Abstract
Although hepatic encephalopathy (HE) is linked to the gut microbiota, stool microbiome analysis has not found differences between HE and no-HE patients. This study aimed to compare sigmoid mucosal microbiome of cirrhotic patients to controls, between HE vs. no-HE patients, and to study their linkage with cognition and inflammation. Sixty cirrhotic patients (36 HE and 24 no-HE) underwent cognitive testing, stool collection, cytokine (Th1, Th2, Th17, and innate immunity), and endotoxin analysis. Thirty-six patients (19 HE and 17 no-HE) and 17 age-matched controls underwent sigmoid biopsies. Multitag pyrosequencing (including autochthonous genera, i.e., Blautia, Roseburia, Fecalibacterium, Dorea) was performed on stool and mucosa. Stool and mucosal microbiome differences within/between groups and correlation network analyses were performed. Controls had significantly higher autochthonous and lower pathogenic genera compared with cirrhotic patients, especially HE patients. HE patients had worse MELD (model for end-stage liver disease) score and cognition and higher IL-6 and endotoxin than no-HE. Mucosal microbiota was different from stool within both HE/no-HE groups. Between HE/no-HE patients, there was no difference in stool microbiota but mucosal microbiome was different with lower Roseburia and higher Enterococcus, Veillonella, Megasphaera, and Burkholderia abundance in HE. On network analysis, autochthonous genera (Blautia, Fecalibacterium, Roseburia, and Dorea) were associated with good cognition and decreased inflammation in both HE/no-HE, whereas genera overrepresented in HE (Enterococcus, Megasphaera, and Burkholderia) were linked to poor cognition and inflammation. Sigmoid mucosal microbiome differs significantly from stool microbiome in cirrhosis. Cirrhotic, especially HE, patients' mucosal microbiota is significantly different from controls with a lack of potentially beneficial autochthonous and overgrowth of potentially pathogenic genera, which are associated with poor cognition and inflammation.
Keywords: systems biology, lactulose, rifaximin, correlation network analysis, multitag pyrosequencing
the pathogenesis of cirrhosis and its complications, specifically bacterial translocation, infections, and hepatic encephalopathy, are closely related to changes in the intestinal microbiota (47, 52). Recent studies have demonstrated differences in the stool microbiome of patients with cirrhosis compared with healthy individuals, especially regarding the presence of resident or autochthonous bacteria (6, 12). However, despite significant differences in the clinical, proinflammatory milieu and cognitive function, there was minimal difference in stool microbiome between cirrhotic patients with hepatic encephalopathy (HE) and those without (No-HE) (6, 43, 44). This was intriguing since HE therapies are hypothesized to act by influencing the gut bacteria (13). Studies in noncirrhotic populations have demonstrated changes in the intestinal mucosa microbiome compared with the stool, but this has not been studied in cirrhosis to date (18, 55).
The aim of the study was to evaluate changes between the stool and colonic mucosal microbiome of cirrhotic patients with and without HE and to link them with changes in peripheral inflammation and cognition. The a priori hypothesis was that there would be a significant difference in the microbiome composition of the colonic mucosa compared with the stool in cirrhotic patients with HE compared with those without HE and that these shifts in the mucosal microbiome would be associated with changes in inflammation and cognition.
MATERIALS AND METHODS
Patients with cirrhosis with or without HE were included for a one-time visit. We excluded patients with a current infection (defined by elevated white blood cell count, clinical suspicion, or fever), with variceal bleeding within the last 4 wk, on gut-absorbable antibiotics, or with alcohol or illicit drug intake within 3 mo (checked by drug and alcohol screens). Patients in the “No-HE” group had never had an episode of HE and were not on any therapy for it. Patients in the “HE” group had suffered at least one HE episode within the last 3 mo and were currently controlled on lactulose alone or lactulose with rifaximin. A group of age-matched healthy controls not currently abusing alcohol or on antibiotics and without systemic diseases were also included.
During the visit, the subjects underwent a physical examination and measurement of body mass index (BMI), detailed analysis of medical records including current medications, a detailed dietary recall and collection of stool and blood samples. All cirrhotic patients underwent a mini-mental status exam and only those scoring above 25 were included in the full study (16). Subsequently a recommended cognitive battery consisting of the following tests was administered: 1) psychometric hepatic encephalopathy score (PHES), 2) block design test (subjects are required to replicate designs with given blocks in a timed manner), and 3) inhibitory control test [ICT; subjects are instructed to respond to alternating presentations of X and Y on the screen (targets) while inhibiting response when X and Y are not alternating (lures)] (4, 50). The PHES consists of number connection test-A/B (subjects are asked to “join the dots” between numbers or numbers and alphabets in a timed fashion), digit symbol (subjects are required to copy corresponding figures from a given list within 2 min), line drawing (time) and (errors) [subjects are required to trace a line between two parallel lines with balance between speed and accuracy; time required and the number of times the subject's line strays beyond the marked lines (errors) are recorded], and serial dotting [subjects are asked to dot the center of a group of blank circles]. The PHES is a validated battery for cognitive dysfunction in cirrhosis and tests for psychomotor speed, visuomotor coordination, attention, and set shifting (40). Block design tests for visuomotor coordination. A high score on block, digit symbol, and ICT targets and a low score on the rest indicate good performance.
Blood was collected from cirrhotic patients for venous ammonia, liver disease severity [using the model for end-stage liver disease (MELD) score; a validated score of international normalized ratio of the prothrombin time (INR), serum bilirubin, and serum creatinine] and pro- and anti-inflammatory cytokines (28). A portion of the serum was stored at −80°C and was subsequently analyzed for innate immunity (IL-1β, IL-6, TNF-α), Th1 (IFN-γ and IL-2), Th2 (IL-4, IL-10, IL-13), and Th17 responses (IL-17 and IL-23), endotoxin, neural function [neuron-specific enolase (NSE) and s100b protein] (53), endothelial activation [soluble intravascular adhesion molecule (sICAM-1) and soluble vascular adhesion molecule (sVCAM-1)], and asymmetric dimethyl arginine (ADMA). These were analyzed in duplicate by using published techniques by AssayGate (Ijamsville, MD) (5, 6).
Interrogation of the microbiome.
Stool was collected and DNA was extracted for microbiome analysis by use of published techniques (38). A subset underwent an unsedated, unprepared flexible sigmoidoscopy during which a pinch biopsy of the rectosigmoid mucosa was obtained, snap-frozen, and stored till the analysis at −80°C. We first used length heterogeneity PCR (LH-PCR) fingerprinting of the 16S rRNA to rapidly survey our samples and standardize the community amplification. We then interrogated the microbial taxa associated with the gut fecal microbiome using multitag pyrosequencing (MTPS) (17). This technique allows for rapid sequencing of multiple samples at one time, yielding thousands of sequence reads per sample.
Microbiome community fingerprinting.
