Abstract
Nesfatin-1, a novel hypothalamic peptide, inhibits nocturnal feeding behavior and gastrointestinal motility in rodents. The effects of nesfatin-1 on gastrointestinal secretory function, including gastric acid production, have not been evaluated. Nesfatin-1 was injected into the fourth intracerebral ventricle (4V) of chronically cannulated rats to identify a nesfatin dose sufficient to inhibit food intake. Nesfatin-1 (2 μg) inhibited dark-phase food intake, in a dose-dependent fashion, for >3 h. Gastric acid production was evaluated in urethane-anesthetized rats. Nesfatin-1 (2 μg) was introduced via the 4V following endocrine stimulation of gastric acid secretion by pentagastrin (2 μg·kg−1·h−1 iv), vagal stimulation with 2-deoxy-d-glucose (200 mg/kg sc), or no stimulus. Gastric secretions were collected via gastric cannula and neutralized by titration to determine acid content. Nesfatin-1 did not affect basal and pentagastrin-stimulated gastric acid secretion, whereas 2-deoxy-d-glucose-stimulated gastric acid production was inhibited by nesfatin-1 in a dose-dependent manner. c-Fos immunofluorescence in brain sections was used to evaluate in vivo neuronal activation by nesfatin-1 administered via the 4V. Nesfatin-1 caused activation of efferent vagal neurons, as evidenced by a 16-fold increase in the mean number of c-Fos-positive neurons in the dorsal motor nucleus of the vagus (DMNV) in nesfatin-1-treated animals vs. controls (P < 0.01). Finally, nesfatin-induced Ca2+ signaling was evaluated in primary cultured DMNV neurons from neonatal rats. Nesfatin-1 caused dose-dependent Ca2+ increments in 95% of cultured DMNV neurons. These studies demonstrate that central administration of nesfatin-1, at doses sufficient to inhibit food intake, results in inhibition of vagally stimulated secretion of gastric acid. Nesfatin-1 activates DMNV efferent vagal neurons in vivo and triggers Ca2+ signaling in cultured DMNV neurons.
Keywords: gastric hormones, intracellular calcium, dorsal motor nucleus of the vagus
nesfatin-1, an 82-amino acid peptide derived from the parent peptide nucleobindin-2, was first identified as a satiety molecule in the hypothalamus by Oh et al. (33). Inhibition of rodent nocturnal feeding behavior by nesfatin-1 administered into the intracranial ventricles has been consistently observed (18, 25, 34, 40, 42). Most centrally acting peptides that influence food intake also regulate the processes of digestion, including gastrointestinal motility and secretory function (45). In rodents, nesfatin-1 inhibits gastroduodenal motility and gastric emptying (3, 41). We postulate that nesfatin-1 may also affect secretion of gastric acid.
Gastric acid secretion is regulated by neural, hormonal, and paracrine pathways (38, 39). Gastrin, a hormone released from antral G cells, binds to gastrin receptors on parietal cells and also stimulates cholecystokinin-2 receptors on gastric enterochromaffin cells, resulting in histamine release. In paracrine fashion, histamine binds its receptor on the gastric parietal cell, stimulating the production of hydrochloric acid (23). The actions of gastrin constitute a major peripheral mechanism for gastric acid production. Central stimulation of gastric acid secretion is mediated by the vagus nerve. Gastric acid secretion can be stimulated experimentally by 2-deoxy-d-glucose (2-DG), a stable glucose analog that cannot undergo further glycolysis. Systemic administration of 2-DG initiates a state of simulated cytoglycopenia, leading to altered hypothalamic input to the dorsal-vagal complex (DVC) in the brain stem and resultant activation of bilateral vagus nerves (10, 19, 30). 2-DG has been widely used to study gastric acid secretion mediated by the vagus nerve (19, 32, 36). Using gastrin and 2-DG to evaluate peripheral and central mechanisms of stimulated acid production, we have explored the effects of centrally administered nesfatin-1 on gastric acid secretion.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats (240–280 g body wt; Charles River Laboratories, Wilmington, MA) were used for all in vivo experiments. Vagal neurons for in vitro studies were harvested from neonatal Sprague-Dawley rats. All animal studies were approved by the University of Michigan Committee on the Use and Care of Animals. Animals were housed in a temperature-controlled environment with a 12:12-h light-dark cycle and access to food and water ad libitum.
Chemicals and solutions.
