Abstract
Pomegranate seed oil (PSO), which is the major source of conjugated linolenic acids such as punicic acid (PuA), exhibits strong anti-inflammatory properties. Necrotizing enterocolitis (NEC) is a devastating disease associated with severe and excessive intestinal inflammation. The aim of this study was to evaluate the effects of orally administered PSO on the development of NEC, intestinal epithelial proliferation, and cytokine regulation in a rat model of NEC. Premature rats were divided into three groups: dam fed (DF), formula-fed rats (FF), or rats fed with formula supplemented with 1.5% of PSO (FF + PSO). All groups were exposed to asphyxia/cold stress to induce NEC. Intestinal injury, epithelial cell proliferation, cytokine production, and trefoil factor 3 (Tff3) production were evaluated in the terminal ileum. Oral administration of PSO (FF+PSO) decreased the incidence of NEC from 61 to 26%. Feeding formula with PSO improved enterocyte proliferation in the site of injury. Increased levels of proinflammatory IL-6, IL-8, IL-12, IL-23, and TNF-α in the ileum of FF rats were normalized in PSO-treated animals. Tff3 production in the FF rats was reduced compared with DF but not further affected by the PSO. In conclusion, administration of PSO protects against NEC in the neonatal rat model. This protective effect is associated with an improvement of intestinal epithelial homeostasis and a strong anti-inflammatory effect of PSO on the developing intestinal mucosa.
Keywords: enteral nutrition, intestinal injury, mucosal inflammation, cytokines
the pomegranate (punica granatum) is one of the oldest edible fruits with a renowned medical history and a symbol of life, longevity, and health (40). Native to the region of Persia and the Himalayas, P. granatum has been widely cultivated and consumed for centuries by many different cultures starting from the Mediterranean region extending to China, India, and southeast Asia (36). Introduced into Latin America in 18th century, P. granatum is now commercially cultivated in dry Southwest of the United States. The fruit gives rise to three parts: the seeds, the juice, and the peels. Despite the growing popularity of juice products, the importance of the oily phase of the seed has been largely overlooked until recent years.
Pomegranate seed oil (PSO) is predominantly composed of triglycerides containing unsaturated fatty acids including high levels of conjugated linolenic acids (CLnA; 78–86%), followed by oleic acid (4–7%, 18:1n-9), linoleic acid (LA; 3–6%, 18:2n-6), palmitic acid (2–3%, 16:0), and stearic acid (2%, 18:0) (52). PSO CLnA consist of up to seven geometric isomers of 9,11,13-octadecatrienoic acid (18:3), separated by a single carbon-carbon linkage (Fig. 1) as opposed to being separated by methylene groups in α-linolenic acid (18:3n-3) (52). The major CLnA in PSO is punicic acid (PuA; 57–71%) (52). Structurally, PuA is a C18 carbon fatty acid containing cis-9, trans-11, cis-13 double bonds (cis-9,trans-11,cis-13-octadecatrienoic acid) (42).
Fig. 1.
Structure of conjugated double bonds in fatty acids.
It has been suggested that the higher intake of conjugated fatty acids is associated with the lower incidence of inflammatory diseases in the Middle East and Asian populations compared with Western countries (8). Experimental studies showed that PSO is able to reduce tumor occurrence in both ex vivo and in vivo rodent models (29, 35, 43). In addition, recent studies indicate that PuA ameliorates TNSB- and DSS-induced colitis (2, 8).
Necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in premature infants. Despite an increasing occurrence of NEC in the United States, the etiology is unknown. The combination of a genetic predisposition, intestinal immaturity, and high immunoreactivity of the intestinal mucosa leads to the development of NEC (48). The type and amount of enteral feeds is critical in this process (22). Unfortunately, no predictive diagnostic tests or effective treatments are available at this time (13).
Beneficial effects of infant formula supplemented with polyunsaturated fatty acids (PUFA) on the development of the central nervous system and visual acuity of neonates are well established (23). However, only limited information is available regarding the effect of PUFA on neonatal intestines. In a clinical study, Carlson et al. (14) found a lower incidence of NEC in infants fed formula containing higher amounts of PUFA compared with controls. In the rat NEC model, supplementation of PUFA into formula reduces the degree of intestinal injury (1, 12). The mechanism of PUFA-induced protection against NEC is not clear, but the decreased expression of Toll-like receptor 4 and platelet-activating factor receptor was observed (38). However, nothing is known about the effect of the CLnA on NEC pathogenesis.
The gut mucosal immune system is capable of producing an array of cytokines important in the development and control of the inflammatory response (32). We (26, 27) and others (20, 41, 53) have shown the dysregulation of inflammatory cytokines in the intestinal mucosa during NEC pathogenesis. Particularly, interleukin (IL)-6, IL-8, IL-12, IL-18, and tumor necrosis factor-α (TNF-α) have an important role and a diagnostic value in sepsis and in NEC (9, 26, 45). IL-23 is critical in the etiology of inflammatory bowel disease (51), but its role in NEC pathogenesis has not been studied yet.
The intestinal epithelium protects the underlying lamina propria against damage and microbe invasion through the production of mucins (Muc) and trefoil factors (Tff). In the rat small intestine, Muc2 is the predominant secretory mucin produced by goblet cells. Mucins are cosecreted with Tff, small peptides exerting multiple biological effects on epithelium. Impaired production of Muc2 and Tff3 has been reported in clinical and experimental NEC (15, 33).
The aim of this study was to determine whether oral administration of PSO protects against experimental NEC and to elucidate the mechanisms underlying the protective actions of PSO. In a rat model of NEC, we investigated the efficacy of PSO on disease development and severity, the effect on intestinal morphology, enterocyte proliferation, mucin expression, and proinflammatory cytokine production.
MATERIALS AND METHODS
Animal model of NEC and diets.
This protocol was approved by the Animal Care and Use Committee of the University of Arizona (A-324801-95081). Sixty neonatal Sprague-Dawley rats (Charles River Laboratory, Pontage, MI) were collected by caesarian section 24 h before their scheduled birth, and the first feeding started 2 h after delivery. Animals were hand fed six times daily with a total volume of 850 μl of rat milk formula per day (19). Experimental NEC was induced by asphyxia (breathing 100% nitrogen gas for 60 s) and cold stress (4°C for 10 min) twice daily (18). Caesarian section-delivered pups were divided into the following experimental groups: neonatal rats formula fed (FF; n = 20), neonatal rats fed with formula containing 1.5% of PSO (FF + PSO; n = 20), and dam-fed littermates fed by surrogate mothers as a baseline control (DF; n = 20). After 96 h, all surviving animals were terminated via decapitation. Animals that developed signs of distress or imminent death before 96 h were terminated and included in the study.
