Abstract
Expression of estrogen and progesterone hormone receptors indicates a favorable prognosis due to the successful use of hormonal therapies such as tamoxifen and aromatase inhibitors. Unfortunately, 15–20% of patients will experience breast cancer recurrence despite continued use of tamoxifen. Drug resistance to hormonal therapies is of great clinical concern so it is imperative to identify novel molecular factors that contribute to tumorigenesis in hormone receptor positive cancers and/or mediate drug sensitivity. The hope is that targeted therapies, in combination with hormonal therapies, will improve survival and prevent recurrence. We have previously shown that the DEK oncogene, which is a chromatin remodeling protein, supports breast cancer cell proliferation, invasion and the maintenance of the breast cancer stem cell population. In this report, we demonstrate that DEK expression is associated with positive hormone receptor status in primary breast cancers and is up-regulated in vitro following exposure to the hormones estrogen, progesterone, and androgen. Chromatin immunoprecipitation experiments identify DEK as a novel estrogen receptor α (ERα) target gene whose expression promotes estrogen-induced proliferation. Finally, we report for the first time that DEK depletion enhances tamoxifen-induced cell death in ER+ breast cancer cell lines. Together, our data suggest that DEK promotes the pathogenesis of ER+ breast cancer and that the targeted inhibition of DEK may enhance the efficacy of conventional hormone therapies.
Introduction
Clinical and pathological characterization of breast cancer, the second leading cause of cancer-related deaths among women in the United States [1], is crucial for identifying the best course of treatment for each patient. Detection of steroid hormone receptor expression, particularly estrogen, progesterone, and androgen receptors, determines whether or not a patient will respond to selective estrogen receptor (ER) modulators (SERMS), aromatase inhibitors, or other anti-hormone therapies. In recent decades, there has been an increase in the percentage of breast cancers that are positive for the expression of ERα (“ER+”) such that nearly 75% of all breast cancers are now ER+ [2]. Continued advancements in our understanding of the biology of these cancers are important in order to generate novel, more effective and perhaps combinatorial treatments.
Recent reports have shown that transcription of the DEK oncogene is up-regulated in breast cancers with particularly strong gene expression detected in lymph node positive and late stage breast cancers, and that DEK expression correlated with increased recurrence rates after 3 years [3]–[6]. Furthermore, work from our laboratory has shown that DEK protein levels are elevated in both cultured cell lines and primary invasive adenocarcinomas and that DEK expression stimulates breast cancer cell proliferation in vitro and in vivo, together with cellular invasion, and growth of the breast cancer stem cell population [7].
DEK is a unique, ubiquitously expressed protein that predominantly binds to chromatin but can also be soluble or secreted as a result of post-translational modifications [8]–[12]. Its ability to bind nucleic acids has led to functional associations with several cellular processes including chromatin remodeling, transcriptional regulation, replication, mRNA splicing, and DNA repair [13]–[19]. Cell free assays have shown that DEK introduces constrained positive supercoils into DNA and can facilitate the ligation of linear DNA molecules in vitro [20], [21]. Importantly, DEK plays a critical role in chromatin organization and the maintenance of genome stability in vivo. It has histone chaperone activity, which regulates epigenetic markers on chromatin, and it is necessary for the maintenance of heterochromatin integrity by facilitating the interaction between Heterochromatin Protein 1α (HP1α) and trimethylated histone H3 (H3K9me3) [22], [23]. These effects on chromatin structure are likely responsible for alterations in gene transcription and DNA replication by regulating accessibility to DNA. In addition, DEK recently was found to promote DNA-PK activity and DNA double-strand break repair by non-homologous end joining (NHEJ) [19]. Therefore, when DEK is up-regulated, as is observed in numerous types of cancer including breast cancer, perturbations to normal genome architecture and integrity are likely contributors to oncogenesis [3], [4], [7], [24]–[28]. In addition to epigenetics and chromatin integrity, the DEK oncogene has also been implicated in regulating the expression, phosphorylation and/or activity of several important signaling molecules and pathways. For example, DEK enhances invasion in breast cancer cell lines by stimulating β-catenin activity [7]. Furthermore, DEK inhibits p53-dependent and –independent apoptosis and has been found to mediate the apoptotic response to clastogenic chemotherapeutic agents such as doxorubicin and cisplatin [7], [28]–[30].
It is well documented that the levels of DEK protein are critical for events such as oncogenesis and genome stability. The over-expression of DEK stimulated tumorigenesis in several tissues, and DEK depletion can result in cell death and impaired DNA double strand break repair [7], [19]. Therefore, cellular DEK expression is tightly controlled in order to maintain proper cell function and viability. However, little is known about cellular regulation of DEK gene expression. NF-Y and YY1 were shown to be responsible for the constitutive transcription of DEK and the DEK-CAN fusion gene (found in t(6;9) acute myeloid leukemias) in transformed cell lines [31]. DEK is also an E2F target gene and consequently up-regulated in cells infected with human papillomavirus (HPV) due to inactivation of Rb and the subsequent activation of E2F transcription factors [27]. Here, we report for the first time that DEK transcription is regulated by steroid hormone receptors, particularly ERα in breast cancer, and that DEK expression promotes hormone-dependent cancer cell proliferation.
Results
Hormone Receptor Positive Primary Breast Cancers Express the DEK Oncogene
We performed immunohistochemical analysis for DEK expression on a tissue microarray that consisted of 30 invasive breast carcinomas and compared expression levels with numerous clinical and pathological variables including patient age, tumor grade, tumor stage, tumor size, lymph node status, HER2 expression, and hormone receptor status. Of those, possible associations between positive DEK expression and both androgen receptor (AR) positivity and patient age greater than 50 years old manifested as a trend. However, there was a strong positive relationship between DEK expression and progesterone and estrogen hormone receptor positive primary invasive breast adenocarcinomas (Table 1; Fig. 1A). This contradicts a recent report by Liu et al. which did not find a correlation between DEK and ER/PR expression in invasive adenocarcinomas [6]. Besides using a different patient population, the major difference between our work and theirs is that they only considered cancers that were “strongly positive” for DEK expression; here, we uncovered an association between ER/PR/AR positivity with any degree of DEK staining.
Table 1. Association of DEK expression with clinical and pathological variables in invasive adenocarcinomas of the breast.
| Clinical & Pathological Variables | N | Number DEK+ | p value* |
| Age | |||
| ≤50 yr | 21 | 15 | |
| >50 yr | 9 | 9 | 0.07 |
| Estrogen Receptor | |||
| Negative | 19 | 13 | |
| Positive | 11 | 11 | 0.04 |
| Progesterone Receptor | |||
| Negative | 20 | 14 | |
| Positive | 10 | 10 | 0.05 |
| Androgen Receptor | |||
| Negative | 21 | 15 | |
| Positive | 9 | 9 | 0.07 |
Chi-Squared statistic.