LH-PCR was done to standardize the community analysis as previously published. Briefly, total genomic DNA was extracted from tissue by using a Bio101 kit from MP Biomedicals, Montreal, Quebec, Canada as per the manufacturer's instructions. About 10 ng of extracted DNA was amplified by PCR by using a fluorescently labeled forward primer 27F [5′-(6FAM) AGAGTTTGATCCTGGCTCA G-3′] and unlabeled reverse primer 355R′ (5′-GCTGCCTCCCGTAGGAGT-3′). Both primers are universal primers for bacteria (29). The LH-PCR products were diluted according to their intensity on agarose gel electrophoresis and mixed with ILS-600 size standards (Promega) and HiDi Formamide (Applied Biosystems, Foster City, CA). The diluted samples were then separated on a ABI 3130xl fluorescent capillary sequencer (Applied Biosystems) and processed using the Genemapper software package (Applied Biosystems). Normalized peak areas were calculated by using a custom PERL script, and operational taxonomic units constituting less than 1% of the total community from each sample were eliminated from the analysis to remove the variable low-abundance components within the communities.
MTPS.
We employed the MTPS process to characterize the microbiome from the fecal and biopsy samples. Specifically, we have generated a set of 96 emulsion PCR fusion primers that contain the 454 emulsion PCR linkers on the 27F and 355R primers and a different eight-base “barcode” between the A adapter and 27F primer. Thus each fecal sample was amplified with unique bar-coded forward 16S rRNA primers, and then up to 96 samples were pooled and subjected to emulsion PCR and pyrosequenced by use of a GS-FLX pyrosequencer (Roche). Data from each pooled sample were “deconvoluted” by sorting the sequences into bins based on the barcodes by using custom PERL scripts. Thus we were able to normalize each sample by the total number of reads from each barcode. We have noted that ligating tagged primers to PCR amplicons distorts the abundances of the communities and thus it is critical to incorporate the tags during the original amplification step.
Microbiome community analysis.
We identified the taxa present in each sample using the Bayesian analysis tool in Version 10 of the Ribosomal Database Project (RDP10). The abundances of the bacterial identifications were then normalized by using a custom PERL script, and genera present at >1% of the community were tabulated. We chose this cutoff because of our a priori assumption that genera present in <1% of the community vary between individuals and have minimal contribution to the functionality of that community and 2,000 reads per sample will only reliably identify community components that are greater than 1% in abundance (17).
Statistical analysis.
Cirrhotic patients with HE were compared with those without HE with respect to BMI, inflammatory markers, cognitive performance, and microbiome constituents. Mucosal microbiota constituents were also compared between controls and cirrhotic patients. Unpaired t-tests were used to compare demographics, cognitive tests, and inflammatory markers. Since the microbiome constituents tend to be sparse and nonparametrically distributed, we used Metastats to compare microbiome between stool and mucosa of patients with and without HE (51). Metastats performs statistical analysis (to investigate metagenomic differences) along with biomarker discovery (to evaluate specific features underlying these differences) based on repeated t statistics and Fisher's tests on random permutations (42). We also performed Metastats analysis between the mucosal microbiota compared with the stool microbiome in patients with HE and without HE and those with HE on or not on rifaximin, and between controls and cirrhotic patients' mucosal microbiota. principal component analysis (PCA) on the abundance tables was also performed (25, 33). Subsequently, we analyzed the correlations between MELD score, BMI, inflammatory markers, cognitive tests, and microbiome constituents using a correlation network analysis obtained through a customized statistical script in R (6) using a P value cutoff of <0.05 and an R value >0.5 to identify the most significant relationships (3, 17).
This study was approved by the Institutional Review Boards of the McGuire Veterans Affairs Medical Center and the Virginia Commonwealth University Medical Center.
RESULTS
A total of 60 patients with cirrhosis and 17 age-matched healthy controls were included in the study. Ten controls were women, the mean age was 52 ± 6 yr, and all of the controls underwent flexible sigmoidoscopy with biopsy. The distribution of HE and No-HE was relatively uniform with 24 patients without HE and 36 with HE. Of the 36 HE patients, 17 were only on lactulose whereas 19 were on both lactulose and rifaximin therapy. All subjects were nonvegetarians and had similar dietary intake and constituents on recall prior to sample collection (mean intake 2,390 kcal and 16% protein intake). HE patients had a significantly higher MELD score and also, as expected, higher ammonia and worse cognitive performance on all tests compared with patients without HE (Table 1). There was higher endotoxin, s100b, IL-6, and ADMA in the HE patient group. Of the 60 patients, 36 (17 patients without HE and 19 with HE) underwent flexible sigmoidoscopy with biopsy the same day of the stool and sample collection. Patients on rifaximin had a worse cognitive performance compared with those only on lactulose on number connection-A (68.3 vs. 52.25 s, P = 0.05) and -B (192.9 vs. 145.8 s, P = 0.03), targets (86 vs. 90%, P = 0.05), serial dotting (94.2 vs. 84.0 s, P = 0.04), line tracing errors (62.5 vs. 44.9, P = 0.05) but not line tracing time (118.0 vs. 127.3, P = 0.41), digit symbol (37 vs. 38 score, P = 0.89), block design (17.5 vs. 19.8 score, P = 0.56), and lures (15.7 vs. 16.9 responses, P = 0.92). Patients currently on rifaximin also had a significantly higher level of IL-6 (51.04 vs. 30.13, P = 0.04) and endotoxin (0.43 vs. 0.20, P = 0.05), with a trend toward higher MELD (18 vs. 16, P = 0.08) compared with those on lactulose alone.
Table 1.