The rat milk formula used for feeding of neonatal rats was prepared primarily from evaporated milk as described previously (19). In the FF group, the major source of lipids in the formula was the mixture of Intralipid (Fresenius Kabi, Uppsala, Sweden) and almond oil. In the FF + PSO diet, almond oil was replaced with 1.5% wt/wt of PSO. The FA composition of the FF and FF + PSO diets expressed as a percent of total FA is shown in Table 1. The FF + PSO diet contained 2.7% of CLnA of the total FA, whereas the CLnA were absent in the FF diet. Concentration of arachidonic acid (AA; 20:4n-6) and docosahexaenoic acid (DHA; 22:6n-3) was similar in both diets.
Table 1.
Fatty acid composition (%wt/wt) of the FF and FF + PSO diets
| Fatty Acids | FF | FF + PSO |
|---|---|---|
| 8:0 | 2.2 | 1.6 |
| 10:0 | 1.7 | 1.6 |
| 12:0 | 2.1 | 2.0 |
| 14:0 | 6.7 | 6.7 |
| 14:1n-5 | 0.7 | 0.7 |
| 15:0 | 1.0 | 1.1 |
| iso-15:0 | 0.3 | 0.4 |
| 16:0 | 25.3 | 25.3 |
| iso-16:0 | 0.2 | 0.3 |
| 16:1 | 1.3 | 1.3 |
| 17:0 | 0.4 | 0.5 |
| anteiso-17:0 | 0.4 | 0.4 |
| 18:0 | 9.3 | 9.2 |
| 18:1n-7 | 1.0 | 1.1 |
| 18:1n-9 | 22.2 | 20.6 |
| 18:2n-6 (LA) | 18.7 | 18.6 |
| 18:3n-3 (LNA) | 1.9 | 1.9 |
| 20:0 | 0.1 | 0.2 |
| cis-9, trans-11 18:2 (rumenic acid) | 0.2 | 0.2 |
| cis-9, trans-11, cis-13 18:3 (PuA) | 0.0 | 1.7 |
| Other 9,11,13 −18:3 isomers | 0.0 | 1.0 |
| 20:1 | 0.2 | 0.1 |
| 20:3n-6 | 0.3 | 0.2 |
| 20:4n-6 (AA) | 1.7 | 1.6 |
| 22:5n-6 | 0.2 | 0.2 |
| 22:5n-3 | 0.2 | 0.2 |
| 22:6n-3 (DHA) | 1.4 | 1.2 |
| Summary CLnA | 0.0 | 2.7 |
| Summary n-6 | 20.9 | 20.6 |
| Summary n-3 | 3.5 | 3.3 |
FF, formula fed; PSO, 1.5% pomegranate seed oil; LA, linoleic acid; LNA, linolenic acid; PuA, punicic acid; AA, arachidonic acid; DHA, docosahexaenoic acid; CLnA, conjugated linolenic acid.
All measurements were performed in the ileum from the same set of rat pups that were used for NEC evaluation.
NEC evaluation.
After termination, a 2-cm piece of distal ileum was removed and fixed in 70% ethanol, paraffin embedded, sectioned at 4–6 μm, and stained with hematoxylin and eosin (H&E) for histological evaluation of NEC. Pathological changes in intestinal architecture were evaluated using our previously published NEC scoring system (18, 33, 50). Histological changes in the ileum were scored by a blinded evaluator and graded as follows: 0 (normal), no damage; 1 (mild), slight submucosal and/or lamina propria separation; 2 (moderate), moderate separation of submucosa and/or lamina propria, and/or edema in submucosal and muscular layers; 3 (severe), severe separation of submucosa and/or lamina propria, and/or severe edema in submucosa and muscular layers, region villous sloughing; 4 (necrosis), loss of villi and necrosis. Intermediate scores of 0.5, 1.5, 2.5 and 3.5 were also utilized to more accurately assess levels of ileal damage when necessary (18, 33, 50). To determine incidence of NEC, animals with histological scores of <2 have not developed NEC; animals with histological scores of ≥2 have developed NEC (33, 50).
Morphometrical measurements and epithelial cell proliferation in the ileum.
A 2-cm section of distal ileum stained with H&E was used for morphometric measurements as previously described (16). Briefly, twenty villi were measured in each histological sample, and 8–10 animals were evaluated per experimental group. Sections from animal with a NEC score of ≥3 were not included in analyses because of the lack of intact tissue to evaluate. Villi were measured from the tip to the crypt base and using an image analysis system (Image-Pro Plus; Media Cybernetics, Silver Spring, MD). Statistical analysis of all morphological data was performed in a blind manner to prevent observer bias.
Expression of proliferating cell nuclear antigen (PCNA) was evaluated in serial sections of the paraffin-embedded samples from the ileum prepared as we previously described (16). After deparaffinization, rehydration and incubation in hydrogen peroxide, sections were blocked with 1.5% appropriate serum (Vector Laboratories, Burlingame, CA), then incubated with 2.0 μg/ml goat polyclonal anti-rat PCNA (Santa Cruz Biotechnology, Santa Cruz, CA), followed by biotinylated secondary antibody (Vector Laboratories), Vectastain Elite ABC reagent (Vector Laboratories), diaminobenzidine activated by hydrogen peroxide, and counterstained with hematoxylin. Control sections were treated with the same procedure except they were incubated with 2.0 mg/ml rabbit Ig (for Casp-3) or 2.0 μg/ml goat Ig (for PCNA) (Sigma, St. Louis, MO). No immunostaining was observed in the controls. The enumeration of positively stained cells was accomplished by counting 15 villi per animal and evaluating six rats per experimental group.
Fatty acid analysis.