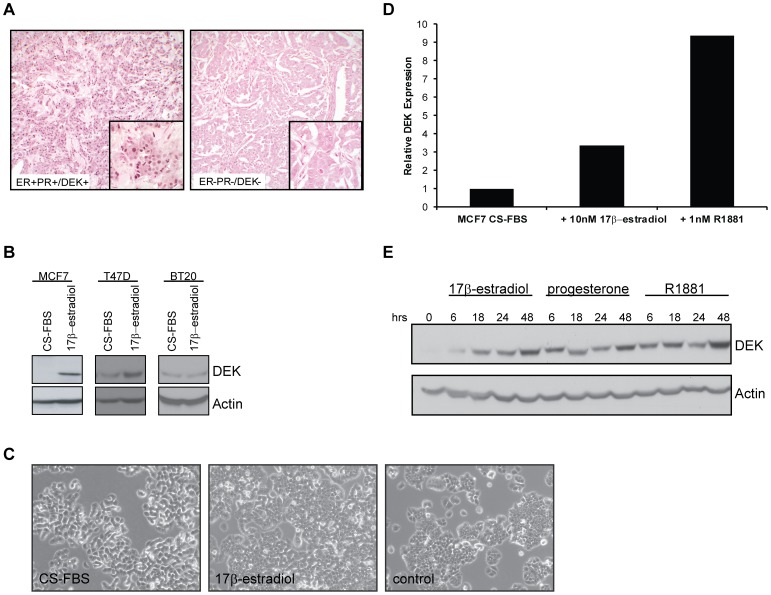
Figure 1. DEK expression is associated with positive hormone receptor status in human primary and cultured breast cancers.
(A) Estrogen receptor (ER) negative tumors were often negative for DEK staining (right) while ER positive tumors were often positive for DEK staining (left). Immunohistochemical staining for DEK in two invasive ductal carcincomas showing positive DEK staining (DEK+) in a hormone receptor positive (ER+/PR+) tumor and lack of DEK expression (DEK-) in a hormone receptor negative tumor (ER−/PR−). Low power images are at 200× and all high power images are at 1000× magnification. (B) Western blotting for DEK showed increased expression following exposure to 17β-estradiol for 48 hours in ER+ MCF7 and T47D cells but not in ER- BT20 cells. (C) Cell morphology of T47D cells in CS-FBS (left), 48 hours of 17β-estradiol treatment (middle), and under normal culture conditions (right). Bright field images of cultured cells were obtained at 100× total magnification. (D) DEK expression increases in 17β-estradiol and R1881 treated cells. Quantitative RT-PCR was performed to detect DEK expression in hormone starved MCF7 cells treated with 10 nM 17β-estradiol or 1 µM methyltrienolone (R1881) for six hours. GAPDH was used as a control and values are normalized to the untreated sample. (E) Western blotting for DEK shows increased protein levels after treatment of hormone starved MCF7 cells with 10 nM 17β-estradiol, 10 nM progesterone, or 1 µM methyltrienolone (R1881) over the course of 48 hours.
DEK is an Estrogen Receptor Target Gene
In order to determine if DEK expression was associated with hormone receptor expression and activity in vitro, two ER+/PR+/AR+ cell lines, MCF7 and T47D, and ER−/PR−/AR− BT20 cells were cultured in hormone depleted charcoal-stripped serum (CS-FBS) then treated with 10 nM 17β-estradiol (E2) to activate the estrogen receptor. DEK expression was significantly up-regulated in the two ER+ cell lines, particularly in MCF7 cells, upon 17β-estradiol exposure but was unchanged in ER− BT20 cells (Fig. 1B). In addition, 17β-estradiol exposure of T47D cells restored the epithelial morphology and induced proliferation (Fig. 1C). Efficacy of 17β-estradiol treatment was confirmed by western blotting for phosphorylated p44/42 (Erk1/2; Figure S1). Further analysis revealed that all three hormones, 17β-estradiol, progesterone, and the synthetic androgen R1881, resulted in increased expression of DEK mRNA and/or protein as early as 6 hours after treatment (Fig. 1D and 1E). Based on the strength of the association between DEK expression and ER status in primary adenocarcinomas (p = 0.04), we decided to focus on the mechanism of 17β-estradiol mediated DEK up-regulation.
Analysis of microarray data in Oncomine indicated that at least 14 independent studies showed a correlation between DEK mRNA expression with estrogen receptor status such that DEK expression was lower in primary ER+ breast cancers compared to ER- breast cancers. In contrast, we showed high levels of DEK protein in ER+ breast cancers (Fig. 1). We were thus intrigued by a report by Coser et al. that revealed DEK mRNA levels increased in MCF7/BUS cells treated with 17β-estradiol in a dose-dependent manner [5], [32]. We also have observed that DEK protein levels were up-regulated in T47D cells at these minimal doses (Figure S2). To determine how rapidly DEK expression increases after 17β-estradiol treatment, MCF7 cells were cultured in CS-FBS and then exposed to 10 nM 17β-estradiol for 30 minutes, 2 hours, and 6 hours and analyzed by quantitative RT-PCR. A nearly four-fold increase in DEK expression was observed at 30 minutes post-treatment and remained high up until the last time point (Fig. 2A). Prolonged exposure to 17β-estradiol resulted in DEK transcript expression returning to baseline levels by 24 hours (Figure S3). Despite the transient mRNA induction, DEK protein levels increased further even after 48 hours of 17β-estradiol treatment (Figure 1E).
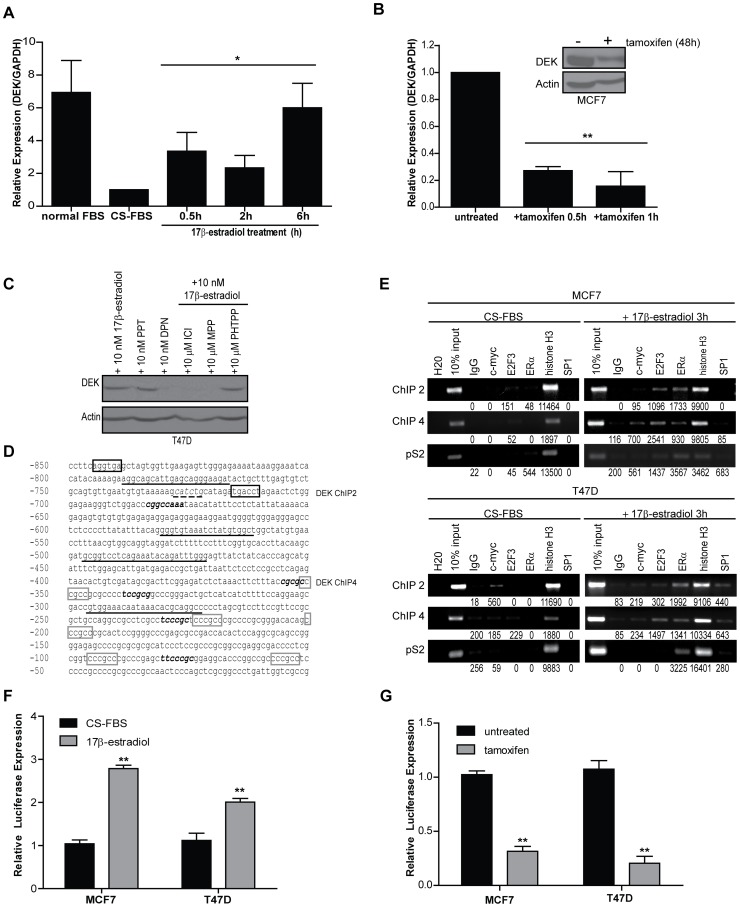
Figure 2. ERα binds to the DEK promoter in response to 17β-estradiol exposure and receptor activity correlates with DEK expression.