Comparison of clinical parameters between patients with and without HE
| Cirrhosis without HE (n = 24) | Cirrhosis with HE (n = 36) | |
|---|---|---|
| Age | 54 ± 6 | 56 ± 4 |
| Sex (male/female) | 20/4 | 30/6 |
| Body mass index | 28.9 ± 4.4 | 29.0 ± 6.7 |
| Ascites, % patients | 2 | 16 |
| Child-Turcotte-Pugh score | 6 ± 4 | 9 ± 5* |
| MELD score | 10.4 ± 4.1 | 17.3 ± 6.8* |
| Venous ammonia | 32.8 ± 12.6 | 48.8 ± 27.5* |
| IL-1β, pg/ml | 14.3 ± 58.7 | 6.2 ± 12.1 |
| IL-2, pg/ml | 15.0 ± 62.3 | 24.4 ± 68.6 |
| Interferon-γ, pg/ml | 10.1 ± 33.1 | 15.9 ± 54.5 |
| TNF-α, pg/ml | 13.9 ± 43.2 | 7.4 ± 8.2 |
| IL-4, pg/ml | 16.3 ± 55.5 | 25.3 ± 94.5 |
| IL-6, pg/ml | 12.2 ± 32.5 | 40.6 ± 63.3* |
| IL-10, pg/ml | 10.4 ± 28.3 | 4.88 ± 5.89 |
| ADMA, μm/ml | 0.38 ± 0.13 | 0.558 ± 0.20* |
| S100b protein, pg/ml | 34.7 ± 27.4 | 57.1 ± 49.4* |
| Endotoxin, EU/ml | 0.06 ± 0.01 | 0.32 ± 0.26* |
| Neuron-specific enolase, pg/ml | 7,461 ± 4,288 | 6,793 ± 3,424 |
| IL-23, pg/ml | 519 ± 1,137 | 1,130 ± 3,289 |
| IL-17, pg/ml | 5.9 ± 16.9 | 17.8 ± 60.4 |
| sVCAM-1, pg/ml | 1,488,817 ± 664,793 | 1,683,186 ± 794,252 |
| sICAM-1, pg/ml | 319,844 ± 268,066 | 304,680 ± 214,037 |
| Number connection-A, s | 35.0 ± 14.7 | 60.3 ± 41.4* |
| Number connection-B, s | 95.9 ± 49.2 | 170 ± 123* |
| Digit symbol, raw score | 57.7 ± 12.0 | 37.4 ± 15.8* |
| Block design, raw score | 30.1 ± 14.9 | 18.7 ± 16.7* |
| Lures, number incorrect | 10.5 ± 7.7 | 16.4 ± 10.3* |
| Targets, % correct | 95.3 ± 8.7 | 88.1 ± 13.7* |
| Serial dotting, s | 64.6 ± 18.7 | 89.3 ± 34.9* |
| Line tracing time, s | 95.0 ± 37.7 | 122.5 ± 56.9* |
| Line tracing errors, number | 28.2 ± 20.4 | 54.0 ± 39.1* |
Values are means ± SD,
P < 0.05. As expected, patients with hepatic encephalopathy (HE) have a worse model for end-stage liver disease (MELD) score andcognitive performance and higher venous ammonia, endotoxin, IL-6, asymmetric dimethyl arginine (ADMA), and S100b protein compared with patients without HE. A high score in Digit symbol, Block design, and Targets indicates good cognitive performance whereas a high score in the remaining cognitive tests suggests poor performance. sICAM-1, soluble intravascular adhesion molecule; sVCAM-1, soluble vascular adhesion molecule.
Comparison between control and patient mucosa microbiota.
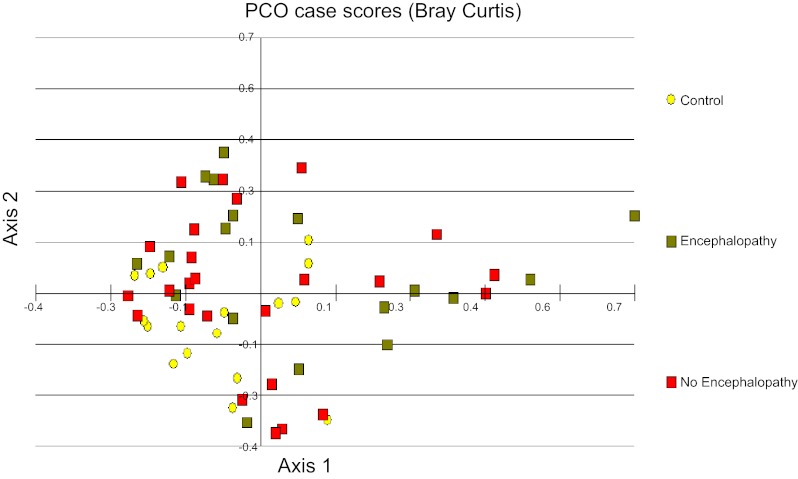
We found significant differences on Metastats between healthy controls and cirrhotic patients mucosa (Table 2), and when the controls were compared with HE group (Table 3) and comparatively fewer differences between controls and no-HE patients (Table 4). There was a significantly higher abundance of autochthonous genera (Dorea, Subdoligranulum, and Incertae Sedis other) and a lower abundance of potentially pathogenic ones (Enterococcus, Proteus, Clostridium, and Burkholderia) in controls compared with cirrhotic patients' mucosa. We also found significant clustering of the controls with each other in the PCA (Fig. 1). Additionally, the no-HE patients clustered around the controls whereas HE patients were scattered further from the central control cluster (Fig. 1). These results indicate that there is a greater variance in the microbiome composition between controls and HE patients compared with controls and no-HE patients.
Table 2.
Control mucosa vs. entire cirrhosis group mucosa comparison using Metastats
| Family_Genus, % abundance | Control Mucosa (n = 17) | Cirrhosis Mucosa (n = 36) | P Value |
|---|---|---|---|
| Burkholderiaceae_Burkholderia | 0.0 | 0.1 | <0.00001 |
| Burkholderiaceae_Ralstonia | 0.0 | 0.1 | 0.001 |
| Clostridiaceae_Clostridium | 0.0 | 0.2 | 0.005 |
| Clostridiaceae_other | 0.0 | 0.2 | 0.009 |
| Enterobacteriaceae_Proteus | 0.0 | 0.1 | <0.00001 |
| Enterococcaceae_Enterococcus | 0.0 | 2.2 | 0.02 |
| Incertae Sedis XIV_other | 1.6 | 0.5 | 0.008 |
| Lachnospiraceae_Dorea | 1.9 | 0.6 | 0.03 |
| Lachnospiraceae_other | 20.3 | 12.7 | 0.03 |
| Ruminococcaceae_Subdoligranulum | 1.1 | 0.4 | 0.02 |
| Veillonellaceae_Acidaminococcus | 0.0 | 0.1 | 0.0001 |
Table 3.
Control mucosa vs. cirrhosis with HE group mucosa comparison using Metastats
| Family_Genus, % abundance | Control Mucosa (n = 17) | HE Mucosa (n = 19) | P Value |
|---|---|---|---|
| Burkholderiaceae_Burkholderia | 0.0 | 0.2 | 0.001 |
| Incertae Sedis XIV_Blautia | 8.6 | 3.0 | 0.006 |
| Incertae Sedis XIV_other | 1.6 | 0.4 | 0.01 |
| Lachnospiraceae_Roseburia | 2.1 | 0.4 | 0.009 |
| Lachnospiraceae_other | 20.3 | 9.1 | 0.005 |
| Ruminococcaceae_Faecalibacterium | 3.5 | 1.6 | 0.02 |
| Ruminococcaceae_Subdoligranulum | 1.1 | 0.2 | 0.002 |
| Streptomycetaceae_Streptomyces | 0.0 | 1.9 | 0.001 |
Table 4.
Control mucosa vs. cirrhosis with no HE group mucosa comparison using Metastats
| Family_Genus, % abundance | Control Mucosa (n = 17) | No HE Mucosa (n = 17) | P Value |
|---|---|---|---|
| Alcaligenaceae_Achromobacter | 0.0 | 0.1 | 0.001 |
| Enterobacteriaceae_Proteus | 0.0 | 0.2 | 0.001 |
| Incertae Sedis XIV_other | 1.6 | 0.5 | 0.008 |
| Lachnospiraceae_Dorea | 1.9 | 0.5 | 0.007 |
| Vibrionaceae_Vibrio | 0.0 | 3.2 | 0.001 |
Fig. 1.