Total lipid from rat milk formula and ileal samples were extracted using a modification of the Bligh and Dyer method (7). Ileum was sampled for fatty acid analysis, with care taken to insure there was no obvious contamination from residual luminal contents. Fatty acid methyl esters (FAME) were prepared using 14% BF3 in methanol (Sigma Chemical). FAME analyses were performed using a Hewlett Packard 5890 Gas Chromatography-Flame Ionization Detector (GC-FID). A BPX-70 column (25 m × 0.22 mm × 0.25 μm; SGE, Austin, TX) was used for the analysis with H2 as the carrier gas. FAME identities were determined using a Varian Star 3400 GC coupled to a Varian Saturn 2000 ion trap MS. FAME identities and double-bond position were determined by chemical ionization mass spectrometry using acetonitrile as reagent gas (37). FA levels are expressed as percentage, weight for weight (%wt/wt).
RNA preparation and real-time PCR.
Total RNA was isolated from ileal tissue (snap frozen in liquid N2) using the RNeasy Plus Mini Kit (Qiagen, Santa Clarita, CA) as described in the manufacturer's protocol. RNA concentration was quantified by ultraviolet spectrophotometry at 260 nm, and the purity and integrity were determined using a NanoDrop (Thermo Fisher Scientific, Wilmington, DE) (34).
RT real-time PCR assays were performed to quantify steady-state mRNA levels of selected cytokines (IL-6, IL-8, IL-12, IL-18, IL-23, and TNF-α). cDNA was synthesized from 0.2 μg of total RNA. Real-time PCR amplification was performed using Primer Express Software (Applied Biosystems, Foster City, CA). Target probe was labeled with fluorescent reporter dye. PreDeveloped TaqMan primers and probes were used for the detection of IL-6, IL-8, IL-12, IL-18, and IL-23. The following sequences was used for TNF-α (GenBank X66539): sense primer- 5′-gtgatcggtcccaacaagga-3′; anti-sense primer- 5′-gggccatggaactgatgaga-3′; and probe- 5′-cccatttgggaacttc-3′.
Reporter dye emission was detected by an automated sequence detector combined with ABI Prism 7700 Sequence Detection System software (Applied Biosystems). Real-time PCR quantification was then performed using TaqMan 18S controls.
Immunohistology and enumeration of Muc2- and Tff3-positive cells.
Serial sections of the ileum were stained for either Muc2 or Tff3. Briefly, after deparaffinization and rehydration, sections were incubated with either rabbit anti-Muc2 polyclonal antibody (Santa Cruz Biotechnology) or rabbit polyclonal Tff3 antibody (54) for 30 min, washed with PBS three times, and incubated with a biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) for 30 min. Vectastain Elite ABC reagent (Vector Laboratories) was then applied, followed by diaminobenzidine as a substrate. Sections were counterstained with hematoxylin, dehydrated, and mounted on coverslips. Muc2- and Tff3-positive cells were counted from nine animals per experimental group, and the total number of epithelial cells per crypt-villus unit was also enumerated. Fifteen crypt-villus units were counted for each animal.
Statistics.
Statistical analyses between DF, FF, and FF + PSO groups were performed using ANOVA followed by Fisher paired least-significant difference and by the Student's t-test at the 95% confidence level. Analysis of NEC score between groups was accomplished using the Kruskal-Wallis test for nonparametric values followed by pairwise comparison using the Mann-Whitney test. The Pearson's chi-squared (χ2) test was used to analyze differences in incidence of NEC between groups. All statistical analyses were conducted using the statistical program StatPlus:mac LE for Macintosh computers (AnalystSoft, Alexandria, VA). All numerical data are expressed as means ± SE.
RESULTS
Oral administration of PSO reduces the severity and incidence of NEC.
There was no difference between experimental groups in the survival of C-section-delivered premature rats. The survival rates for these studies were as follows: DF 90% (18/20); FF 90% (18/20); and FF + PSO 95% (19/20). The degree of intestinal injury and the incidence of NEC were evaluated in prematurely born rats fed formula with or without PSO. Damage to the villi and submucosa was evident in the FF group and supplementation of PSO into formula significantly reduced ileal damage (Fig. 2) to a median histological NEC score of 1.5 compared with 2.0 in the FF group (P ≤ 0.01). The incidence of NEC was markedly decreased from 61% (11/18) in the FF group to 26% (5/19) in the FF + PSO group. The null hypothesis of no difference in NEC incidence between formula-fed groups was rejected (P = 0.033); PSO reduced the incidence of NEC by 57% (61% vs. 26%). In DF rats, the median histological score was 0.75 and incidence of NEC was 0% (0/18).
Fig. 2.
Severity of necrotizing enterocolitis (NEC) in neonatal rat model. A: histological NEC score in the dam-fed group (DF) (△; n = 18), formula-fed group (FF) (●; n = 18), and group that was formula fed with 1.5% pomegranate seed oil added (FF + PSO) (◆; n = 19) groups are shown. Ileal damage was assessed using the histological scoring scale of 0 (normal) to 4 (necrosis) as previously described (18, 33, 50). Animals with scores ≥2 are considered NEC positive. B: representative histology shows area of damage to ileal architecture in the FF group (histological score 2.0) compared with the ileum of FF + PSO (histological score 1.5) or DF controls (histological score 0.5). Magnification: ×200.
CLnAs are incorporated into the ileum.
The FF diet had no detectable PuA or other cis-trans isomers of 9,11,13 C18:3 such as the eleostearic acid isomers (Table 1). In contrast, FF + PSO contained 1.7% and 1.0% PuA and 9,11,13–18:3 isomers; these CLnA proportions are within the range of values reported very recently for several cultivars of pomegranate seeds (21). A conjugated linoleic acid (CLA), rumenic acid (cis-9, trans-11 18:2) was found at 0.2% in both groups.
PuA and other isomers were found in ileal lipids of the FF + PSO and, unexpectedly, at low levels in the FF group (Table 2). The PuA concentration was found to be only 28% of the total CLnA isomers detected (1.10 of 3.86 total) even though it was >60% in the feed. Rumenic acid was more than fourfold greater in the FF + PSO group (0.33) compared with the FF group.
Table 2.