Nesfatin-1 was purchased from Phoenix Pharmaceuticals (Burlingame, CA); Neurobasal A medium, B27 supplement, penicillin-streptomycin, l-glutamine, trypsin type I, β-FGF, fura 2-AM, goat serum, and ProLong antifade with 4,6-diamidino-2-phenylindole from Life Technologies, formerly Invitrogen (Carlsbad, CA); Triton X-100 from Thermo Fisher (Waltham, MA); 5-thio-d-glucose from MP Biomedicals (Solon, OH); ketamine, poly-l-lysine, pentagastrin, 2-DG, NiCl2, verapamil, ω-conotoxin GVIA, and mibefradil dihydrochloride from Sigma-Aldrich (St. Louis, MO); xylazine from Lloyd (Shenandoah, IA); optimal cutting temperature (OCT) compound from Sakura Finetek USA (Torrance, CA); and rabbit anti-c-Fos antibody from Calbiochem (San Diego, CA), formerly Oncogene (Cambridge, MA). FITC-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA) was used as secondary antibody. Intracerebroventricular cannulas were purchased from and cut to 7.5 mm by Plastics One (Roanoke, VA).
Fourth intracerebral ventricle cannula placement.
Male Sprague-Dawley rats (240–280 g body wt) were anesthetized with ketamine (90 mg/kg ip) and xylazine (10 mg/kg ip) and placed on a stereotaxic instrument (Stoelting, Wood Dale, IL). A 22-gauge single-lumen cannula was implanted into the fourth intracerebral ventricle (4V) according to coordinates published by Paxinos and Watson (35): midline, 12.8 mm caudal to bregma, 7.5 mm deep, with the incisor bar set 3.3 mm below the interaural line. The cannula was secured with craniofacial cement. A sterile internal cannula was placed to maintain patency and prevent infection. On postprocedure day 3, cannula position was tested by 4V injection of 210 μg of 5-thio-d-glucose in 3 μl of PBS. A >100% rise in blood glucose in response to 5-thio-d-glucose was considered confirmation of correct cannula position. Experiments were conducted beginning on postprocedure day 7, allowing adequate recovery from the procedure and confirmation of cannula position.
Dark-phase food intake.
To establish a nesfatin-1 dose sufficient to inhibit food intake for use in the remainder of the studies, chronically cannulated rats were injected via the 4V with nesfatin-1 in 2 μl of PBS at 6 PM, the onset of the dark phase. The nesfatin-1 dose was varied from 0 to 2 μg on the basis of previous dose-response studies of third ventricular nesfatin injection (33). Rats were singly caged in familiar conditions with free access to food and water. Food intake was monitored for up to 24 h and calculated as grams of food consumed per 300 g body wt.
Gastric acid output.
The effects of nesfatin-1 on in vivo gastric acid secretion were studied using an acute gastric fistula method previously described (29). Rats with chronically implanted 4V cannulas were fasted for 16 h and then anesthetized with urethane (1.5 g/kg ip). Venous access was achieved via the jugular vein. Through a small midline abdominal incision, the stomach and duodenum were exposed. The esophagus was ligated at the gastroesophageal junction, with care taken to preserve the vagal trunks. A double-lumen cannula was inserted into the stomach via the duodenum before ligation of the pylorus. The stomach was flushed with 50–100 ml of saline to remove any solid content. After collection of gastric outputs in 5 ml of perfusate every 15 min for 30 min to establish baseline acid secretion, test substances were administered via the 4V, intravenously, or subcutaneously. The gastric lumen was subsequently rinsed with 5 ml of saline every 15 min for 120 min. Acid production was measured by titration of gastric rinse solution with 0.1 N NaOH to neutrality and expressed as microequivalents of HCl per 15 min.
Brain sections and c-Fos immunofluorescence.
To investigate the activation of brain stem vagal neurons by nesfatin-1, chronically cannulated and freely fed rats were treated with 4V nesfatin-1 (0.5 μg in 2 μl of PBS, n = 3) or vehicle (2 μl of PBS, n = 3) during the dark phase. After 90 min, the rats were deeply anesthetized and euthanized prior to transcardial perfusion with PBS and 4% paraformaldehyde. Brains were removed, postfixed in 4% paraformaldehyde, transferred to a solution of 20% sucrose in PBS for 5 days, and finally embedded with 20% sucrose-OCT compound (2:1) on dry ice. A Leica cryostat was used to section tissue blocks in 40-μm slices through the dorsal motor nucleus of the vagus (DMNV). Each section was stored in 300 μl of PBS with 0.02% sodium azide in each well of the 24-well plate.
