Abstract
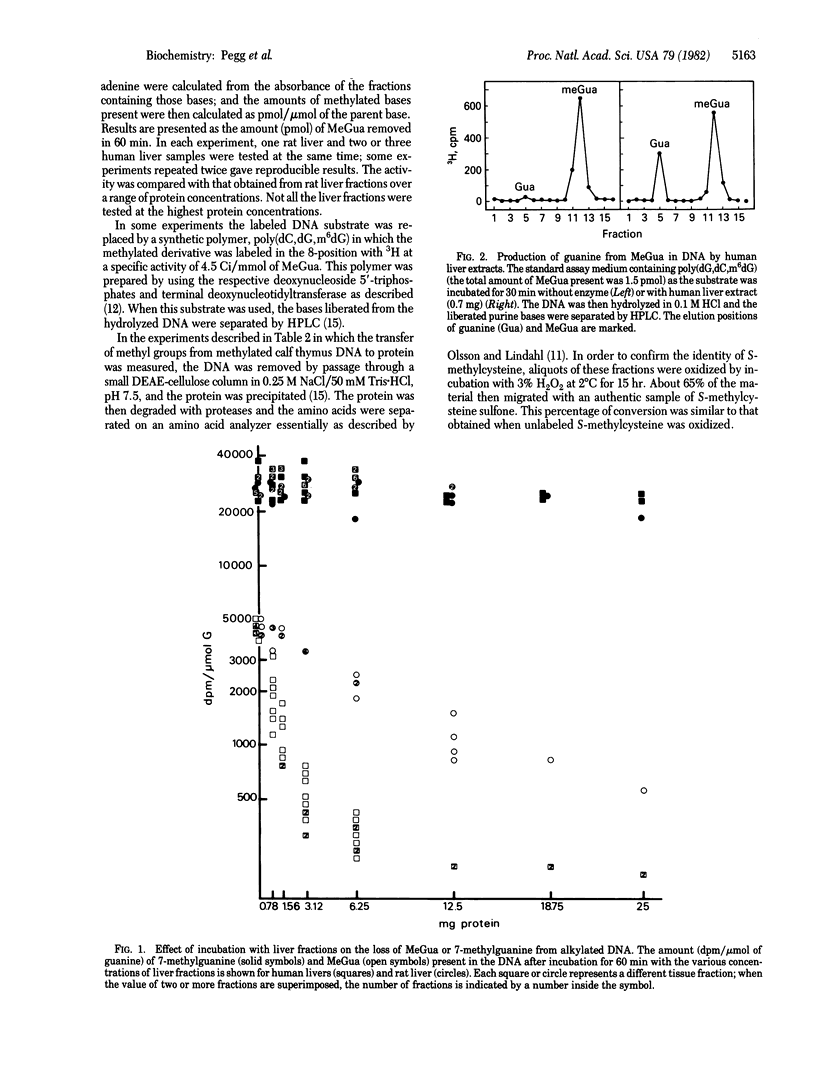
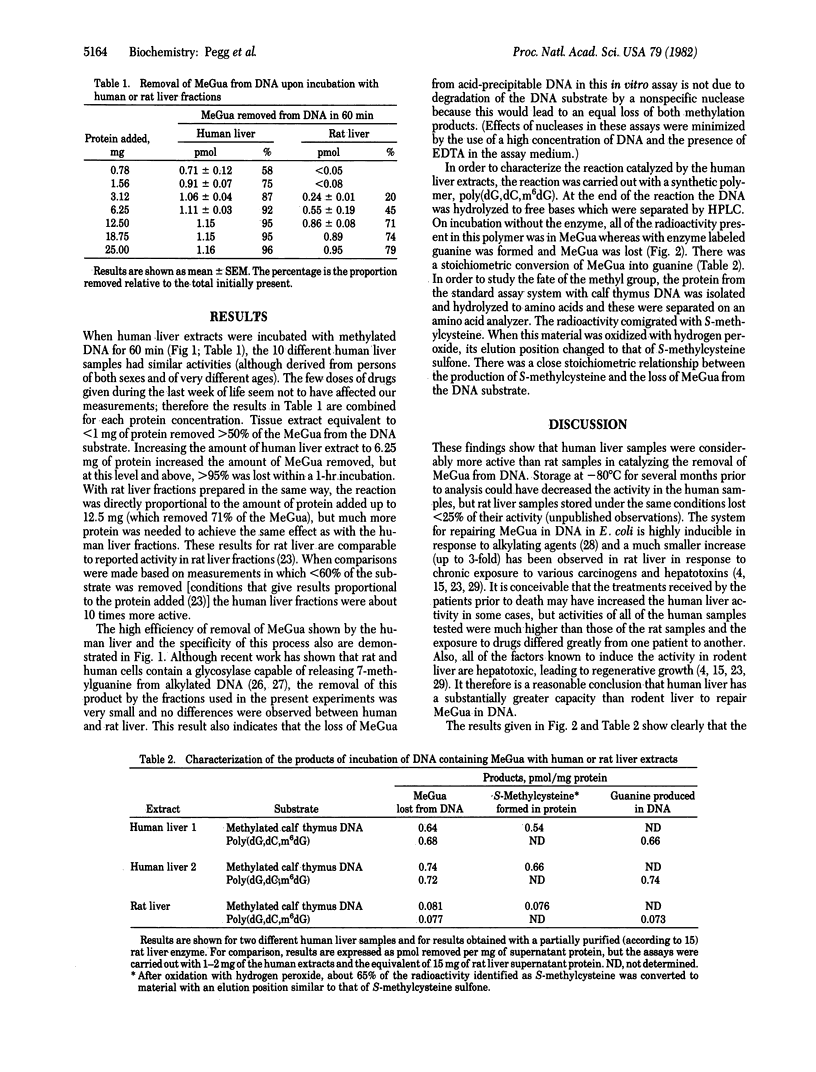
In in vitro assays using methylated DNAs as substrates, human liver fractions were shown to be able to catalyze the removal of O6-methylguanine. The amount of removal was proportional to the amount of protein added, and the loss of O6-methylguanine occurred with stoichiometric formation of guanine in the DNA and S-methylcysteine in protein. This indicates that human liver contains a protein similar to that previously found in bacteria exposed to alkylating agents. This protein acts as a transmethylase, transferring the intact methyl group from O6-methylguanine in DNA to a cysteine residue on that protein. A similar activity is present in rodent liver, but it was found that human liver was about 10 times more active in carrying out this reaction. In contrast, there was no difference between the human and rat liver extracts in catalyzing the loss of another methylation product, 7-methylguanine, from alkylated DNA. The liver is the organ most likely to be alkylated after exposure to exogenous potential alkylating agents such as dimethylnitrosamine. The present results show that human liver has a significant capacity to repair O6-methylguanine in DNA, which has been implicated as a critical product in carcinogenesis and mutagenesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altamirano-Dimas M., Sklar R., Strauss B. Selectivity of the excision of alkylation products in a xeroderma pigmentosum-derived lymphoblastoid line. Mutat Res. 1979 Apr;60(2):197–206. doi: 10.1016/0027-5107(79)90184-2. [DOI] [PubMed] [Google Scholar]
- Bogden J. M., Eastman A., Bresnick E. A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res. 1981 Jul 10;9(13):3089–3103. doi: 10.1093/nar/9.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cairns J. The Leeuwenhoek Lecture, 1978. Bacteria as proper subjects for cancer research. Proc R Soc Lond B Biol Sci. 1980 Jun 24;208(1171):121–133. doi: 10.1098/rspb.1980.0046. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Foote R. S., Mitra S., Pal B. C. Demethylation of O6-methylguanine in a synthetic DNA polymer by an inducible activity in Escherichia coli. Biochem Biophys Res Commun. 1980 Nov 28;97(2):654–659. doi: 10.1016/0006-291x(80)90314-9. [DOI] [PubMed] [Google Scholar]