Fatty acid composition (%wt/wt) in ileal lipids
| Fatty Acids | FF | FF + PSO |
|---|---|---|
| 14:0 | 1.71 ± 0.83 | 1.32 ± 0.56 |
| 15:0 | 0.47 ± 0.15 | 0.43 ± 0.08 |
| 16:0 | 25.01 ± 3.24 | 22.16 ± 1.82 |
| 16:1n-9 | 0.33 ± 0.10 | 0.20 ± 0.05 |
| 16:1n-7 | 0.65 ± 0.21 | 0.39 ± 0.10 |
| 17:0 | 0.43 ± 0.10 | 0.40 ± 0.05 |
| 18:0 | 16.23 ± 1.95 | 16.37 ± 2.15 |
| 18:1 | 12.74 ± 3.89 | 10.15 ± 1.21 |
| 18:1 | 1.76 ± 0.27 | 1.27 ± 0.10 |
| 18:2n-6 (LA) | 20.54 ± 3.25 | 24.34 ± 2.53 |
| 18:3n-6 | 0.38 ± 0.21 | 0.26 ± 0.11 |
| 18:3n-3 (LNA) | 0.62 ± 0.71 | 0.86 ± 0.42 |
| 20:0 | 0.97 ± 0.13 | 1.43 ± 0.23 |
| cis-9, trans-11 18:2 (rumenic acid) | 0.07 ± 0.15 | 0.33 ± 0.08 |
| cis-9, trans-11, cis-13 18:3 (PuA) | 0.07 ± 0.15 | 1.10 ± 0.42 |
| Other 9,11,13- 18:3 isomers | 0.21 ± 0.48 | 2.76 ± 1.00 |
| 20:1 | 0.40 ± 0.25 | 0.44 ± 0.10 |
| 20:2 | 0.50 ± 0.23 | 0.31 ± 0.10 |
| 20:3n-6 | 1.50 ± 0.78 | 0.99 ± 0.43 |
| 20:4n-6 (AA) | 8.16 ± 3.72 | 7.34 ± 1.60 |
| 22:0 | 1.19 ± 0.31 | 1.47 ± 0.28 |
| 22:4n-6 | 0.83 ± 0.28 | 0.67 ± 0.18 |
| 24:0 | 1.64 ± 0.57 | 1.74 ± 0.34 |
| 24:1 | 0.93 ± 0.31 | 0.61 ± 0.19 |
| 22:5n-3 | 0.38 ± 0.16 | 0.34 ± 0.09 |
| 22:6n-3 (DHA) | 2.29 ± 0.79 | 2.32 ± 0.44 |
Results are expressed as means ± SD.
PSO improves intestinal morphology.
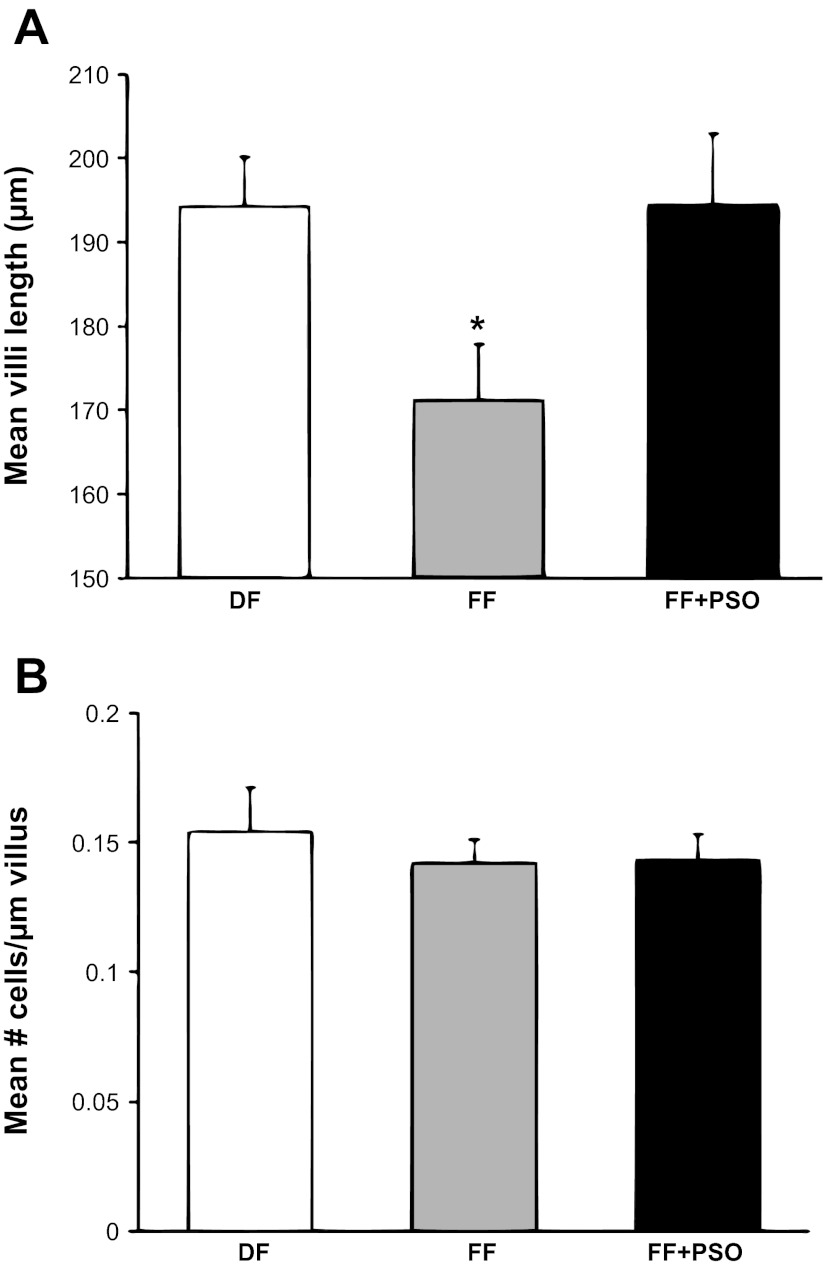
Intestinal morphology was determined by light microscopy (Fig. 3A). The FF group had significantly shorter villi compared with healthy controls (171.2 ± 6.7 μm vs. 194.3 ± 5.9 μm). In the formula-fed pups, supplementation of PSO into formula significantly increased villi length (Fig. 3B), reaching values similar to those in the DF group (194.4 ± 8.6 μm; P ≤ 0.01 vs. DF or FF + PSO). To determine whether the increase in villus height in the FF + PSO group was a result of hypertrophy or hyperplasia, the total number of epithelial cells in each measured villus was counted, and results from these measurements were expressed as number of epithelial cells per micrometer of villus. There were no statistically significant differences seen between two groups, indicating that PSO caused hyperplasia of the epithelial cells and not hypertrophy (Fig. 3C).
Fig. 3.