Free-floating slices were first rinsed with PBS with 0.5% Triton for 15 min and incubated with a blocking buffer (10% normal goat serum, 3% BSA, 0.4% Triton X-100, and 1% glycine, pH 7.4) for 1 h at room temperature. Sections were then treated with rabbit anti-c-Fos antibody (1:10,000 dilution) at 4°C for 40 h, washed with PBS, and treated with FITC-conjugated goat anti-rabbit IgG (1:400 dilution) for 1 h at room temperature in the dark. After incubation with the secondary antibody, sections were washed in PBS for 15 min and incubated with 4,6-diamidino-2-phenylindole (1:6,000 dilution) for 3 min. Finally, sections were diverted to slides, dried for 1 h, mounted with ProLong Gold antifade reagent, and covered with coverslips. All antibodies were diluted in 5% goat serum blocking buffer. Slides were viewed using a fluorescence microscope (Nikon Eclipse Ti-U). The average numbers of c-Fos-positive (green) neurons per section per brain nucleus were assessed.
In vitro culture of DMNV neurons.
Neurons of the DMNV were harvested from neonatal Sprague-Dawley rats, as previously described (49). After the animals were euthanized, the brain was rapidly removed and placed into a chilled electrolyte solution. The brain stem medulla was then sectioned transversely into a 1-mm slice. The DMNV was identified under a dissecting microscope as the area immediately ventral to the nucleus of the solitary tract (NTS) and dorsal to the XII nucleus. DMNV tissue was excised and digested with trypsin type I (0.4 mg/ml) at 30°C for 20 min. Tissue was then gently dissociated by pipette trituration and plated onto poly-l-lysine-coated 25-mm chamber slides in 35-mm culture dishes. Neurons were maintained in serum-free culture medium containing Neurobasal A medium, 2% B27 supplement, 2 mM glutamine, 1% penicillin, 1% streptomycin, and 5 ng/ml β-FGF. One-half of the medium was replaced on day 1 and every 3rd day thereafter. Cells were utilized between day 5 and day 10.
Intracellular Ca2+ concentration measurements.
Intracellular Ca2+ concentration ([Ca2+]i) was measured by dual-wavelength fura 2-AM microfluorometry and imaging, as previously reported (2, 49). DMNV neurons plated on coverslips were loaded with 1 μM fura 2-AM and incubated for 60 min at 37°C. Coverslips were placed into a perfusion chamber mounted on the stage of an inverted fluorescence microscope (Nikon) and perfused with HBSS containing CaCl2. Superfusion of buffer and reagents was kept constant at 1 ml/min at 30°C. For Ca2+-free conditions, HBSS without CaCl2 was used. After 60 s of exposure to buffer, cells were perfused with one of the following reagents: nesfatin-1, NiCl2, verapamil, ω-conotoxin GVIA, and mibefradil dihydrochloride. At the conclusion of each experiment, cells were exposed to 55 mM KCl; cells that were unresponsive to KCl were excluded. [Ca2+]i was measured using a Nikon inverted fluorescence microscope (Mager Scientific, Dexter, MI) with ×40 oil-immersion objective and TILLvision digital imaging system (TILL Photonics, Pleasanton, CA). [Ca2+]i was determined for each individual cell from the ratio of fluorescence intensity at 550 nm following excitation at 340 nm to that following excitation at 380 nm. Measurements were corrected for background fluorescence intensity. The change in fluorescence ratio (ΔF) in response to a stimulus was calculated for each cell and divided by that cell's basal fluorescence ratio (F0) to yield percent change in cytoplasmic Ca2+ compared with baseline (ΔF/F0). Cells with ΔF/F0 >10% in response to a given stimulus were considered responsive.
Data analysis.
Data were analyzed with GraphPad Prism software (GraphPad Software, San Diego, CA) using ANOVA or Student's t-test as appropriate. Values are means ± SE. Significance was accepted at P < 0.05.
RESULTS
Effect of nesfatin-1 on food intake.