Principal component analysis (PCO) of sigmoid mucosal microbiome between controls and patients with and without hepatic encephalopathy (HE). The first principal component accounts for 19% of the variance and the second component accounts for 17% of the variance for a total of 36% of the variance. We found significant clustering of the controls with each other. Additionally, the no-HE patients cluster around the controls whereas HE patients were scattered further from the central control cluster. Yellow: controls; red: no hepatic encephalopathy; green: hepatic encephalopathy patients.
Comparison between all patients' mucosa to stool microbiome.
We found a significant change in the microbiome of the mucosa compared with stool in the entire group by use of Metastats (Table 5). This change persisted when the comparison between stool and mucosa was performed for the HE and the no-HE group. The composition of the mucosal microbiome in the entire population differed considerably from the corresponding stool microbiome. Prominent bacterial genera found at a higher abundance in the mucosa belonged to Firmicutes (Blautia, Incertae Sedis XI), Actinobacteria (Propionibacterium and Streptomyces), and Proteobacteria (Vibrio). Interestingly most bacteria found in higher abundances in stool were Firmicutes (Leuconostoc, Roseburia, Veillonella, and Incertae Sedis XIV). These differences persisted when the group was divided into HE and no-HE (Tables 6 and 7). Propionibacterium and Vibrio genera were significantly more abundant in the mucosa than in the stool in both HE and no-HE.
Table 5.
Cirrhosis mucosa vs. cirrhosis stool comparison using Metastats
| Family_Genus, % abundance | Mucosa (n = 36) | Stool (n = 36) | P Value |
|---|---|---|---|
| Incertae Sedis XIV_Blautia | 4.1 | 1.5 | 0.002 |
| Vibrionaceae_Vibrio | 3.1 | 0.0 | 0.001 |
| Propionibacteriaceae_Propionibacterium | 1.3 | 0.0 | 0.001 |
| Streptomycetaceae_Streptomyces | 1.5 | 0.0 | 0.001 |
| Incertae Sedis XI_other | 0.5 | 0.0 | 0.001 |
| Incertae Sedis XIV_other | 0.3 | 1.2 | 0.005 |
| Veillonellaceae_Veillonella | 0.2 | 2.2 | 0.01 |
| Leuconostocaceae_Leuconostoc | 0.0 | 1.0 | 0.001 |
| Bacteroidales_incertae_sedis_other | 0.0 | 0.2 | 0.001 |
| Lachnospiraceae_Roseburia | 0.2 | 1.0 | 0.03 |
There was a significantly higher Blautia, Vibrio, Incertae Sedis XI, Propionibacterium, and Streptomyces abundance and lower Incertae Sedia XIV, Veillonella, Bacteroides, and Roseburia in the stool.
Table 6.
HE mucosa vs. HE stool
| Family_Genus, % abundance | HE Mucosa (n = 19) | HE stool (n = 19) | P Value |
|---|---|---|---|
| Incertae Sedis XIV_Blautia | 5.2 | 1.6 | 0.004 |
| Vibrionaceae_Vibrio | 4.4 | 0 | 0.01 |
| Propionibacteriaceae_Propionibacterium | 1.1 | 0 | 0.001 |
| Incertae Sedis XI_other | 0.8 | 0 | 0.001 |
| Vibrionaceae_other | 0.6 | 0 | 0.001 |
| Incertae Sedis XIV_other | 0.3 | 1.4 | 0.005 |
| Fusobacteriaceae_other | 0 | 1.1 | 0.001 |
Table 7.
No HE mucosa vs. No HE stool
| Family_Genus, % abundance | No-HE Mucosa (n = 17) | No-HE Stool (n = 17) | P Value |
|---|---|---|---|
| Leuconostocaceae_Leuconostoc | 0 | 1.0 | 0.002 |
| Bacteroidales_incertae_sedis_other | 0 | 1.0 | 0.001 |
| Alcaligenaceae_other | 0 | 0.8 | 0.001 |
| Streptomycetaceae_Streptomyces | 2.5 | 0 | 0.001 |
| Propionibacteriaceae_Propionibacterium | 1.8 | 0 | 0.001 |
| Vibrionaceae_Vibrio | 1.3 | 0 | 0.001 |
| Burkholderiaceae_Ralstonia | 0.5 | 0 | 0.001 |
Comparison between HE and No-HE patients' microbiome.
Next we compared the stool and mucosal microbiome of the HE and No-HE groups. We again found no appreciable difference in the stool microbiome between patients with and without HE despite the higher sample size in this study. However, there was a significant difference in the mucosal microbiome between HE and no-HE patients (Table 8). Specifically, Firmicutes such as members of genera Veillonella, Megasphaera, Bifidobacterium, and Enterococcus were higher in HE whereas Roseburia was more abundant in the no-HE group.
Table 8.
Comparison between mucosal microbiome abundances between HE and no-HE groups using Metastats
| Family_Genus, % abundance | HE Mucosa (n = 17) | No-HE Mucosa (n = 19) | P Value |
|---|---|---|---|
| Lachnospiraceae_Roseburia | 0.5 | 2.5 | 0.002 |
| Veillonellaceae_Veillonella | 0.7 | 0 | 0.001 |
| Burkholderiaceae_other | 0.8 | 0 | 0.001 |
| Veillonellaceae_Megasphaera | 2.4 | 0 | 0.001 |
| Streptomycetaceae_Streptomyces | 2.7 | 0 | 0.001 |
| Fusobacteriaceae_other | 3.5 | 0 | 0.001 |
| Bifidobacteriaceae_Bifidobacterium | 3.8 | 0 | 0.001 |
| Enterococcaceae_Enterococcus | 7.7 | 0 | 0.001 |
Comparison between patients on lactulose alone compared with those on lactulose and rifaximin.
As found between HE and no-HE patients, there was no difference in the stool microbiome of patients on rifaximin and lactulose compared with those on lactulose alone. The mucosal microbiome in rifaximin-treated patients, however, was significantly different (Table 9). There was a significantly decreased abundance of autochthonous bacteria (Roseburia and Blautia) and Veillonellaceae but an increased abundance of Propionibacterium in the rifaximin group.
Table 9.
Comparison of the mucosal microbiome between patients on lactulose alone compared with those on lactulose and rifaximin using Metastats
| Family_Genus, % abundance | Lactulose Alone | Rifaximin and Lactulose | P Value |
|---|---|---|---|
| Incertae Sedis XIV_Blautia | 4.2 | 1.5 | 0.008 |
| Lachnospiraceae_Roseburia | 1.9 | 0 | 0.005 |
| Propionibacteriaceae_Propionibacterium | 1.1 | 2.3 | 0.03 |
| Veillonellaceae_Other | 1.1 | 0 | 0.03 |
| Rikenellaceae_Alistipes | 1.8 | 0 | 0.03 |
Correlation network analysis.