Ileal structure in neonatal rat model of NEC. Morphological measurements of villus length (A) and number of villi epithelial cells (B) in the ileum of FF or FF + PSO. Values are means ± SE; n = 8–10 animals/experimental group. *P ≤ 0.01 vs. DF or FF + PSO.
PSO induces epithelial proliferation in the ileum.
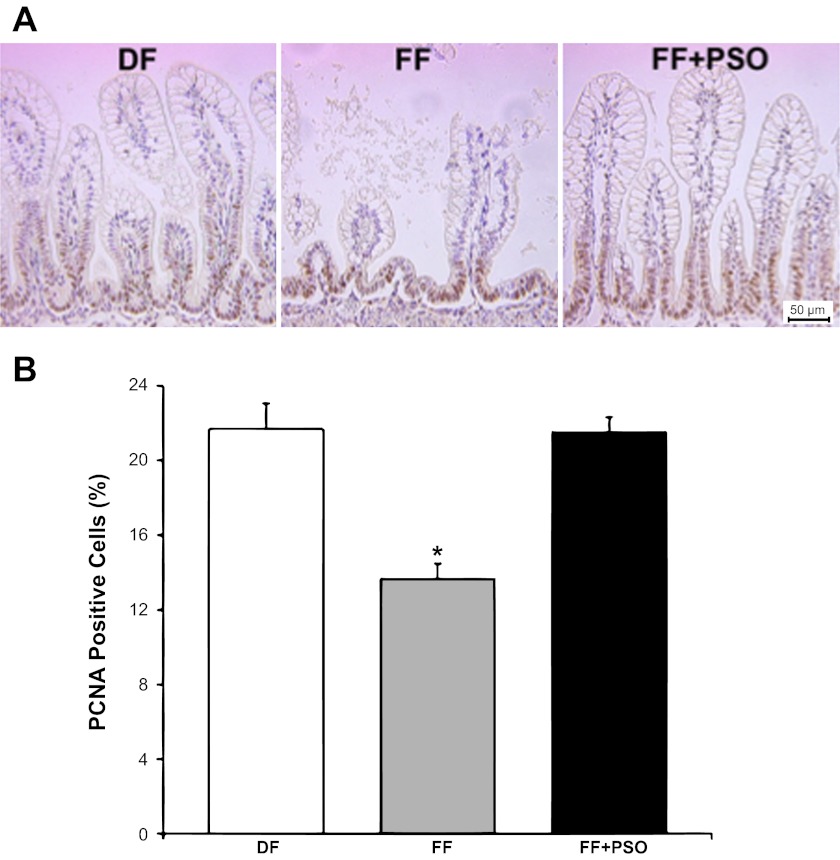
To determine whether the PSO induces mitogenic effects on epithelial cells in the ileum, the cell proliferation marker PCNA was evaluated by immunohistochemistry, and PCNA-positive cells were enumerated (16). PCNA-positive cells were detected in the crypt epithelium and the lower half of villi (Fig. 4A). The number of PCNA-positive cells was significantly reduced in the FF group (13.6%) compared with healthy controls, indicating a significant decrease in the intestinal epithelial cell proliferation in formula-fed pups (Fig. 4B; *P ≤ 0.01 vs. DF or FF + PSO). Supplementation of PSO into the formula normalized this effect, and the number of PCNA-positive cells in the terminal ileum was similar to values seen in the DF group (21.5% in the FF + PSO vs. 21.7% in the DF group).
Fig. 4.
Effect of PSO on expression of the proliferating cell nuclear antigen (PCNA) in the ileum of neonatal rats. A: PCNA-positive representative slides from DF, FF, and FF + PSO groups are shown. Magnification: ×200. B: enumeration of PCNA-positive cells in neonatal rat ileum is shown in the graph. Cells were counted in 15 villi/animal, and results are expressed as percentage of PCNA-positive cells/100 epithelial cells ± SE; n = 6 rats/experimental group. *P ≤ 0.01 vs. DF or FF + PSO.
PSO reduces inflammatory response in the ileum.
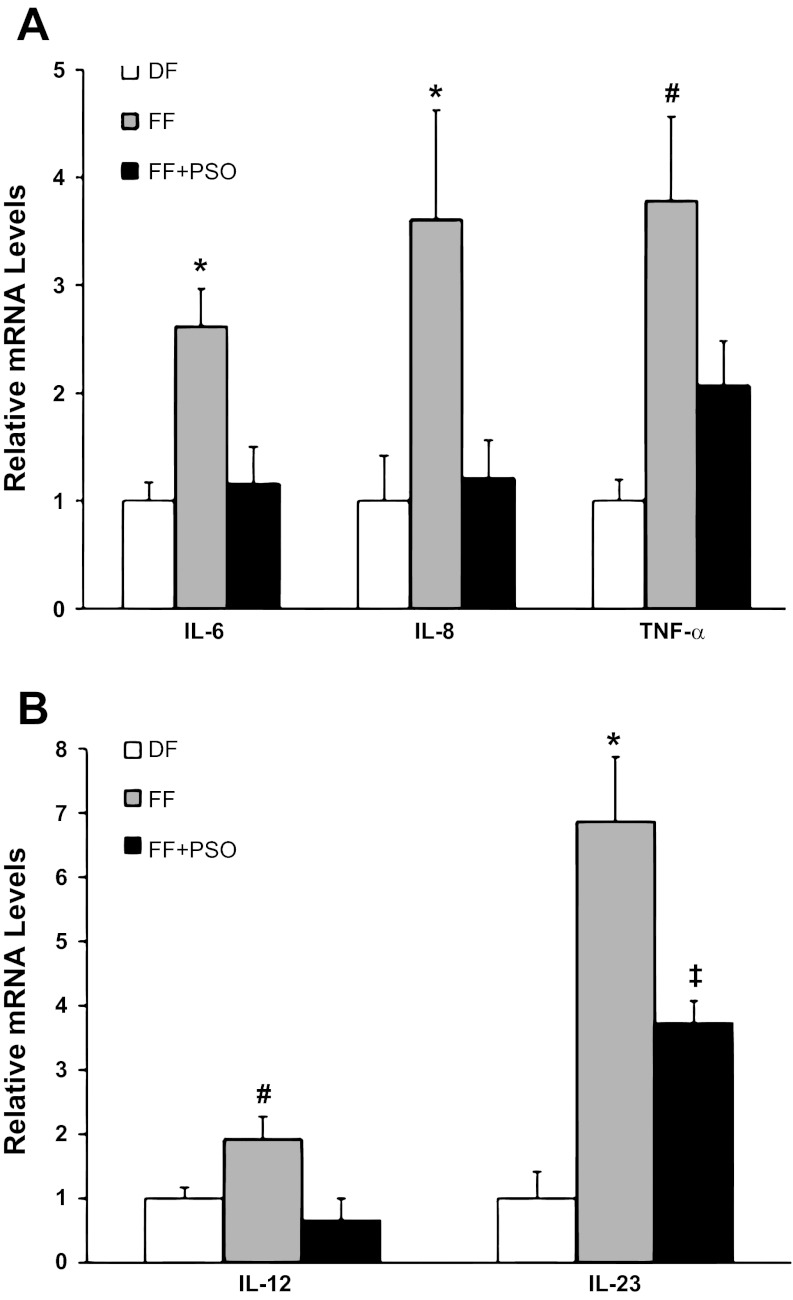
Proinflammatory and effector cytokines IL-6, IL-8, IL-12, IL-18, IL-23, and TNF-α are the major cytokines associated with NEC pathogenesis and neonatal sepsis (26, 53). Thus gene expression of these selected cytokines was determined in the terminal ileum using RT-PCR. Expression of IL-6, IL-8, and TNF-α (Fig. 5A) was significantly increased in animals with NEC (FF) and normalized in the FF + PSO group to levels found in DF animals (*P ≤ 0.01, #P ≤ 0.05). Gene expression of IL-12 and IL-23 (Fig. 5B) was markedly increased in NEC animals compare to DF or FF + PSO (*P ≤ 0.01), but IL-23 mRNA levels in the FF + PSO group was significantly higher compared with DF rats (‡P ≤ 0.05). There was no effect on ileal gene expression of IL-18 (results not shown) between experimental groups.