Intracerebroventricular injection of nesfatin-1 was performed to confirm suppression of dark-phase food intake and to establish dosing relevant to studies of gastric acid secretion. In freely fed, chronically cannulated rats, 4V administration of 2 and 0.2 μg of nesfatin-1 reduced cumulative food intake by 31% and 34%, respectively, for the first 3 h (P < 0.05). At 6 h, cumulative food intake was decreased by 27% in the 2-μg, but not the 0.2-μg, nesfatin-1 group compared with PBS-treated controls (P < 0.01). Sixteen- and 24-h cumulative food intake did not differ significantly between groups.
Effect of nesfatin-1 on in vivo gastric acid secretion.
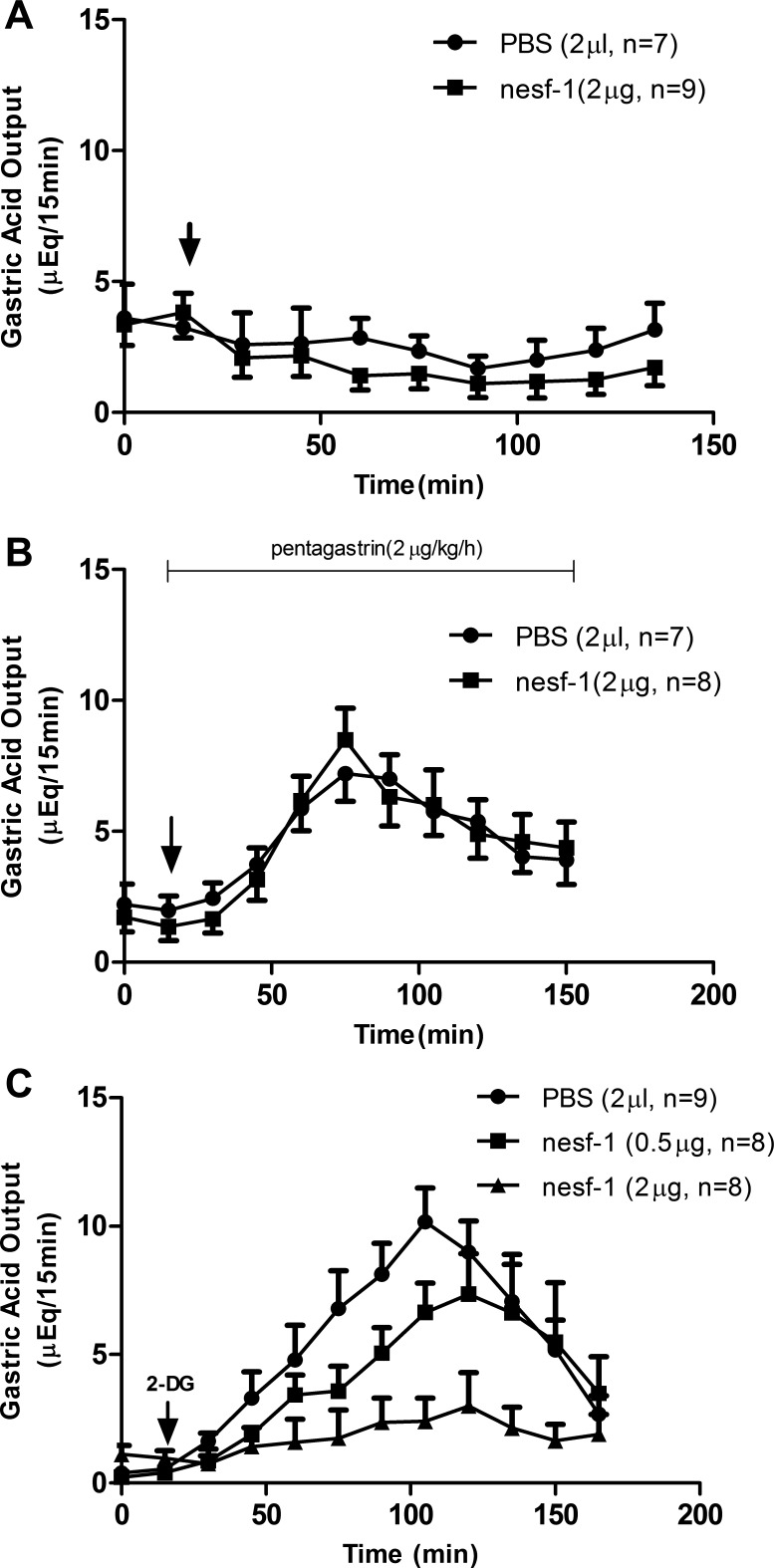
4V administration of nesfatin-1 at a dose sufficient to inhibit food intake (2 μg) had no effect on basal gastric acid secretion (Fig. 1A). Similarly, pentagastrin (2 μg·kg−1·h−1 iv)-stimulated gastric acid secretion was unaffected by 4V nesfatin-1 administration (Fig. 1B). Vagal stimulation of gastric acid secretion was induced by 2-DG (200 mg/kg sc). Administration of 4V nesfatin-1 caused a dose-dependent inhibition of 2-DG-stimulated gastric acid output. Peak stimulated acid production was reduced by 61% and 26% in response to 2 and 0.5 μg of nesfatin-1, respectively (Fig. 1C).