(A) 17β-estradiol treatment causes a rapid 4-fold increase in DEK transcription. Quantitative RT-PCR was performed to detect DEK expression in hormone starved MCF7 cells treated with 10 nM 17β-estradiol during the time course shown. GAPDH was used as a control and values are normalized to the untreated sample and represented as fold-change. Results shown depict the average of two to five independent experiments. An asterisk (*) indicates p≤0.05 as determined by one-way ANOVA. (B) Treatment with 3 µg/ml tamoxifen results in a dramatic decrease in DEK expression. Quantitative RT-PCR was performed to detect DEK expression in MCF7 cells grown in low serum treated with 3 µg/ml tamoxifien during the time course shown. GAPDH was used as a control and values are normalized to the untreated sample and represent fold change. Results shown depict the average of replicate experiments. Two asterisks (**) indicates p≤0.01 as determined by one-way ANOVA. (C) ERα, not ERβ, stimulates 17β-estradiol induced DEK expression as determined by western blot analysis. T47D cells were grown in CS-FBS for 7 days then treated with either 10 nM of agonists or 10 µM of antagonist in the presence of 10 nM 17β-estradiol. (D) DEK gene promoter −850 bp upstream of the 5′ UTR. Primers used for ChIP are depicted by underlined sequences. The ERE half sites, described as “DEK ChIP 2,” are highlighted in black boxes at positions −845 and −716 bp. The putative ERα/SP1 binding sites are highlighted with gray boxes drawn starting at position −352 and were amplified as “DEK ChIP 4.” E2F and E2F3 binding sites, previously characterized by Carro et al [27], are indicated by bold, italicized text. The putative c-myc binding site is underlined with a dashed line. (E) ERα binds to the DEK promoter in response to 17β-estradiol treatment. Chromatin was isolated from MCF7 and T47D cells cultured in CS-FBS that were either untreated or treated for 3 hours with 10 nM 17β-estradiol. Chromatin was then subjected to immunoprecipitation using antibodies for IgG (negative control), c-myc, E2F3, ERα, histone H3 (positive control), or SP1. “Input” represents 10% of the DNA used in the immunoprecipitation. Two loci were tested in the DEK promoter, “DEK ChIP 2″ and “DEK ChIP 4″ (see above). The pS2 gene promoter was used as a positive control for ERα binding following 17β-estradiol treatment. Raw densitometry values are indicated under the gel images. (F) DEK reporter assays show transcriptional up-regulation in response to 17β-estradiol treatment. MCF7 and T47D cells were transfected with a luciferase reporter construct under the control of a 1200 bp fragment of the DEK promoter and the first exon. Cells were treated with 10 nM 17β-estradiol for 24 hours. Data represent the average fold induction of luciferase expression above untreated (CS-FBS) levels from triplicate experiments. Two asterisks (**) indicate p≤0.01 as determined by Student’s t-test. (G) DEK reporter assays show transcriptional down-regulation in response to tamoxifen treatment. MCF7 and T47D cells were transfected with a luciferase reporter construct as in (F). Cells were treated with tamoxifen for 24 hours. Data represent the average fold reduction of luciferase expression below untreated levels from triplicate experiments. Two asterisks (**) indicate p≤0.01 as determined by Student’s t-test.
Activity of the estrogen receptor can be chemically inhibited by a family of drugs called selective estrogen receptor modulators (SERMs), an example of which is the commonly used chemotherapeutic drug tamoxifen. MCF7 cells treated with 3 µg/ml tamoxifen rapidly down-regulated DEK expression as determined by quantitative RT-PCR (Fig. 2B). This translated into substantially decreased levels of DEK protein as detected by western blotting after 48 hours of treatment with tamoxifen (Fig. 2B, inset).
In order to ascertain if ERα or ERβ activation was responsible for 17β-estradiol mediated DEK expression, we used a combination of ER agonists and antagonists that differentially altered the activity of the two receptors. Treatment with 17β-estradiol (ERα/β agonist) and propylpyrazole triol (PPT; ERα agonist) both stimulated DEK expression whereas diarylpropionitrile (DPN; ERβ agonist) did not. Likewise, treatment with ICI-182780 (fulvestrant; ERα/β antagonist) and methyl-piperidino-pyrazole (MPP; ERα antagonist) inhibited 17β-estradiol induced DEK expression whereas pyrazolo [1,5-a] pyrimidine (PHTPP; ERβ antagonist) did not (Fig. 2C). Collectively, this indicates that ERα, not ERβ, is responsible for DEK up-regulation following exposure to 17β-estradiol.
We next determined whether 17β-estradiol could induce ERα binding to the DEK promoter. In silico analysis using both the Pattern Search for Transcription Factor Binding Sites (PATCH) and Transcription Element Search Software (TESS) software programs identified a cluster of putative ERα/SP1 binding sites, which are non-canonical ERα binding sites, within 1 kb of the transcriptional start site of the DEK gene (Fig. 2D, gray boxes) and both AR and PR binding sites within 3 kb of the transcriptional start site and within the first intron (data not shown) [33]–[36]. Additional searches revealed a canonical estrogen response element (ERE) approximately 800 bp upstream of the transcriptional start site (Fig. 2D, half sites indicated by black boxes). To determine if ERα can bind directly to select sites in the DEK promoter, we performed chromatin immunoprecipitation (ChIP). Briefly, chromatin was collected from hormone starved and 17β-estradiol treated MCF7 and T47D cells and subjected to immunoprecipitation with antibodies for IgG (negative control), histone H3 (positive control), the known DEK regulator E2F3, the β-catenin target c-myc, ERα, and SP1 [27]. We attempted to detect the multiple ERα/SP1 sites in the first 250 bp of the DEK promoter but the GC-rich region was technically difficult to amplify. Two other locations were tested within the DEK promoter (ChIP 2 and ChIP 4) as well as the promoter of the pS2 gene, a known ERα target gene. First, we determined that c-myc can bind the DEK promoter in starved T47D cells and low levels of E2F3 can bind the DEK promoter in hormone starved MCF7 cells (Fig. 2E, left panels). This relatively high degree of c-myc binding may explain why the baseline level of DEK in T47D cells grown in CS-FBS was higher with a modest increase in DEK expression following 17β-estradiol treatment compared to MCF7 cells (Fig. 1B). None of the other transcription factors were bound to the DEK promoter under conditions of hormone starvation. Interestingly, ERα was detected at both loci in the DEK promoter in the presence of 17β-estradiol, possibly in a complex with SP1. This suggests that DEK is an ERα target gene, which is up-regulated rapidly after exposure to 17β-estradiol. Luciferase reporter assays confirmed that transcription from the DEK promoter was up-regulated following 17β-estradiol treatment (Fig. 2F) and down-regulated in response to tamoxifen-mediated inhibition of estrogen receptor activity (Fig. 2G). Together, this suggests that the regions amplified in ChIP 2 and 4 contain putative binding sites for ERα and there may be additional sites outside of these regions that result in steroid hormone induced up-regulation of DEK oncogene expression. Therefore, detailed mutational studies will be critical to uncover their functional importance, and that of associated transcription factor complexes, in human breast cancer cells and cell lines.
