotSHOT). Biotechniques 29: 52–54, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Walsh JH. Role of gastrin as a trophic hormone. Digestion 47 Suppl 1: 11–16; discussion 49–52, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Wang TC, Koh TJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Dockray GJ. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest 98: 1918–1929, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Hypergastrinemia in response to gastric inflammation suppresses somatostatin. Am J Physiol Gastrointest Liver Physiol 282: G175–G183, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg 142: 709–723; discussion 724–708, 1955 [PMC free article] [PubMed] [Google Scholar]