- Gomez M. I., Swann P. F., Magee P. N. The absorption and metabolism in rats of small oral doses of dimethylnitrosamine. Implication for the possible hazard of dimethylnitrosamine in human food. Biochem J. 1977 Jun 15;164(3):497–500. doi: 10.1042/bj1640497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G., Lawley P. D., Olsen I. Mode of action of methylating carcinogens: comparative studies of murine and human cells. Carcinogenesis. 1981;2(5):403–411. doi: 10.1093/carcin/2.5.403. [DOI] [PubMed] [Google Scholar]
- Herron D. C., Shank R. C. Methylated purines in human liver DNA after probable dimethylnitrosamine poisoning. Cancer Res. 1980 Sep;40(9):3116–3117. [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Margison G. P., Pegg A. E. Enzymatic release of 7-methylguanine from methylated DNA by rodent liver extracts. Proc Natl Acad Sci U S A. 1981 Feb;78(2):861–865. doi: 10.1073/pnas.78.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medcalf A. S., Lawley P. D. Time course of O6-methylguanine removal from DNA of N-methyl-N-nitrosourea-treated human fibroblasts. Nature. 1981 Feb 26;289(5800):796–798. doi: 10.1038/289796a0. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B., Renard A., Verly W. G. Repair of O6-ethylguanine in DNA by a chromatin fraction from rat liver: transfer of the ethyl group to an acceptor protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6766–6770. doi: 10.1073/pnas.78.11.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R. Alkylation of DNA and tissue specificity in nitrosamine carcinogenesis. J Supramol Struct Cell Biochem. 1981;17(3):259–273. doi: 10.1002/jsscb.380170307. [DOI] [PubMed] [Google Scholar]
- Montesano R., Brésil H., Planche-Martel G., Margison G. P., Pegg A. E. Effect of chronic treatment of rats with dimethylnitrosamine on the removal of O6-methylguanine from DNA. Cancer Res. 1980 Feb;40(2):452–458. [PubMed] [Google Scholar]
- Montesano R., Magee P. N. Metabolism of diethylnitrosamine by human liver slices in vitro. Nature. 1970 Oct 10;228(5267):173–174. doi: 10.1038/228173a0. [DOI] [PubMed] [Google Scholar]
- Montesano R., Pegg A. E., Margison G. P. Alkylation of DNA and carcinogenicity of N-nitroso compounds. J Toxicol Environ Health. 1980 Sep-Nov;6(5-6):1001–1008. doi: 10.1080/15287398009529922. [DOI] [PubMed] [Google Scholar]