Furthermore, the Wnt/β-catenin target c-myc also bound to the DEK promoter in the region of ChIP4 and E2F3 bound to both ChIP2 and ChIP4, as well as on the pS2 promoter following treatment with 17β-estradiol. Given the concomitant binding of the E2F3 and c-myc transcription factors that are both pro-proliferative with ERα binding, we hypothesized that DEK promotes cellular proliferation following 17β-estradiol exposure.
DEK is Necessary for 17β-estradiol-stimulated Cell Proliferation
Estrogen is a well-characterized mitogenic signal that stimulates cellular proliferation in primary and cultured breast cancer cells, including the MCF7 cell line [37]–[39]. The DEK oncogene also has been implicated in proliferation and survival in several different cell and tissue types in the absence of estrogen modulation [30], [40]. To determine if the up-regulation of DEK in response to 17β-estradiol was functionally relevant for breast cancer pathogenesis, MCF7 cells were infected with lentiviruses carrying non-targeting shRNA (NTsh) or DEKshRNA (DEKsh2) as previously described (Fig. 3A inset) [7]. After selection with puromycin, the cells were plated in CS-FBS media with or without 10 nM 17β-estradiol, and BrdU incorporation was monitored as a measure of DNA replication. There was no difference in BrdU incorporation between the two hormone starved cell lines. However, DEK knockdown resulted in a significant decrease in the proliferative response to 17β-estradiol compared to control NTsh cells (Fig. 3A, p<0.05). Taken together, this data demonstrates that DEK is necessary for hormone-stimulated proliferation in the ER+ MCF7 breast cancer cell line.
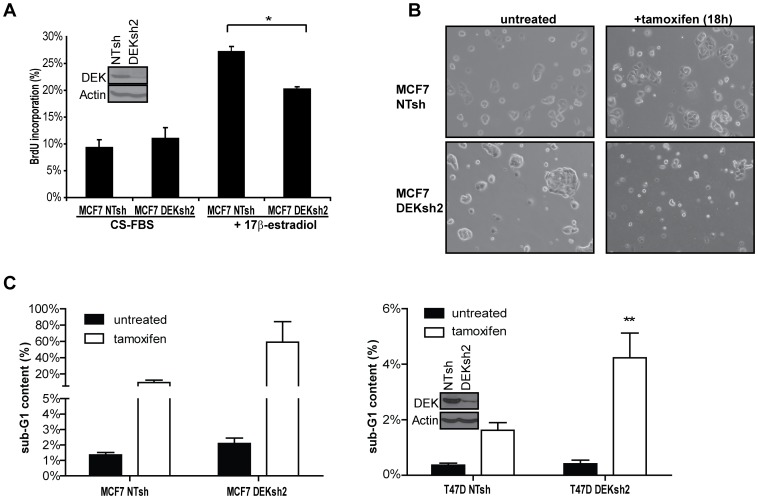
Figure 3. DEK is necessary for 17β-estradiol stimulated cell proliferation and modulates sensitivity to tamoxifen.
(A) DEK expression is required for 17β-estradiol stimulated cellular proliferation. Hormone starved MCF7 cells transduced with non-targeting shRNA (NTsh) or DEK shRNA (DEKsh2) were untreated (CS-FBS) or exposed to 10 nM 17β-estradiol, then cultured in BrdU. The percentage of BrdU positive cells was determined by flow cytometry. Asterisk (*) denotes p<0.05 using Student’s t-test. (B and C) DEK depletion by shRNA (DEKsh2) works synergistically with tamoxifen to induce apoptosis in breast cancer cell lines. (B) Bright field images (100× magnification) of MCF7 cells expressing either NTsh or DEKsh2 were cultured in low serum media and either untreated or treated with tamoxifen for 18 hours. (C) DEK depletion by shRNA (DEKsh2) enhances the cytotoxic effect of tamoxifen. DEK proficient and deficient MCF7 (left) and T47D (right) cells were grown in low serum media then treated with 3 µg/ml tamoxifen for 22 hours. Cells were labeled with 7AAD then analyzed for sub-G1 content by flow cytometry as a measure of apoptosis. Results shown are the average of triplicate experiments. Two asterisks (**) indicate p<0.01 as determined using a 2-way ANOVA test for significance. For MCF7 cells, p = 0.08. (A and B insets) DEK shRNA knockdown is shown by western blot analysis for normally cultured cells that were transduced with lentivirus carrying either non-targeting shRNA (NTsh) or DEK specific shRNA (DEKsh2).
Loss of DEK Sensitizes ER+ Breast Cancer Cells to Tamoxifen
The estrogen receptor antagonist tamoxifen is used therapeutically to inhibit proliferation and promote apoptosis in ER+ breast cancers. Tamoxifen treatment decreased DEK expression (Fig. 2B), but DEK was still expressed to detectable levels. To determine if the remaining DEK protein in tamoxifen treated cells was important for the cellular response to therapy, control and DEK-depleted (DEKsh2) cells were treated with tamoxifen and analyzed for apoptosis, as detected by sub-G1 DNA content using flow cytometry and visual analysis with bright field microscopy. As previously reported, loss of DEK by itself increased the percentage of apoptotic cells in the population (Fig. 3C) [7]. In addition to this baseline increase in apoptosis, DEK depletion by shRNA enhanced the apoptotic response to tamoxifen treatment in MCF7 and T47D cells (Fig. 3B, 3C). These findings were confirmed by DAPI staining to visualize condensed and fragmented DNA in apoptotic cells that remained attached to the culture surface, and western blotting indicated the presence of increased levels of cleaved caspase 8 (Fig. S4). Combined, these data suggest that the two methodologies, DEK depletion and tamoxifen treatment, can act synergistically to eliminate ER+ cancer cells in culture.