Fig. 1.
Effects of nesfatin-1 (nesf-1) on gastric acid secretion. A and B: effect of nesfatin-1 or PBS on basal and pentagastrin-stimulated acid secretion. Arrow indicates injection of nesfatin-1 into the 4th intracerebral ventricle (4V). C: 2-deoxy-d-glucose (2-DG, 200 mg/kg sc)-stimulated acid secretion decreased with 4V nesfatin-1 injection in a dose-dependent manner [P < 0.05, 0.5 and 2 μg nesfatin-1 vs. PBS (by ANOVA)].
Effect of nesfatin-1 on c-Fos expression in DMNV neurons.
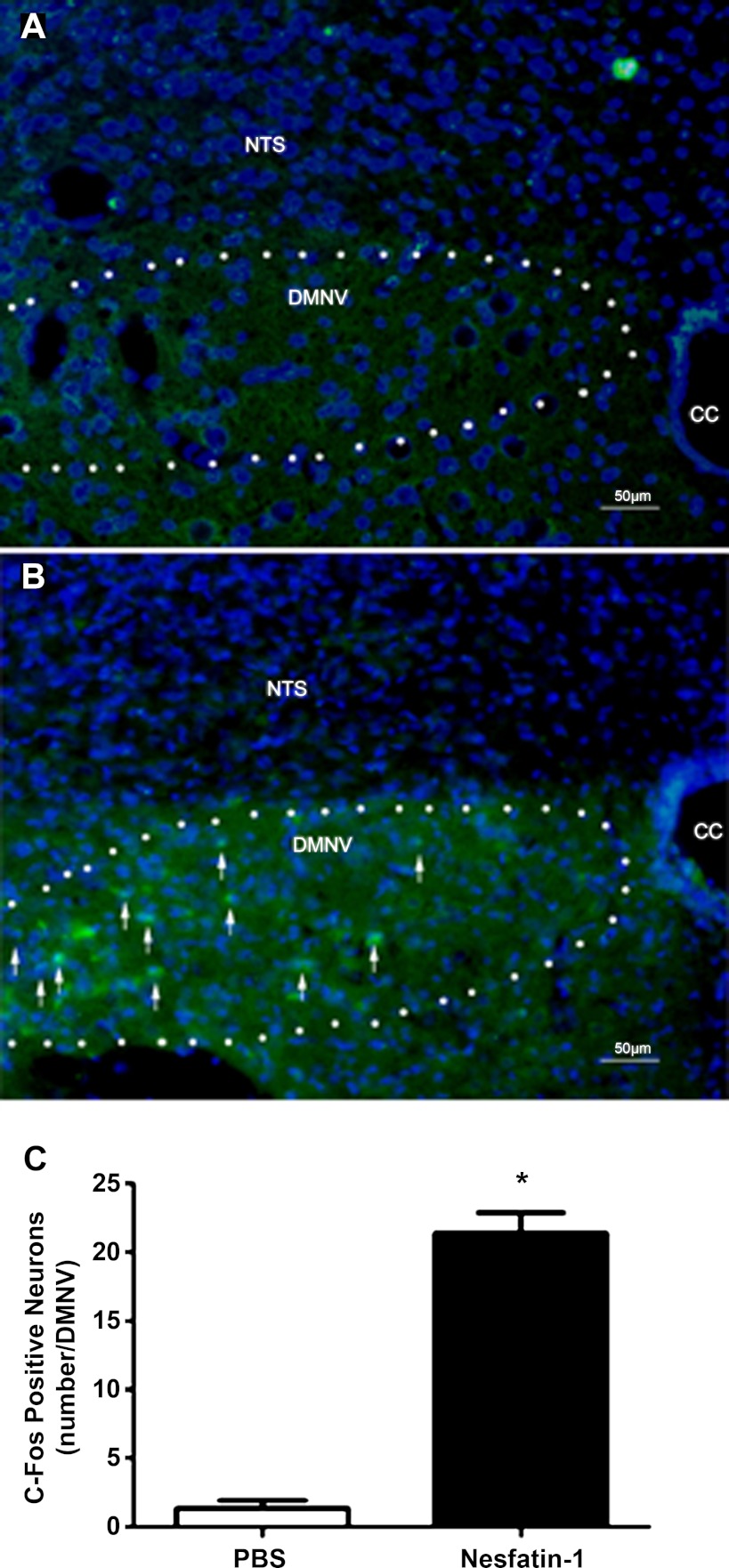
Given the ability of nesfatin-1 to inhibit vagally mediated gastric acid production, c-Fos immunofluorescence was used to determine whether 4V nesfatin-1 injection caused activation of vagal neurons. In controls treated with PBS (2 μl) via the 4V (n = 3), levels of c-Fos positivity in DMNV neurons were low (Fig. 2A). In animals treated with 0.5 μg of nesfatin-1 via the 4V (n = 3), we observed a 16-fold increase in the mean number of c-Fos-positive neurons per DMNV compared with controls (P < 0.01; Fig. 2, B and C). Neurons in the adjacent NTS did not exhibit changes in c-Fos positivity associated with nesfatin-1 injection. These observations suggest that 4V administration of nesfatin-1 results in stimulation of a population of DMNV neurons.