Fig. 5.
IL-6, IL-8, IL-12, IL-23, and TNF-α mRNA levels in neonatal rat ileum. A: IL-6, IL-8, and TNF-α mRNA levels. B: IL-12 and IL-23 mRNA levels. The mean steady-state mRNA levels for the DF group were assigned a value of 1.0, and mean mRNA levels for the FF and FF + PSO groups were determined relative to this number. Values are means ± SE; n = 10–12 animals/experimental group. *P ≤ 0.01 vs. DF or FF + PSO; #P ≤ 0.05 vs. DF or FF + PSO; ‡P ≤ 0.05 vs. DF.
Evaluation of Muc2 and Tff3 production.
Ileal Muc2 production was evaluated using immunohistochemistry, and enumeration of Muc2-positive cells in the ileum was compared among all experimental groups (Table 3). The signal of Muc2 was similar in all experimental groups. Ileal Tff3 was evaluated by enumeration of Tff3 positively stained cells. There was a significant decrease of Tff3-positive cells in the ileal tissue from the FF and FF + PSO groups compared with DF controls (P ≤ 0.01).
Table 3.
Muc2- and Tff3-positive cells in the ileum of neonatal rats
Results are expressed as means mucin 2 (Muc2)- or (Tff3)-positive cells/100 epithelial cells ± SE (n = 9 animals/experimental group).
P ≤ 0.01 vs. dam-fed animals (DF).
DISCUSSION
In this study, for the first time, we report that supplementation of PSO into milk formula reduces the incidence and severity of NEC in a neonatal rat model of NEC. The beneficial effects of PSO against NEC are associated with the improved enterocyte proliferation and reduced expression of proinflammatory cytokines in the site of injury. Oral administration of PSO does not affect Muc2 and Tff3 secretion in the terminal ileum.
Neonatal NEC is a multifactorial disease, which primarily affects prematurely born infants. Decreasing gestational age of newborns clearly correlates with increasing incidence of NEC (24). Another critical factor in NEC pathogenesis is the type and composition of infant diet. Breastfeeding has an advantage over infant formula feeding in the reduction of both human (39) and experimental NEC (17). Arachidonic acid (AA) and docosahexaenoic acid (DHA) have been shown to reduce NEC (1, 11), whereas the effect of conjugated FAs on the developing intestine and NEC pathogenesis have not been studied yet.
There are two types of structurally related conjugated octadecaenoic acids available in human diets, CLA (18:2) and CLnA (18:3). The CLA have been intensively studied for their beneficial biological activities in a variety of disease models (6), including mouse and pig models of colitis (3, 5), and a clinical trial in patients with Crohn's disease (4). In contrast, information concerning the biological function of CLnA is still scarce (55). Human milk contains a significant amount of the CLA (44), but the presence of the CLnA has yet to be established. In the present study, we showed that our rat milk formula contains only traces of the CLA (0.2%) and that the CLnA are absent. Supplementation of formula with 1.5% of PSO significantly increased CLnA levels but had no effect on the concentration of CLA, AA, and DHA in our formula (Table 1). Thus we were able to evaluate the direct effect of orally administered CLnA in the rat NEC model.
A clinical study by Carlson et al. (14) revealed that preterm infants fed a formula supplemented with AA and DHA had a lower incidence of NEC (14). In studies with rodent NEC models, first Akisu et al. (1) and then Caplan et al. (12) demonstrated that supplementation of PUFA into formula reduces the incidence of experimental NEC. Comparison of three different preparations of PUFA (AA + DHA, egg phospholipids, and DHA alone) showed a similar efficacy of each treatment (38). Interestingly, very low levels of either AA or DHA (0.7% or 0.5% of the total fatty acids) were sufficient to protect intestinal tissue against injury (38). The presence of either CLA or CLnA was not detected in the above-mentioned studies. Results from our study showed that supplementation of PSO into formula markedly reduced the incidence of NEC (from 61% to 26%) and severity of ileal damage in the rat NEC model. Because concentrations of CLA, AA, and DHA were the same in both FF and FF + PSO diets, we conclude that CLnA is responsible for the protective effect of the PSO.
Maternal milk affects the exposure to FA in the newborn intestine (31). Cao et al. (10) have shown that conjugated FA in maternal milk can be proportionally incorporated into liver phospholipids (PL) and change their FA composition. In our study, both CLA and CLnA were incorporated into ileum in a diet-dependent manner. Intestinal tissue from FF + PSO pups exhibited more then 10-fold greater CLnA compared with the control FF group. Moreover, our results show that there is active metabolism of PuA at the intestine of these immature rats. PSO feeding led to shift in CLnA profile from 60% PuA in the FF + PSO feeds to about 28% in the ileal lipids, whereas there was more than a fourfold increase in ileal rumenic acid (Table 2). These data are consistent and indicate that conversion at the level of the ileum is operative in very immature rats.