Discussion
Herein we report for the first time a functional association between 17β-estradiol exposure and DEK expression in ER+ breast cancers and identify DEK as a novel ERα target gene. DEK expression was associated with the presence of steroid hormone receptors in primary tumors and its expression was upregulated in response to steroid hormone treatment, with a particular focus on 17β-estradiol in ER+ MCF7 and T47D cells in vitro. DEK transcription was upregulated rapidly, within 30 minutes of 17β-estradiol treatment, but then fell to baseline levels with prolonged exposure (Figures 2 and S3). This transient synthesis of DEK message may explain why microarray studies in Oncomine, using tissues persistently exposed to estrogen, have typically reported lower DEK expression in ER+ cancers when compared to ER- cancers. Prolonged DEK up-regulation at the protein, but not transcript, level therefore also suggests post-transcriptional regulatory mechanisms following hormone exposure, but the regulation of DEK protein stability and turnover in breast cancer cells is unknown. DEK induction by 17β-estradiol signaling also was functionally important since the RNAi-mediated loss of DEK expression in ER+ MCF7 cells diminished 17β-estradiol induced cell proliferation. Together, our work suggests that, in vivo, hormone signaling, such as 17β-estradiol exposure, might result in the up-regulation of DEK protein levels to promote proliferation in ER+ cancers.
DEK expression was also observed in several primary ER- breast cancers and was previously published to be highly expressed in ER- breast cancer cell lines compared to immortalized mammary epithelial cells [7]. This may be because DEK is a known E2F target gene and thus upregulated upon Rb pathway deregulation, a frequent event in breast cancer [27], [41], [42]. E2F3 also was observed to bind the DEK promoter in response to 17β-estradiol treatment, but this binding was minimal in the growth-restricting CS-FBS containing media (Fig. 2E). Of note, E2F is a known ERα target gene; therefore, more than one mechanism may contribute to DEK up-regulation following the mitogenic signal of steroid hormone exposure [43]. First, ERα binding to the DEK promoter could directly enhance transcription. Second, activated ERα could drive the expression of E2F proteins, the binding of which to the DEK promoter might then further increase expression in an additive or synergistic fashion (Fig. 4). We postulate that DEK is transcriptionally up-regulated via ER-dependent and -independent mechanisms in a majority of breast cancers, and studies to dissect the relative contributions of E2F and ERα are currently under way. Importantly, the up-regulation of DEK in ER+ and ER- cancers suggest that targeting DEK expression may be a therapeutic option for breast cancers with different molecular signatures.
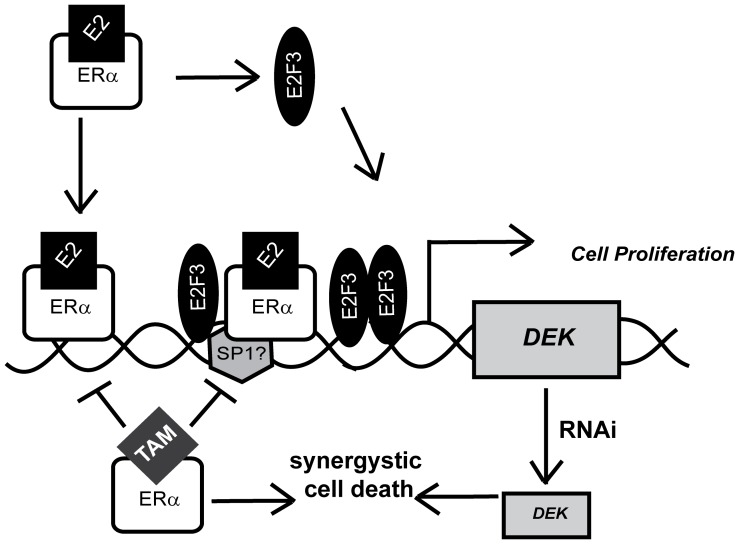
Figure 4. Model for DEK transcriptional up-regulation following 17β-estradiol exposure.
Upon 17β-estradiol exposure, ERα is activated and binds to the DEK promoter at least at two locations – an ERE half site at −716 bp and at ERα/Sp1 binding sites more proximal to the transcriptional start site. A second potential mechanism of up-regulation is the ERα-mediated up-regulation of E2F proteins (particularly E2F3) that also increase DEK transcription. Increased levels of DEK then promote proliferation. DEK expression can be targeted with the anti-estrogen tamoxifen to inhibit cell proliferation. The knockdown of DEK by RNAi can increase tamoxifen sensitivity of ER+ cell lines by synergistically inducing an apoptotic response.
Previous reports have linked the role of DEK in DNA repair to resistance to clastogenic agents such as hydroxyurea, etoposide, doxorubicin, camptothecin, and neocarzinostatin [11], [19], [28], [44]. We report here, for the first time, that DEK depletion can also act synergistically with another class of chemotherapeutic drugs, SERMs, whose activity is unrelated to the DNA damage response. DEK depletion using RNAi enhanced the cytotoxic activity of tamoxifen in ER+ cell lines. Due to its ability to modulate apoptotic responses to multiple classes of drugs, chemical- or RNAi-based therapeutic DEK targeting approaches in cancer cells could substantially enhance the efficacy of existing therapies. Importantly, DEK depletion by RNAi has been shown to induce a dramatic cytotoxic effect in cancer cells but has relatively little, and no, toxicity in normal and differentiated cells, respectively [4], [30]. Therefore, DEK targeting combined with tamoxifen may be a promising method to enhance drug efficacy or to overcome drug resistance in ER+ breast cancers with limited effects on adjacent normal epithelia.
Methods
Cell Culture
Cell lines were obtained from the American Type Culture Collection (ATCC) and cultured as directed by the ATCC. For hormone responsiveness, cells were starved in media without phenol red or insulin and with 10% charcoal-stripped FBS (CS-FBS) for seven days prior to treatment with 10 nM 17β-estradiol (Sigma-Aldrich, St. Louis, MO, USA), 10 nM progesterone (Sigma-Aldrich, St. Louis, MO), 1 µM methyltrienolone (R1881; Sigma-Aldrich, St. Louis, MO), 10 nM propylpyrazole triol (PPT; Tocris Bioscience, Bristol, United Kingdom), 10 nM diarylpropionitrile (DPN; Tocris Bioscience, Bristol, United Kingdom), 10 µM ICI-182780 (ICI; Tocris Bioscience, Bristol, United Kingdom), 10 µM methyl-piperidino-pyrazole (MPP; Tocris Bioscience, Bristol, United Kingdom), and 10 µM pyrazolo [1,5-a] pyrimidine (PHTPP; Tocris Bioscience, Bristol, United Kingdom). For treatment with 3 µg/ml tamoxifen (Sigma-Aldrich, St. Louis, MO), cells were cultured as directed by the ATCC with the exception of 5% FBS instead of 10% FBS. Images were obtained with a Leica DMIL microscope (Leica Microsystems, Bannockburn, IL) and SPOT imaging software (Diagnostic Instruments, Sterling Heights, MI).