Fig. 2.
c-Fos immunofluorescence in dorsal-vagal complex (DVC). A and B: merged c-Fos (green) and 4,6-diamidino-2-phenylindole (blue) immunofluorescence in rat DVC sections treated with PBS (A) and nesfatin-1 (B). Few neurons of the dorsal motor nucleus of the vagus nerve (DMNV) were c-Fos-positive in controls, whereas a significantly greater proportion were c-Fos-positive in the nesfatin-1-treated animals (arrows). Neurons of the nucleus of the solitary tract (NTS) exhibit low c-Fos positivity in both groups. CC, central canal. C: quantification of c-Fos immunoreactivity in DMNV neurons. Average numbers of c-Fos-positive neurons per section per brain nucleus were significantly increased by nesfatin-1 exposure: *P < 0.01.
Nesfatin-1 induces [Ca2+]i increments in DMNV neurons.
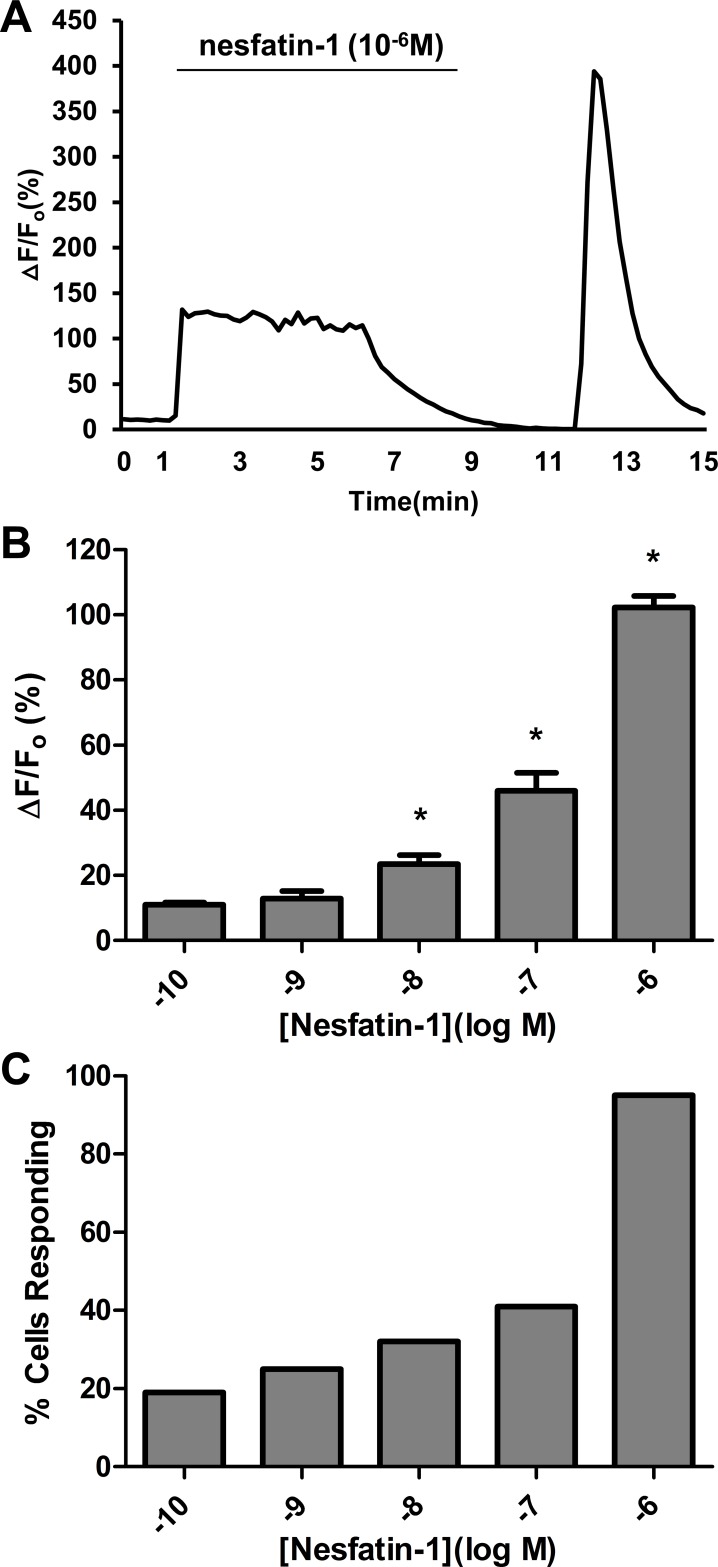
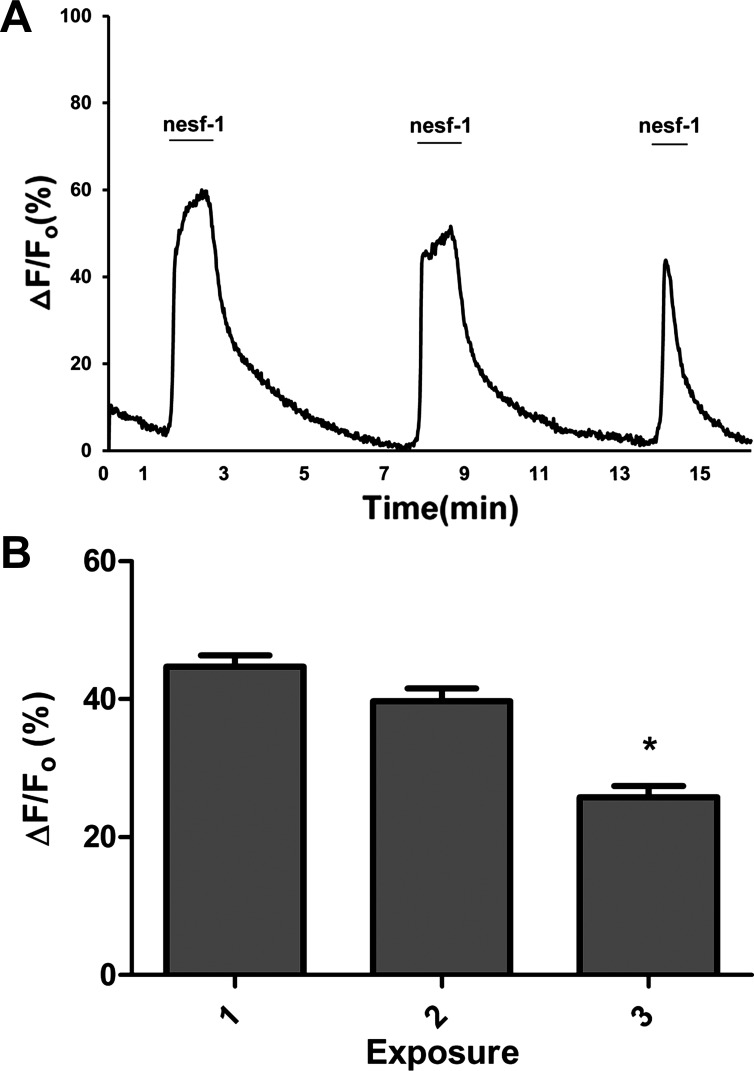
Nesfatin-1-induced Ca2+ transients were evaluated in primary cultured DMNV neurons to confirm DMNV neuron response to nesfatin-1. Superfusion of fura 2-loaded DMNV neurons with 10−6 M nesfatin-1 for 300 s induced [Ca2+]i increments in 95% of cells (n = 102). The increment in [Ca2+]i reached a peak ΔF/F0 (102 ± 6%) within 30 s, maintained a plateau for the duration of nesfatin-1 administration, and