- Newbold R. F., Warren W., Medcalf A. S., Amos J. Mutagenicity of carcinogenic methylating agents is associated with a specific DNA modification. Nature. 1980 Feb 7;283(5747):596–599. doi: 10.1038/283596a0. [DOI] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E. Enzymatic removal of O6-methylguanine from DNA by mammalian cell extracts. Biochem Biophys Res Commun. 1978 Sep 14;84(1):166–173. doi: 10.1016/0006-291x(78)90278-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Hui G. Formation and subsequent removal of O6-methylguanine from deoxyribonucleic acid in rat liver and kidney after small doses of dimethylnitrosamine. Biochem J. 1978 Sep 1;173(3):739–748. doi: 10.1042/bj1730739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Perry W. Alkylation of nucleic acids and metabolism of small doses of dimethylnitrosamine in the rat. Cancer Res. 1981 Aug;41(8):3128–3132. [PubMed] [Google Scholar]
- Pegg A. E., Perry W., Bennett R. A. Effect of partial hepatectomy on removal of O6-methylguanine from alkylated DNA by rat liver extracts. Biochem J. 1981 Jul 1;197(1):195–201. doi: 10.1042/bj1970195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Perry W. Stimulation of transfer of methyl groups from O6-methylguanine in DNA to protein by rat liver extracts in response to hepatotoxins. Carcinogenesis. 1981;2(11):1195–1200. doi: 10.1093/carcin/2.11.1195. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Becker Y. Kinetics of O6-methylguanine repair in human normal and ataxia telangiectasia cell lines and correlation of repair capacity with cellular sensitivity to methylating agents. Cancer Res. 1981 Dec;41(12 Pt 1):5114–5120. [PubMed] [Google Scholar]
- Singer B., Brent T. P. Human lymphoblasts contain DNA glycosylase activity excising N-3 and N-7 methyl and ethyl purines but not O6-alkylguanines or 1-alkyladenines. Proc Natl Acad Sci U S A. 1981 Feb;78(2):856–860. doi: 10.1073/pnas.78.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B. N-nitroso alkylating agents: formation and persistence of alkyl derivatives in mammalian nucleic acids as contributing factors in carcinogenesis. J Natl Cancer Inst. 1979 Jun;62(6):1329–1339. [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Stumpf R., Margison G. P., Montesano R., Pegg A. E. Formation and loss of alkylated purines from DNA of hamster liver after administration of dimethylnitrosamine. Cancer Res. 1979 Jan;39(1):50–54. [PubMed] [Google Scholar]
- von Bahr C., Groth C. G., Jansson H., Lundgren G., Lind M., Glaumann H. Drug metabolism in human liver in vitro: establishment of a human liver bank. Clin Pharmacol Ther. 1980 Jun;27(6):711–725. doi: 10.1038/clpt.1980.102. [DOI] [PubMed] [Google Scholar]