Tissue Microarray
A breast tissue microarray (BRC961C_F, Pantomics, Inc., Richmond, CA) was stained by immunohistochemistry using the M.O.M. kit (M.O.M. Peroxidase Kit; Vector Laboratories, Burlingame, CA) and monoclonal DEK antibody (1∶60, BD Biosciences, San Jose, CA). The tissue microarray contained intact duplicate samples including normal breast (n = 2), invasive adenocarcinomas (n = 30, 28 ductal, one ductal papillary and one mucinous) and non-malignant breast disease including hyperplasia (n = 3), fibrocystic changes (n = 4) and fibroadenomas (n = 3). All patients were female with the exception of the one case of ductal papillary adenocarcinoma. Due to sample size and histological variability, we focused on the invasive adenocarcinomas for characterization. Breast cancer risk increases with age; the age of 50 years old was chosen in this study to (1) attempt to delineate between inherited breast cancers and sporadic breast cancers and (2) because overall risk and incidence increases after age 50 [45]. DEK staining was blindly scored as positive or negative based on the presence of any brown-stained nuclei. Tumors considered positive for hormone receptors showed staining for the receptor in ≥5% of cells.
Western Blotting
Western blotting was performed as previously described [30], [46]. Membranes were probed with antibodies to DEK (1∶1000, BD Biosciences, San Jose, CA), phospho-p44/42 (1∶2000, Cell Signaling, Danvers, MA), total p44/42 (1∶2000, Cell Signaling, Danvers, MA), caspase 8 (1∶1000, Cell Signaling, Danvers MA), or Actin (1∶10,000; gift of James Lessard, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH).
Quantitative RT-PCR
Total mRNA was isolated, reverse transcribed and cDNA was amplified with SYBR Green PCR master mix using an ABI-7300 quantitative PCR machine (Applied Biosystems, Carlsbad, CA). The following forward (F) and reverse (R) primer pairs were used at a concentration of 0.4 ng/µl each: GAPDH F-(5′-GGTCTCCTCTGACTTCAACA) R-(5′-ATACCAGGAAATGAGCTTGA), and DEK F-(5′-TGTTAAGAAAGCAGATAGCAGCACC-3′) R-(5′-ATTAAAGGTTCATCATCTGAACTATCCTC-3′).
Chromatin Immunoprecipitation (ChIP)
Chromatin immunoprecipitation was performed as previously described [47]. Briefly, MCF7 and T47D cells cultured in CS-FBS media were treated with 10 nM 17β-estradiol for 3 hours then crosslinked with formaldehyde for 10 minutes. Crosslinking was reversed by adding glycine to a final concentration of 0.125 M for 10 minutes. Cells were harvested, washed in PBS with protease inhibitors and resuspended in cell lysis buffer (5 mM PIPES (pH 8.0), 85 mM KCl, 0.5% Nonidet P-40, and protease inhibitors) for 30 min and centrifuged. Nuclei were isolated via centrifugation and resuspended in nuclei lysis buffer (50 mm Tris-Cl (pH 8.1), 10 mm EDTA, 1% SDS, and protease inhibitors) for 30 min. The chromatin was sonicated three times, for 10 seconds each time, to generate DNA fragments with a range of 100–1000 bp. Ten percent of the chromatin was kept as an input, and 70 µg was used for immuneprecipitation in combination with 1 µg of antibody and RIPA lysis buffer with protease inhibitors to a final volume of 500 µl. After three hours at 4°C, 20 µl of protein A slurry was added overnight. IPs were washed once in RIPA with 125 mM NaCl then three times with RIPA + protease inhibitors and two times with TE before incubating beads with extraction buffer (0.1 m NaHCO3, 1% SDS, 0.3 m NaCl, 10 mg/ml RNase A) at 65°C overnight to de-crosslink. Immunoprecipitated DNA was purified using the PCR purification kit (Qiagen, Valencia, CA) and resuspended in 50 µl of sterile water. The purified DNA was PCR-amplified and run on ethidium bromide stained agarose gels. The antibodies used were IgG1 (BD Biosciences, San Jose, CA), c-myc (9E10, Sigma-Aldrich, St. Louis, MO), E2F3 (C18, Santa Cruz Biotechnology, Santa Cruz, CA), ERα (Ab10, clone TE111.5D11; Thermo Scientific, Rockford, IL), histone H3 (ChIP grade; Abcam, Cambridge, MA), and SP1 (Millipore, Billerica, MA). The primers to amplify the DEK promoter at ChIP 2 are (F) AGG CAG CAT TGA GCA GGG AAG AT and (R) GGG TGT AAA TCT ATG TGG CT and at ChIP 4 are (F) GCG GTC CTC AGA AAT ACA GAT TTG GG and (R) GTG GAA ACA ATA AAC ACG CAG GCC. The primer sequences to amplify the pS2 gene promoter are: (F) CCG GCC ATC TCT CAC TAT GAA and (R) CCT TCC CGC CAG GGT AAA TAC.
Luciferase Assays
The first exon of the DEK gene and 1200 bp of the proximal promoter were inserted upstream of a luciferase reporter gene in the pGL3-basic plasmid. The DEK reporter construct and Renilla (pRL-TK) were transfected into MCF7 and T47D cells cultured in CS-FBS using Mirus TransIT-LT1 (Mirus Bio LLC, Madison, WI). Twenty-four hours post-transfection, cells were treated with vehicle (ethanol; EtOH), 17β-estradiol, or tamoxifen for an additional 24 hours then collected and analyzed using the Dual Luciferase Reporter assay system (Promega Corporation, Madison WI). Results represent fold-change compared to untreated (EtOH) control cells.
Lentiviral Transduction
Cells were transduced with the lentiviral pLKO.1 constructs (Sigma Aldrich Mission shRNA library, Sigma-Aldrich, St. Louis, MO) and selected in puromycin. DEKsh2 represents construct pLKO.1_DEK832 (targeting DEK mRNA at nucleotide position 832). DEKsh2 functionality was published previously [7], [11].
Flow Cytometry
Cells were labeled for BrdU incorporation and analyzed by flow cytometry according to manufacturer’s instructions (BD Biosciences, San Jose, CA). Cells were counter-stained with 7-amino-actinomycin D (7-AAD) to analyze sub-G1 content with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Cells were gated based on forward- and side-scatter to minimize cellular debris that could skew the results.
Statistics
The χ2 test was used to identify correlations between DEK expression and clinico-pathological variables on the tissue microarray; otherwise, statistical significance was assayed using Student’s t-test or ANOVA, as indicated in the figure legends. All in vitro experiments represent the average of triplicate experiments and errors bars depict standard error. In the figures, one asterisk (*) indicates p<0.05, and two asterisks (**) indicates p<0.01.
Supporting Information
17β-estradiol treatment results in increased DEK expression and phosphorylation of p44/42 (Erk1/2). MCF7 cells cultured under hormone starvation conditions were either untreated or treated for 24 hours with 10 nM 17β-estradiol. Whole cell lysates were subjected to western blotting and probed with antibodies for DEK, phospho-p44/42, total p44/42, and Actin.
(TIF)
Dose response of DEK expression to 17β-estradiol treatment. (A) Microarray studies using MCF7/BUS cells treated with varying concentrations of 17β-estradiol show that DEK expression is increased following treatment with 17β-estradiol. This data was originally published by Coser, K.R. et al [32]. (B) Western blotting of T47D cells grown in CS-FBS for seven days indicated that even 10 pM of 17β-estradiol for 48 hours is sufficient to stimulate DEK expression.