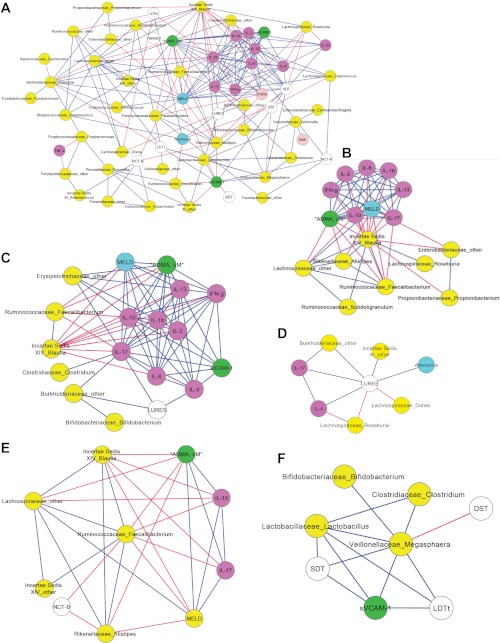
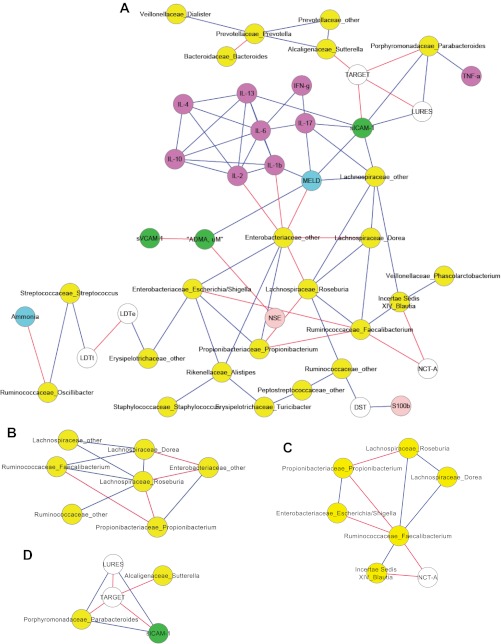
We performed a Spearman correlation using a custom R package to analyze linkages between the cognitive performance and inflammatory markers and the mucosal microbiome in HE and No-HE patients. We did not perform the analysis with the stool microbiome since there was no significant difference between the two groups' stool microbiome when using Metastats. The overall view of the two networks shows a distinct increase in the connectivity within the HE network (Fig. 2A) compared with the No-HE network (Fig. 3A). Certain bacterial genera were negatively correlated with inflammation and endothelial activation and linked to good cognitive performance across both networks. These were Fecalibacterium, Roseburia, other Lachnospiraceae, and Blautia. We also found a significant dense correlation network surrounding IL-17 and MELD with other inflammatory markers and cognitive performance in both networks. Replicating our prior experience, we found members of the Alcaligeneceae and Porphyromonadaceae families associated with poor cognitive performance in the No-HE network (6). What was interesting is that the genera present in higher abundance in the HE patients' mucosa (Tables 5–7) was associated with higher inflammation, worse cognition, and worse endothelial activation in the correlation network (Fig. 2A). Specifically, the subnetworks centered on Megaspheara, Veillonella, Burkholderia, and Bifidobacterium showed that they were associated with poor cognition, higher MELD, higher inflammation, and endothelial activation. These genera were not present in the no-HE network. In contrast, Roseburia, which was higher in the no-HE group, was associated with beneficial effects, i.e., less inflammation and endothelial activation and better cognition in both networks. Figures 2A and 3A are the correlation networks for the HE and no-HE groups' mucosal microbiome, respectively.
Fig. 2.
Correlation network and subnetworks of the mucosal microbiome of HE patients. Connecting lines in red indicate a significant negative whereas those in blue mean a significantly positive correlation. Nodes in yellow are bacterial genera, purple are inflammatory cytokines, white are cognitive tests, blue are clinical variables, green are markers of endothelial activation, and light pink are neuroglial markers. A high score on digit symbol (DST) and targets indicates good cognition whereas a high score on the rest of the cognitive tests indicates poor performance. SDT, serial dotting; LDTt, line tracing test time; NCT-A/B, number connection test A/B. A: correlation network of the mucosal microbiome of HE patients. As can be seen, autochthonous genera belonging to the Ruminococcaceae, Lachnospiraceae, and Incertae Sedis families are associated with good cognition, lower model for end-stage liver disease (MELD) score, lower ammonia, and decreased inflammation. Subnetworks from this complex network are displayed in the following panels. B: subnetwork of the HE mucosa microbiome showing the negative correlation of the autochthonous bacteria to MELD score and inflammation. C: subnetwork of the HE mucosa microbiome showing the negative correlation of the inflammatory cytokines, particularly IL-17 with autochthonous bacteria and positive correlation with lures (indicating worse cognition with increased inflammation), endothelial activation [soluble intravascular adhesion molecule (sICAM-1)], MELD score, and nonautochthonous bacterial genera (Burkholderiaceae, Erysipelothricaceae). D: a high lure number indicates poor cognition. This subnetwork of the HE mucosa microbiome shows that lures are negatively correlated with autochthonous bacterial genera (Roseburia and Dorea) whereas they are correlated positively with Burkholderiaceae and Incertae Sedis XI and as expected with ammonia and inflammatory cytokines. E: a high number on NCT-B indicates poor performance. This subnetwork of the HE mucosal microbiome shows a negative correlation, i.e., good NCT-B performance with the abundances of Ruminococcaceae Fecalibacterium. This autochthonous genus has been associated with lower MELD score and lower inflammation (IL-17 and IL-10) and is positively correlated with other beneficial autochthonous bacteria. F: Megasphaera was significantly more abundant in HE; in this subnetwork Megasphaera abundance is significantly correlated with soluble vascular adhesion molecule (sVCAM-1) (marker of endothelial activation) and with poor cognitive performance (a high score on SDT and LDTt indicates poor whereas a high score on DST indicates good cognitive performance).
Fig. 3.