subsequently declined to baseline (Fig. 3A). All neurons responded simultaneously, indicating that the effects of nesfatin-1 were direct, rather than the result of propagation through gap junctions or synapses. DMNV neurons responded to nesfatin-1 in a dose-dependent fashion over a range of concentrations from 10−10 to 10−6 M (n = 443, P < 0.05). Increasing doses of nesfatin-1 resulted in Ca2+ increments of greater magnitude (Fig. 3B), as well as a greater proportion of neurons reaching the response threshold (Fig. 3C). For the remaining experiments, 10−7 M was chosen as a working concentration for nesfatin-1. Repetitive exposure to nesfatin-1 produced progressive decrements in peak ΔF/F0 responses (Fig. 4A). The average ΔF/F0 values for the three exposures were 45 ± 3%, 40 ± 2%, and 26 ± 2%, respectively (n = 76, P < 0.05 for peak 3 compared with peak 1; Fig. 4B).
Fig. 3.
DMNV neuron response to nesfatin-1. A: representative trace of a single DMNV neuron response to 10−6 M nesfatin-1. Exposure to nesfatin-1 for 300 s caused an increment in intracellular Ca2+ concentration ([Ca2+]i) that peaked within 30 s at >100% over baseline, maintained a plateau for the duration of nesfatin-1 exposure, and subsequently returned to baseline. B and C: dose-dependent