(TIF)
DEK expression is transiently upregulated with 17β-estradiol treatment. DEK expression increases rapidly in 17β-estradiol treated cells but returns to baseline levels upon prolonged exposure. Quantitative RT-PCR was performed to detect DEK expression in MCF7 cells grown in CS-FBS treated with 10 nM 17β-estradiol for 6, 24, and 48 hours. Expression was normalized to GAPDH transcript levels.
(TIF)
DEK depletion is synergistic with tamoxifen treatment to induce apoptosis in the MCF7 breast cancer cell line. (A) MCF7 cells transduced with non-targeting (NTsh) or DEK-targeting (DEKsh2) lentiviral shRNA constructs were grown in reduced serum and either untreated (EtOH) or treated with tamoxifen for 18 hours. Cells were fixed in 2% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI). Apoptotic cells are indicated with white arrows and show the condensation and fragmentation of DNA. Percentages of apoptotic cells are shown in the bottom right corner of each image. (B) Western blotting of whole cell lysates from MCF7 NTsh and DEKsh2 cells indicates increased levels of cleaved caspase 8, a marker of apoptosis, in DEKsh2 cells treated with tamoxifen. Actin was used for normalization and the numbers below indicate the fold-change in cleaved caspase 8 levels, compared to untreated NTsh cells, as determined by densitometry.
(TIF)
Acknowledgments
We thank James Lessard (Cincinnati Children’s Hospital Medical Center) for the Actin antibody and the Viral Vector Core at CCHMC for production of shRNA-containing lentivirus. We also thank Esther Peterson (Albert Einstein College of Medicine) for technical assistance. We would like to acknowledge the assistance of the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center.
Funding Statement
This work was supported by Kirschstein National Research Service Awards (NRSA), award numbers F32CA139931 and T32HL091805 from the National Cancer Institute, to LMPV; and Public Health Service grant CA116316 to SIW. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. American Cancer Society I, Breast Cancer Facts & Figures 2007–2008 (2007) [Google Scholar]
- 2. Brown SB, Mallon EA, Edwards J, Campbell FM, McGlynn LM, et al. (2009) Is the biology of breast cancer changing? A study of hormone receptor status 1984–1986 and 1996–1997. Br J Cancer 100: 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abba MC, Sun H, Hawkins KA, Drake JA, Hu Y, et al. (2007) Breast cancer molecular signatures as determined by SAGE: correlation with lymph node status. Mol Cancer Res 5: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wise-Draper TM, Mintz-Cole RA, Morris TA, Simpson DS, Wikenheiser-Brokamp KA, et al. (2009) Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo. Cancer Res 69: 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, et al. (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu S, Wang X, Sun F, Kong J, Li Z, et al. (2012) DEK overexpression is correlated with the clinical features of breast cancer. Pathol Int 62: 176–181. [DOI] [PubMed] [Google Scholar]
- 7. Privette Vinnedge LM, McClaine R, Wagh PK, Wikenheiser-Brokamp KA, Waltz SE, et al. (2011) The human DEK oncogene stimulates beta-catenin signaling, invasion and mammosphere formation in breast cancer. Oncogene 30: 2741–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Waldmann T, Scholten I, Kappes F, Hu HG, Knippers R (2004) The DEK protein-an abundant and ubiquitous constituent of mammalian chromatin. Gene 343: 1–9. [DOI] [PubMed] [Google Scholar]
- 9. Mor-Vaknin N, Kappes F, Dick AE, Legendre M, Damoc C, et al. DEK in the synovium of patients with juvenile idiopathic arthritis: characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum 63: 556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mor-Vaknin N, Punturieri A, Sitwala K, Faulkner N, Legendre M, et al. (2006) The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol 26: 9484–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kappes F, Fahrer J, Khodadoust MS, Tabbert A, Strasser C, et al. (2008) DEK is a poly(ADP-ribose) acceptor in apoptosis and mediates resistance to genotoxic stress. Mol Cell Biol 28: 3245–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kappes F, Damoc C, Knippers R, Przybylski M, Pinna LA, et al. (2004) Phosphorylation by protein kinase CK2 changes the DNA binding properties of the human chromatin protein DEK. Mol Cell Biol 24: 6011–6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soares LM, Zanier K, Mackereth C, Sattler M, Valcarcel J (2006) Intron removal requires proofreading of U2AF/3′ splice site recognition by DEK. Science 312: 1961–1965. [DOI] [PubMed] [Google Scholar]
- 14. McGarvey T, Rosonina E, McCracken S, Li Q, Arnaout R, et al. (2000) The acute myeloid leukemia-associated protein, DEK, forms a splicing-dependent interaction with exon-product complexes. J Cell Biol 150: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Hir H, Gatfield D, Izaurralde E, Moore MJ (2001) The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. Embo J 20: 4987–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexiadis V, Waldmann T, Andersen J, Mann M, Knippers R, et al. (2000) The protein encoded by the proto-oncogene DEK changes the topology of chromatin and reduces the efficiency of DNA replication in a chromatin-specific manner. Genes Dev 14: 1308–1312. [PMC free article] [PubMed] [Google Scholar]
- 17. Campillos M, Garcia MA, Valdivieso F, Vazquez J (2003) Transcriptional activation by AP-2alpha is modulated by the oncogene DEK. Nucleic Acids Res 31: 1571–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sammons M, Wan SS, Vogel NL, Mientjes EJ, Grosveld G, et al. (2006) Negative regulation of the RelA/p65 transactivation function by the product of the DEK proto-oncogene. J Biol Chem 281: 26802–26812. [DOI] [PubMed] [Google Scholar]
- 19. Kavanaugh GM, Wise-Draper TM, Morreale RJ, Morrison MA, Gole B, et al. The human DEK oncogene regulates DNA damage response signaling and repair. Nucleic Acids Res 39: 7465–7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waldmann T, Eckerich C, Baack M, Gruss C (2002) The ubiquitous chromatin protein DEK alters the structure of DNA by introducing positive supercoils. J Biol Chem 277: 24988–24994. [DOI] [PubMed] [Google Scholar]
- 21. Waldmann T, Baack M, Richter N, Gruss C (2003) Structure-specific binding of the proto-oncogene protein DEK to DNA. Nucleic Acids Res 31: 7003–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, et al. A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev 24: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kappes F, Waldmann T, Mathew V, Yu J, Zhang L, et al. (2011) The DEK oncoprotein is a Su(var) that is essential to heterochromatin integrity. Genes Dev 25: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondoh N, Wakatsuki T, Ryo A, Hada A, Aihara T, et al. (1999) Identification and characterization of genes associated with human hepatocellular carcinogenesis. Cancer Res 59: 4990–4996. [PubMed] [Google Scholar]
- 25. Lu ZL, Luo DZ, Wen JM (2005) Expression and significance of tumor-related genes in HCC. World J Gastroenterol 11: 3850–3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Evans AJ, Gallie BL, Jewett MA, Pond GR, Vandezande K, et al. (2004) Defining a 0.5-mb region of genomic gain on chromosome 6p22 in bladder cancer by quantitative-multiplex polymerase chain reaction. Am J Pathol 164: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carro MS, Spiga FM, Quarto M, Di Ninni V, Volorio S, et al. (2006) DEK Expression is controlled by E2F and deregulated in diverse tumor types. Cell Cycle 5: 1202–1207. [DOI] [PubMed] [Google Scholar]
- 28. Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, et al. (2009) Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res 69: 6405–6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibata T, Kokubu A, Miyamoto M, Hosoda F, Gotoh M, et al.. (2010) DEK oncoprotein regulates transcriptional modifiers and sustains tumor initiation activity in high-grade neuroendocrine carcinoma of the lung. Oncogene. [DOI] [PubMed]
- 30. Wise-Draper TM, Allen HV, Jones EE, Habash KB, Matsuo H, et al. (2006) Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions. Mol Cell Biol 26: 7506–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sitwala KV, Adams K, Markovitz DM (2002) YY1 and NF-Y binding sites regulate the transcriptional activity of the dek and dek-can promoter. Oncogene 21: 8862–8870. [DOI] [PubMed] [Google Scholar]
- 32. Coser KR, Chesnes J, Hur J, Ray S, Isselbacher KJ, et al. (2003) Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray. Proc Natl Acad Sci U S A 100: 13994–13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krishnan V, Wang X, Safe S (1994) Estrogen receptor-Sp1 complexes mediate estrogen-induced cathepsin D gene expression in MCF-7 human breast cancer cells. J Biol Chem 269: 15912–15917. [PubMed] [Google Scholar]
- 34.PATCH 1.0; Pattern Search for Transcription Factor Binding Sites website. Available: http://www.gene-regulation.com/cgi-bin/pub/programs/patch/bin/patch.cgi. Accessed 2008 May 12.