Correlation network and subnetworks of the mucosal microbiome of patients without HE. Connecting lines in red indicate a significant negative whereas those in blue mean a significantly positive correlation. Nodes in yellow are bacterial genera, purple are inflammatory cytokines, white are cognitive tests, blue are clinical variables, green are markers of endothelial activation, and light pink are neuroglial markers. A high score on DST and targets indicates good cognition whereas a high score on the rest of the cognitive tests indicates poor performance. A: correlation network of the mucosal microbiome of patients without HE. Autochthonous genera belonging to the Ruminococcaceae, Lachnospiraceae, and Incertae Sedis families are associated with good cognition and lower MELD, ammonia, and inflammation. B: this subnetwork of patients without HE shows that bacteria genera belonging to autochthonous families (Ruminococcaceae and Lachnospiraceae) are positively correlated with each other while negatively correlated with potentially pathogenic Enterobacteriaceae and Propionibacterium. C: this subnetwork of the no-HE mucosal microbiome again shows the positive correlation of the autochthonous bacteria with each other and a negative correlation with time required to complete NCT-A, which indicates good cognitive performance. D: a high score on targets and low score on lures indicates good cognitive performance. We again found a correlation between poor performance on lures and targets with genera belonging to Porphyromonadaceae and Alcaligenaceae.
DISCUSSION
We found a significant alteration in the colonic mucosal microbiome compared with stool in cirrhosis. We did not find any significant change in the stool microbiome between patients with or without HE but found a dramatic change in the mucosal microbiome between the two groups and with healthy controls.
Specifically, we found a higher abundance of the beneficial genus Roseburia in patients without HE whereas a higher abundance of members of the genera Enterococcus, Veillonella, Megasphaera, Bifidobacterium, and Burkholderia was found in the HE patients' mucosa. An increase in members of the genus Enterococcus have been previously reported in the fecal microbiome of patients with liver cirrhosis compared with control patients (31). In the present study, an increase in the levels of Bifidobacterium genera in HE patients was unexpected since members of this genus have been used as probiotics and the presence of this bacterium is correlated with suppression of inflammation (37). This could be partly explained by the use of lactulose as a carbon and energy source by Bifidobacterium species (which only the cirrhosis group was on) and also by the relatively greater species diversity within this genus, some of which may be not related to overall benefit (11, 49). The correlation network linking the mucosal microbiome to cognition, endothelial activation, inflammation, and disease severity was richer in connectivity in the HE group, suggesting functional shifts in the microbial community that correlate with disease.
The differences between the mucosa and stool microbiome has been shown in several disease conditions such as Crohn's disease as well as in healthy volunteers (46). Prior studies have also shown that the influence of the fecal microbes may be less than that of the mucosal microbiome on immunity and overall health (18, 30). The intestinal barrier has a strong immunological interface comprising mucus, epithelium, and the mucosa-associated immune cells. The bacterial biofilm is usually restricted to the outer mucus layer (24, 35). However, there is evidence of cross talk between the mucosal immune system and the gut bacterial species that can usually differentiate between commensals and pathogens (8). We replicated prior noncirrhotic studies by showing a significant difference between the mucosal and stool microbiome in the overall population and when divided into HE and no-HE. The study of the mucosal microbiome in cirrhosis is relevant because most premortal events in cirrhosis, such as spontaneous bacterial peritonitis and spontaneous bacteremia, are related to intestinal bacterial translocation (1, 47). Alteration in permeability, bacterial overgrowth, and poor motility, along with deficiency of antimicrobial peptides, further increases the risk of bacterial translocation in cirrhosis (41, 48, 52). The underlying suppression of the mucosal immunity in cirrhosis with the resultant proinflammatory milieu leads to endotoxemia and complications of cirrhosis and HE (47, 52).
We found significant differences between controls and cirrhotic mucosal microbiota that were more pronounced when HE patients were compared with controls. These results showed a significantly higher autochthonous genera abundance (Dorea, Subdoligranulum, Incertae Sedis XIV, Blautia, Roseburia, Faecalibacterium) and lower abundance of pathogenic genera (Enterococcus, Burkholderia, Proteus) in controls compared with cirrhotic patients.
The reason for marked changes in the mucosal gut microbiome between control and patients with cirrhosis and hepatic encephalopathy is not clear. A potential explanation could be the altered synthesis and secretion of bile acids and other biliary components as shown in a preliminary study with worsening cirrhosis severity that could have a significant effect on the gut mucosal physiology, microbiome function, and systemic inflammation (26). A decrease in bile acids, antibacterial peptides, and mucins in the colon may allow for the selection of potentially pathogenic bacteria to adhere and grow in association with the colonic mucosa of patients with cirrhosis allowing for increased translocation of bacterial toxic products (21, 23). This hypothesis is consistent with an increase in serum endotoxin and proinflammatory cytokines (i.e., IL-6) observed in HE patients compared with those without. Certain species of Fecalibacterium, along with other bacteria belonging to Clostridium cluster XIV, have dampening effects on inflammation, in some cases due to NF-κB suppression (10, 19). Interestingly, there was a significantly higher abundance of butyrate-producing genera such as Roseburia, Fecalibacterium, Lachnospiraceae, and Subdoligranulum in less-affected patients (controls and no-HE cirrhotic patients) compared with HE patients. This distribution has been seen in controls when compared against patients with inflammatory bowel disease and colon cancer and may be related to butyrate being the preferred energy source for colonocytes (7, 32). There is also a redundancy in the function of the human microbiome in which several bacterial classes can perform essential metabolic functions, i.e., carbohydrate metabolism. Specifically, Ruminococcus, with its cellulolytic activity, has been shown to ferment plant-based material in the gut and is beneficial from an energy standpoint to the host (15). The high abundance of key genera in controls and in no-HE patients reflects the “healthy human microbiome” in which there is consistently the presence of the families Lachnospiraceae and Ruminococcaceae in healthy volunteers compared with any disease. Further research into the metabolomics of these microbiota is needed to evaluate the functional consequences of these changes (3).
We confirmed our prior study demonstrating that there was no appreciable difference in the fecal bacterial composition of patients with and without HE, including those on rifaximin or not (6). This was intriguing because patients with HE were significantly different from those without HE from a standpoint of liver disease, clinical severity, inflammation, and cognitive function. Therefore the changes in the mucosal bacterial composition were sought and were found to be significantly different between the groups and relatively similar to the ones between controls and HE patients. Autochthonous bacteria such as Roseburia have evolved to survive in the mucosal niches without eliciting a host immune reaction despite the abundant antimicrobial peptides (36). In contrast, genera such as Enterococcus are usually present in the fecal stream, not the mucosa (36). Interestingly we found an increase in abundance of potentially pathogenic genera, Enterococcus, Burkholderia, and Veillonellaceae constituents, in HE patients. Prior stool studies have shown an increased abundance of Veillonellaceae in cirrhosis compared with noncirrhotic patients (12). This shift in HE patients' mucosa with higher Enterococcus, Veillonellaceae, and Burkholderia abundance may reflect a disease-associated reduction in the normally present autochthonous bacteria that would allow the growth of these potentially pathogenic genera in the mucosa. Patients on rifaximin had worse cognition and higher endotoxin and IL-6 compared with those without rifaximin, which is to be expected since rifaximin is initiated in those whose HE is not controlled with lactulose. It is likely that the cross-sectional nature of our patients was the reason behind the poor mitigation of endotoxemia by rifaximin as shown in other studies (27). Therefore, their mucosal microbiome also reflected the worse underlying disease, i.e., a significantly decreased abundance of the autochthonous bacteria. Some of these genera are associated with severe infections in cirrhotic and noncirrhotic patients (34, 54). Furthermore, these genera were only seen in HE patients' network and were associated with a higher MELD score, worse endothelial activation, worse cognitive performance (lures, serial dotting, and digit symbol tests), and higher systemic inflammation (IL-17) in the HE group. We found differences between controls and cirrhotic patients on Metastats and PCA and the abundances have biological plausibility; i.e., autochthonous genera were overrepresented in the no-HE and control groups, whereas pathogenic ones were overrepresented in the HE group and were correlated with cognitive, inflammatory, and endothelial phenotypes in the direction that was expected (25, 42).