In the healthy intestine, epithelial homeostasis and tissue integrity is maintained by balancing the rate of cell proliferation and turnover. Milk-borne factors and nutrients can affect neonatal intestinal growth and intestinal cellular proliferation (16). In the FF group, a significant reduction of villus length was detected in the intact portions of the ileum compared with DF controls. Feeding of formula supplemented with PSO resulted in normalization of villus length. There was no significant difference in the number of enterocytes per micrometer of villus between experimental groups, suggesting that hyperplasia rather than hypertrophy is occurring. Intestinal epithelial proliferation was decreased in the FF group compared with the DF group, and this effect was completely reversed in rats fed with FF + PSO diet. These results indicate that PSO protects the epithelial barrier and preserves the intestinal architecture.
Recent studies show that CLnA may reduce inflammation and enhance the immune response (28). The small intestine is the largest immune organ in the body. Our and other laboratories showed that proinflammatory cytokines are important factors contributing to NEC pathogenesis (26, 27, 53, 56). Patients with NEC have increased serum levels of TNF-α and IL-6 (53). In addition, excessive IL-8 response is observed in the intestinal mucosa during NEC (47). Previously, we showed increased levels of IL-12, IL-18, and TNF-α in the rat NEC model (25, 26). In the present study, we found that gene expression of proinflammatory IL-6, IL-8, and TNF-α mRNA was markedly increased in the ileum of NEC rats compared with controls. Supplementation of the PSO into formula reduced these levels to values seen in controls.
IL-23 is a member of the IL-12 cytokine family secreted by activated dendritic cells and macrophages (49). Because of the shared p40 subunit between IL-12 (p40/p35) and IL-23 (p40/p19), original studies showing the critical role of IL-12 and T helper 1 (Th1) responses in intestinal inflammation (before IL-23 discovery) had to be reevaluated (46). It is clear now, that both IL-23 and IL-12 contribute to intestinal inflammation (30) by inducing Th17 and Th1 effector responses. However, the role of IL-23 in NEC pathogenesis has not been reported yet. Our results showed sevenfold increase in IL-23 and twofold increase in IL-12 gene expression in the ileum of rats with NEC compared with controls (DF). Feeding formula with PSO significantly reduced IL-23 and IL-12 mRNA levels, but IL-23 levels still did not completely normalize to values seen in the DF groups. These results suggest that PSO protects against NEC injury via downregulation of the inflammatory response in the developing intestinal mucosa.
In conclusion, this study demonstrates the orally administered PSO protects against NEC injury in the rat model. The protective effect is associated with improvement of enterocyte proliferation, protection of intestinal architecture, and reduction of inflammatory response in the site of injury, the terminal ileum. We hypothesize that PuA is important in the maintenance of structural and functional integrity of intestinal mucosa. This study introduces a novel nutritional factor, which should be considered for supplementation into formula for premature neonates.
GRANTS
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant HD039657 (to B. Dvorak) and a gift from Mead Johnson Nutrition (to B. Dvorak).
DISCLOSURES
B. Dvorak has grants with Mead Johnson, Meiji Dairies, and NIH. There are no conflicts of interest. J.T. Brenna has grants with NIH and Danone, and no conflict of interest.
AUTHOR CONTRIBUTIONS
Author contributions: C.F.C.-B., C.L.S., C.K.A.-R., and P.L. performed experiments; C.F.C.-B., C.L.S., P.L., J.T.B., and B.D. analyzed data; C.F.C.-B. and B.D. drafted manuscript; J.B.-R., R.H., J.T.B., Z.E.J., and B.D. edited and revised manuscript; J.T.B., Z.E.J., and B.D. interpreted results of experiments; Z.E.J. and B.D. conception and design of research; B.D. prepared figures; B.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Current affiliation for Christine F. Coursodon-Boyiddle: Ventana Medical Systems, Tucson, Arizona.
REFERENCES
- 1. Akisu M, Baka M, Coker I, Kultursay N, Huseyinov A. Effect of dietary n-3 fatty acids on hypoxia-induced necrotizing enterocolitis in young mice. N-3 fatty acids alter platelet-activating factor and leukotriene B4 production in the intestine. Biol Neonate 74: 31–38, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Bassaganya-Riera J, Diguardo M, Climent M, Vives C, Carbo A, Jouni ZE, Einerhand AW, O'Shea M, Hontecillas R. Activation of PPARgamma and delta by dietary punicic acid ameliorates intestinal inflammation in mice. Br J Nutr: 1–9, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Bassaganya-Riera J, Hontecillas R. CLA and n-3 PUFA differentially modulate clinical activity and colonic PPAR-responsive gene expression in a pig model of experimental IBD. Clin Nutr 25: 454–465, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Bassaganya-Riera J, Hontecillas R, Horne WT, Sandridge M, Herfarth HH, Bloomfeld R, Isaacs KL. Conjugated linoleic acid modulates immune responses in patients with mild to moderately active Crohn's disease. Clin Nutr. 2012. April 19 [Epub ahead of print]. doi:10.1016/j.clnu.2012.03.002. [DOI] [PubMed]
- 5. Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology 127: 777–791, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem 17: 789–810, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 8. Boussetta T, Raad H, Letteron P, Gougerot-Pocidalo MA, Marie JC, Driss F, El-Benna J. Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS One 4: e6458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buck C, Bundschu J, Gallati H, Bartmann P, Pohlandt F. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics 93: 54–58, 1994 [PubMed] [Google Scholar]
- 10. Cao Y, Chen J, Yang L, Chen ZY. Differential incorporation of dietary conjugated linolenic and linoleic acids into milk lipids and liver phospholipids in lactating and suckling rats. J Nutr Biochem 20: 685–693, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Caplan MS, Jilling T. The role of polyunsaturated fatty acid supplementation in intestinal inflammation and neonatal necrotizing enterocolitis. Lipids 36: 1053–1057, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Caplan MS, Russell T, Xiao Y, Amer M, Kaup S, Jilling T. Effect of polyunsaturated fatty acid (PUFA) supplementation on intestinal inflammation and necrotizing enterocolitis (NEC) in a neonatal rat model. Pediatr Res 49: 647–652, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Carlisle EM, Poroyko V, Caplan MS, Alverdy JA, Liu D. Gram negative bacteria are associated with the early stages of necrotizing enterocolitis. PLoS One 6: e18084, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlson SE, Montalto MB, Ponder DL, Werkman SH, Korones SB. Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr Res 44: 491–498, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 291: G938–G949, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 288: G755–G762, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Dvorak B, Halpern MD, Holubec H, Dvorakova K, Dominguez JA, Williams CS, Meza YG, Kozakova H, McCuskey RS. Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model. Pediatr Res 53: 426–433, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, Stepankova R, Payne CM, McCuskey RS. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am J Physiol Gastrointest Liver Physiol 282: G156–G164, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Dvorak B, McWilliam DL, Williams CS, Dominguez JA, Machen NW, McCuskey RS, Philipps AF. Artificial formula induces precocious maturation of the small intestine of artificially reared suckling rats. J Pediatr Gastroenterol Nutr 31: 162–169, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Edelson MB, Bagwell CE, Rozycki HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics 103: 766–771, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Elfalleh W, Ying M, Nasri N, Sheng-Hua H, Guasmi F, Ferchichi A. Fatty acids from Tunisian and Chinese pomegranate (Punica granatum L.) seeds. Int J Food Sci Nutr 62: 200–206, 2011 [DOI] [PubMed] [Google Scholar]
- 22. Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res 62: 510–514, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Guesnet P, Alessandri JM. Docosahexaenoic acid (DHA) and the developing central nervous system (CNS)—Implications for dietary recommendations. Biochimie 93: 7–12, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol 23: 278–285, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Halpern MD, Clark JA, Saunders TA, Doelle SM, Hosseini DM, Stagner AM, Dvorak B. Reduction of experimental necrotizing enterocolitis with anti-TNF-α. Am J Physiol Gastrointest Liver Physiol 290: G757–G764, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, McWilliam DL, Payne CM, McCuskey RS, Besselsen DG, Dvorak B. Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res 51: 733–739, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Halpern MD, Khailova L, Molla-Hosseini D, Arganbright K, Reynolds C, Yajima M, Hoshiba J, Dvorak B. Decreased development of necrotizing enterocolitis in IL-18-deficient mice. Am J Physiol Gastrointest Liver Physiol 294: G20–G26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hennessy AA, Ross RP, Devery R, Stanton C. The health promoting properties of the conjugated isomers of alpha-linolenic acid. Lipids 46: 105–119, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Hora JJ, Maydew ER, Lansky EP, Dwivedi C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J Med Food 6: 157–161, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med 203: 2473–2483, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Innis SM. Human milk: maternal dietary lipids and infant development. Proc Nutr Soc 66: 397–404, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 95: 55–65, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khailova L, Dvorak K, Arganbright KM, Halpern MD, Kinouchi T, Yajima M, Dvorak B. Bifidobacterium bifidum improves intestinal integrity in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 297: G940–G949, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khailova L, Mount Patrick SK, Arganbright KM, Halpern MD, Kinouchi T, Dvorak B. Bifidobacterium bifidum reduces apoptosis in the intestinal epithelium in necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G1118–G1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kohno H, Suzuki R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci 95: 481–486, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol 109: 177–206, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Lawrence P, Brenna JT. Acetonitrile covalent adduct chemical ionization mass spectrometry for double bond localization in non-methylene-interrupted polyene fatty acid methyl esters. Anal Chem 78: 1312–1317, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Lu J, Jilling T, Li D, Caplan MS. Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr Res 61: 427–432, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 336: 1519–1523, 1990 [DOI] [PubMed] [Google Scholar]
- 40. Mahdihassan S. Outline of the beginnings of alchemy and its antecedents. Am J Chin Med 12: 32–42, 1984 [DOI] [PubMed] [Google Scholar]
- 41. McElroy SJ, Weitkamp JH. Innate immunity in the small intestine of the preterm infant. Neoreviews 12: e517–e526, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meerts IA, Verspeek-Rip CM, Buskens CA, Keizer HG, Bassaganya-Riera J, Jouni ZE, van Huygevoort AH, van Otterdijk FM, van de Waart EJ. Toxicological evaluation of pomegranate seed oil. Food Chem Toxicol 47: 1085–1092, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Mehta R, Lansky EP. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur J Cancer Prev 13: 345–348, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Molto-Puigmarti C, Castellote AI, Lopez-Sabater MC. Conjugated linoleic acid determination in human milk by fast-gas chromatography. Anal Chim Acta 602: 122–130, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Morecroft JA, Spitz L, Hamilton PA, Holmes SJ. Plasma cytokine levels in necrotizing enterocolitis. Acta Paediatr Suppl 396: 18–20, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Morrison PJ, Ballantyne SJ, Kullberg MC. Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology 133: 397–408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, Llanos A, Claud EC, Walker WA. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One 6: e17776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13: 715–725, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Ran-Ressler RR, Khailova L, Arganbright KM, Adkins-Rieck CK, Jouni ZE, Koren O, Ley RE, Brenna JT, Dvorak B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS One 6: e29032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis 16: 1808–1813, 2010 [DOI] [PubMed] [Google Scholar]
- 52. Sassano G, Sanderson P, Franx J, Groot P, van Straalen J, Bassaganya-Riera J. Analysis of pomegranate seed oil for the presence of jacaric acid. J Sci Food Agric 89: 1046–1052, 2009 [Google Scholar]
- 53. Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, Premachandra BR. Neonatal gut barrier and multiple organ failure: role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg 42: 454–461, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci USA 88: 11017–11021, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vroegrijk IO, van Diepen JA, van den Berg S, Westbroek I, Keizer H, Gambelli L, Hontecillas R, Bassaganya-Riera J, Zondag GC, Romijn JA, Havekes LM, Voshol PJ. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem Toxicol 49: 1426–1430, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Weitkamp JH, Koyama T, Rock MT, Correa H, Goettel JA, Matta P, Oswald-Richter K, Rosen MJ, Engelhardt BG, Moore DJ, Polk DB. Necrotising enterocolitis is characterised by disrupted immune regulation and diminished mucosal regulatory (FOXP3)/effector (CD4, CD8) T cell ratios. Gut. 2012. January 20 [Epub ahead of print]. doi:10.1136/gutjnl-2011-301551. [DOI] [PMC free article] [PubMed]