- 35.Schug J (2003) Using TESS to Predict Transcription Factor Binding Sites in DNA Sequence, in Current Protocols in Bioinformatics, A.D. Baxevanis, Editor, J. Wiley and Sons. [DOI] [PubMed]
- 36.TESS - Transcription Element Search System website. Available: http://www.cbil.upenn.edu/tess/. Accessed 2008 Oct 23.
- 37. Papendorp JT, Schatz RW, Soto AM, Sonnenschein C (1985) On the role of 17 alpha-estradiol and 17 beta-estradiol in the proliferation of MCF7 and T47D-A11 human breast tumor cells. J Cell Physiol 125: 591–595. [DOI] [PubMed] [Google Scholar]
- 38. Podhajcer OL, Bravo AI, Dain L, Guman N, Bover L, et al. (1988) In vitro analysis of the cellular proliferative response to 17-beta-estradiol of human breast cancer. Cancer 61: 1807–1812. [DOI] [PubMed] [Google Scholar]
- 39. van der Burg B, Rutteman GR, Blankenstein MA, de Laat SW, van Zoelen EJ (1988) Mitogenic stimulation of human breast cancer cells in a growth factor-defined medium: synergistic action of insulin and estrogen. J Cell Physiol 134: 101–108. [DOI] [PubMed] [Google Scholar]
- 40. Ageberg M, Gullberg U, Lindmark A (2006) The involvement of cellular proliferation status in the expression of the human proto-oncogene DEK. Haematologica 91: 268–269. [PubMed] [Google Scholar]
- 41. Wise-Draper TM, Allen HV, Thobe MN, Jones EE, Habash KB, et al. (2005) The human DEK proto-oncogene is a senescence inhibitor and an upregulated target of high-risk human papillomavirus E7. J Virol 79: 14309–14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bosco EE, Knudsen ES (2007) RB in breast cancer: at the crossroads of tumorigenesis and treatment. Cell Cycle 6: 667–671. [DOI] [PubMed] [Google Scholar]
- 43. Wang W, Dong L, Saville B, Safe S (1999) Transcriptional activation of E2F1 gene expression by 17beta-estradiol in MCF-7 cells is regulated by NF-Y-Sp1/estrogen receptor interactions. Mol Endocrinol 13: 1373–1387. [DOI] [PubMed] [Google Scholar]
- 44. Riveiro-Falkenbach E, Soengas MS (2010) Control of tumorigenesis and chemoresistance by the DEK oncogene. Clin Cancer Res 16: 2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ACS, Breast Cancer Facts & Figures 2011–2012 (2011) Atlanta, American Cancer Society, Inc.
- 46. Andreassen PR, Margolis RL (1994) Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J Cell Biol 127: 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stengel KR, Thangavel C, Solomon DA, Angus SP, Zheng Y, et al. (2009) Retinoblastoma/p107/p130 pocket proteins: protein dynamics and interactions with target gene promoters. J Biol Chem 284: 19265–19271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
17β-estradiol treatment results in increased DEK expression and phosphorylation of p44/42 (Erk1/2). MCF7 cells cultured under hormone starvation conditions were either untreated or treated for 24 hours with 10 nM 17β-estradiol. Whole cell lysates were subjected to western blotting and probed with antibodies for DEK, phospho-p44/42, total p44/42, and Actin.
(TIF)
Dose response of DEK expression to 17β-estradiol treatment. (A) Microarray studies using MCF7/BUS cells treated with varying concentrations of 17β-estradiol show that DEK expression is increased following treatment with 17β-estradiol. This data was originally published by Coser, K.R. et al [32]. (B) Western blotting of T47D cells grown in CS-FBS for seven days indicated that even 10 pM of 17β-estradiol for 48 hours is sufficient to stimulate DEK expression.
(TIF)
DEK expression is transiently upregulated with 17β-estradiol treatment. DEK expression increases rapidly in 17β-estradiol treated cells but returns to baseline levels upon prolonged exposure. Quantitative RT-PCR was performed to detect DEK expression in MCF7 cells grown in CS-FBS treated with 10 nM 17β-estradiol for 6, 24, and 48 hours. Expression was normalized to GAPDH transcript levels.
(TIF)
DEK depletion is synergistic with tamoxifen treatment to induce apoptosis in the MCF7 breast cancer cell line. (A) MCF7 cells transduced with non-targeting (NTsh) or DEK-targeting (DEKsh2) lentiviral shRNA constructs were grown in reduced serum and either untreated (EtOH) or treated with tamoxifen for 18 hours. Cells were fixed in 2% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI). Apoptotic cells are indicated with white arrows and show the condensation and fragmentation of DNA. Percentages of apoptotic cells are shown in the bottom right corner of each image. (B) Western blotting of whole cell lysates from MCF7 NTsh and DEKsh2 cells indicates increased levels of cleaved caspase 8, a marker of apoptosis, in DEKsh2 cells treated with tamoxifen. Actin was used for normalization and the numbers below indicate the fold-change in cleaved caspase 8 levels, compared to untreated NTsh cells, as determined by densitometry.
(TIF)