In the correlation network for patients with HE, a richer and more robust interaction was seen between the microbiome, cognition, inflammation, and endothelial activation compared with those without HE. We confirmed the results of prior studies that Alcaligeneceae and Porphyromonadaceae were associated with poor cognitive performance (6). One interesting finding was that the autochthonous bacteria belonging to Lachnospiraceae, Ruminococcaceae, and Incertae Sedis XIV had similar beneficial linkages, regardless of the setting. This means that the presence of these bacteria is associated with better cognitive functioning and decreased inflammation and endothelial activation regardless of the early or advanced disease stage. This replicates studies showing that Fecalibacterium and Lachnospiraceae spp. are associated with reduced intestinal inflammation in Crohn's disease and extends this finding into cirrhosis (45, 46). There is also evidence that these bacteria are correlated with markers for reduced inflammation of Th-17 cells in the colon (2). Prior studies have shown that intestinal inflammation can initiate the IL-17/IL-23 system, which is upregulated in Crohn's disease (8, 14, 22). Correspondingly, we found correlations with markers for the IL-17/IL-23 inflammatory response system in cirrhosis, in both HE and non-HE patients. There was a negative correlation between autochthonous bacteria and IL-17, and proinflammatory cytokines, indicating that the gut-based inflammation may be modulated in the presence of these bacteria. Prior studies have also shown that HE is associated with significantly worse systemic inflammation that can potentially improve with therapy (5, 44). Interestingly, we also found that levels of the anti-inflammatory cytokine, IL-10, were correlated with poor outcomes similar to the proinflammatory cytokines; this may reflect a mixed scenario between the systemic inflammatory response and the compensatory anti-inflammatory response syndrome that can be seen in cirrhosis (9). It can therefore be speculated that modulation of both pro- and anti-inflammatory cytokines could be a potential mechanism behind the microbiome-associated changes in brain function in HE.
The present study only relied on the presence of bacteria, but it is also possible that their end-products, such as the beneficial short-chain fatty acids or the relatively toxic indoles and phenols, may influence clinical outcomes (20, 39). A study of the functional component of the microbes would be important to analyze these effects (20). The present analysis is also only correlative; therefore no conclusions regarding causality can be made, but this generates hypotheses to be tested in subsequent studies. It is also possible, given the high MELD score in HE patients, that this was related simply to severity of the liver disease in HE patients.
We conclude that there is a significant difference in sigmoid mucosal microbiome of the cirrhotic patients compared with healthy controls. There are also differences between the colonic and stool microbiome in cirrhosis, which persists even when patients are subdivided into those with and without HE. We also found that the colonic mucosal microbiome of HE patients (who were also more advanced from a liver disease perspective) is significantly different from patients without HE but the stool microbiome was essentially the same. There is a lower abundance of autochthonous bacterial genera coupled with a higher level of potentially pathogenic bacteria such as Enterooccus and Burkholderia in the HE patients' colonic mucosa. Autochthonous bacteria, Lachnospiraceae, Ruminococcacae, and Incertae Sedis XIV, are associated with better cognition, lower severity of liver disease, and decreased inflammation and endothelial activation in both HE and no-HE groups. However, genera overrepresented in the HE patients' mucosa were associated with a proinflammatory milieu, higher MELD score, and poor cognition. Therefore, the colonic mucosal microbiome of patients with HE is significantly different from patients without HE and is associated with the proinflammatory milieu, endothelial activation, and poor cognitive performance that is inherent in this patient population. Further studies are needed to determine the longitudinal effect of interventions on both the colonic and stool microbiome in patients with cirrhosis, accounting for liver disease severity so that newer and specific therapeutic targets can be evaluated.
GRANTS
This work was partly supported by the grant UO1-AT004428 from the National Center for Complementary and Alternative Medicine, grant RO1AA020203 from the National Institute on Alcohol Abuse and Alcoholism, grant RO1DK087913 from the National Institute of Diabetes and Digestive and Kidney Diseases, Grant BX001328 from the Veterans Administration, and the McGuire Research Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S.B. and D.M.H. conception and design of research; J.S.B., K.D., M.B.W., P.M., N.A.N., M.S., and P.M.G. analyzed data; J.S.B., P.B.H., J.M.R., M.S., and P.M.G. interpreted results of experiments; J.S.B. and P.M.G. prepared figures; J.S.B. and P.M.G. drafted manuscript; J.S.B., P.B.H., J.M.R., D.M.H., K.D., and P.M.G. edited and revised manuscript; J.S.B., P.B.H., J.M.R., D.M.H., K.D., M.B.W., P.M., N.A.N., M.S., and P.M.G. approved final version of manuscript; J.M.R., K.D., M.B.W., P.M., N.A.N., M.S., and P.M.G. performed experiments.
REFERENCES
- 1. Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33: 279–288, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331: 337–341, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, Schubert CM, Sikaroodi M, Heuman DM, Crossey MME, Bell DE, Hylemon PB, Fatouros PP, Taylor-Robinson SD. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metab Brain Dis 27: 205–215, 2012 [DOI] [PubMed] [Google Scholar]
- 4. Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, Hischke D, Hammeke TA, Pinkerton SD, Saeian K. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 135: 1591–1600, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT, Fuchs M, Luketic V, Sanyal AJ. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology 140: 478–487, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, Sikaroodi M, Gillevet PM. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 302: G168–G175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barcenilla A, Pryde SE, Martin JC, Duncan SH, Stewart CS, Henderson C, Flint HJ. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol 66: 1654–1661, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barnes MJ, Powrie F. Immunology. The gut's Clostridium cocktail. Science 331: 289–290, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Berry PA, Antoniades CG, Carey I, McPhail MJ, Hussain MJ, Davies ET, Wendon JA, Vergani D. Severity of the compensatory anti-inflammatory response determined by monocyte HLA-DR expression may assist outcome prediction in cirrhosis. Intensive Care Med 37: 453–460, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkila J, Monti D, Satokari R, Franceschi C, Brigidi P, De Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5: e10667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouhnik Y, Attar A, Joly FA, Riottot M, Dyard F, Flourie B. Lactulose ingestion increases faecal bifidobacterial counts: a randomised double-blind study in healthy humans. Eur J Clin Nutr 58: 462–466, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Yang F, Lu H, Wang B, Lei D, Wang Y, Zhu B, Li L. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 54: 562–572, 2011 [DOI] [PubMed] [Google Scholar]
- 13. Cordoba J. New assessment of hepatic encephalopathy. J Hepatol 54: 1030–1040, 2011 [DOI] [PubMed] [Google Scholar]
- 14. D'Elios MM, Del Prete G, Amedei A. Targeting IL-23 in human diseases. Expert Opin Ther Targets 14: 759–774, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Flint HJ, Bayer EA. Plant cell wall breakdown by anaerobic microorganisms from the mammalian digestive tract. Ann NY Acad Sci 1125: 280–288, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198, 1975 [DOI] [PubMed] [Google Scholar]
- 17. Gillevet P, Sikaroodi M, Keshavarzian A, Mutlu EA. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem Biodivers 7: 1065–1075, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Green GL, Brostoff J, Hudspith B, Michael M, Mylonaki M, Rayment N, Staines N, Sanderson J, Rampton DS, Bruce KD. Molecular characterization of the bacteria adherent to human colorectal mucosa. J Appl Microbiol 100: 460–469, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients 3: 637–682, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol 302: G1–G9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hofmann AF, Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proc Natl Acad Sci USA 103: 4333–4334, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huber S, Flavell RA. Checks and balances: IL-23 in the intestine. Immunity 33: 150–152, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 103: 3920–3925, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jolliffe IT. Principal Component Analysis. New York: Springer-Verlag, 1986 [Google Scholar]
- 26. Kakiyama G, Ridlon JM, Hylemon PB, Pandak WM, Gillevet PM, Heuman DM, Daita K, Bajaj JS. The gut microbiome modulates fecal bile acid profile in patients with cirrhosis (Abstract). J Hepatol 56: S51, 2012 [Google Scholar]
- 27. Kalambokis GN, Tsianos EV. Rifaximin reduces endotoxemia and improves liver function and disease severity in patients with decompensated cirrhosis. Hepatology 55: 655–656, 2012 [DOI] [PubMed] [Google Scholar]
- 28. Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology 33: 464–470, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lane DJ. 16s/23s rRNA sequencing. In: Nucleic Acid Techniques in Bacterial Systematics, edited by Goodfellow M. West Sussex, UK: Wiley, 1991, p. 115–175 [Google Scholar]
- 30. Lepage P, Seksik P, Sutren M, de la Cochetiere MF, Jian R, Marteau P, Dore J. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD. Inflamm Bowel Dis 11: 473–480, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Wu D, Ahmed A, Li X, Ma Y, Tang L, Mo D, Xin Y. Comparison of the gut microbe profiles and numbers between patients with liver cirrhosis and healthy individuals. Curr Microbiol 65: 7–13, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294: 1–8, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71: 8228–8235, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol 8: 979–985, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Mutlu EA, Gillevet PM, Rangwala H, Sikaroodi M, Naqvi A, Engen PA, Kwasny M, Lau CK, Keshavarzian A. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 302: G966–G978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nava GM, Stappenbeck TS. Diversity of the autochthonous colonic microbiota. Gut Microbes 2: 99–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ohtsuka Y, Ikegami T, Izumi H, Namura M, Ikeda T, Ikuse T, Baba Y, Kudo T, Suzuki R, Shimizu T. Effects of Bifidobacterium breve on inflammatory gene expression in neonatal and weaning rat intestine. Pediatr Res 71: 46–53, 2012 [DOI] [PubMed] [Google Scholar]
- 38. Ridlon JM, McGarr SE, Hylemon PB. Development of methods for the detection and quantification of 7alpha-dehydroxylating clostridia, Desulfovibrio vulgaris, Methanobrevibacter smithii, and Lactobacillus plantarum in human feces. Clin Chim Acta 357: 55–64, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Riggio O, Mannaioni G, Ridola L, Angeloni S, Merli M, Carla V, Salvatori FM, Moroni F. Peripheral and splanchnic indole and oxindole levels in cirrhotic patients: a study on the pathophysiology of hepatic encephalopathy. Am J Gastroenterol 105: 1374–1381, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Romero Gomez M, Cordoba J, Jover R, del Olmo J, Fernandez A, Flavia M, Company L, Poveda MJ, Felipo V. [Normality tables in the Spanish population for psychometric tests used in the diagnosis of minimal hepatic encephalopathy]. Med Clin (Barc) 127: 246–249, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, Dal Lago A, Ojetti V, Ainora ME, Santoro M, Ghirlanda G, Gasbarrini A. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol 105: 323–327, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol 12: R60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol 54: 640–649, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Shawcross DL, Wright G, Olde Damink SW, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metab Brain Dis 22: 125–138, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Sokol H, Lay C, Seksik P, Tannock GW. Analysis of bacterial bowel communities of IBD patients: what has it revealed? Inflamm Bowel Dis 14: 858–867, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA 105: 16731–16736, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis 28: 26–42, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, Bevins CL, Stange EF, Wehkamp J. Intestinal bacterial translocation in cirrhotic rats is related to compromised Paneth cell antimicrobial host defence. Hepatology 55: 1154–1163, 2012 [DOI] [PubMed] [Google Scholar]
- 49. Ventura M, Turroni F, Zomer A, Foroni E, Giubellini V, Bottacini F, Canchaya C, Claesson MJ, He F, Mantzourani M, Mulas L, Ferrarini A, Gao B, Delledonne M, Henrissat B, Coutinho P, Oggioni M, Gupta RS, Zhang Z, Beighton D, Fitzgerald GF, O'Toole PW, van Sinderen D. The Bifidobacterium dentium Bd1 genome sequence reflects its genetic adaptation to the human oral cavity. PLoS Genet 5: e1000785, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34: 768–773, 2001 [DOI] [PubMed] [Google Scholar]
- 51. White JR, Nagarajan N, Pop M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput Biol 5: e1000352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 41: 422–433, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Wiltfang J, Nolte W, Otto M, Wildberg J, Bahn E, Figulla HR, Pralle L, Hartmann H, Ruther E, Ramadori G. Elevated serum levels of astroglial S100beta in patients with liver cirrhosis indicate early and subclinical portal-systemic encephalopathy. Metab Brain Dis 14: 239–251, 1999 [DOI] [PubMed] [Google Scholar]
- 54. Wong F, Bernardi M, Balk R, Christman B, Moreau R, Garcia-Tsao G, Patch D, Soriano G, Hoefs J, Navasa M. Sepsis in cirrhosis: report on the 7th meeting of the International Ascites Club. Gut 54: 718–725, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zoetendal EG, von Wright A, Vilpponen-Salmela T, Ben-Amor K, Akkermans AD, de Vos WM. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl Environ Microbiol 68: 3401–3407, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]