relationship between nesfatin-1 and Ca2+ response, expressed as maximal average change in fluorescence ratio (ΔF/F0, where ΔF is change in fluorescence ratio and F0 is basal fluorescence ratio) and percentage of cells responding (n = 120, 71, 71, 79, and 102, respectively). *P < 0.05 vs. the next-lower concentration.
Fig. 4.
DMNV neuron response to repetitive nesfatin-1 exposure. A: representative trace of a single DMNV neuron response to 3 sequential 60-s exposures to 10−7 M nesfatin-1. B: no significant difference in ΔF/F0 for exposures 1 and 2 and significantly lower magnitude of response to exposure 3 than exposure 1 (n = 76). *P < 0.05.
Mechanism of nesfatin-1-induced [Ca2+]i increments in DMNV neurons.
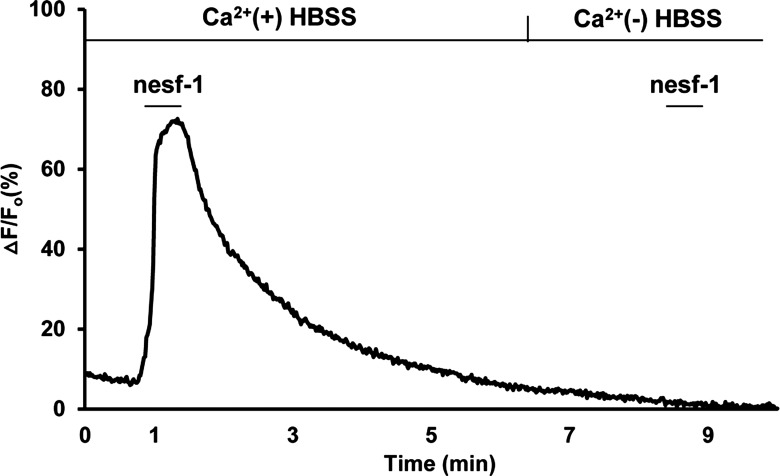
The mechanism by which nesfatin-1 causes increased cytoplasmic Ca2+ concentrations in DMNV neurons was evaluated by nesfatin-1 exposure under a variety of conditions. First, the involvement of extracellular Ca2+ in nesfatin-induced Ca2+ transients was examined. In the absence of extracellular Ca2+, nesfatin-1 failed to increase [Ca2+]i in any of the cells (Fig. 5). This observation suggests that nesfatin-1-induced Ca2+ transients are the result of Ca2+ influx across the plasma membrane.
Fig. 5.
Representative trace of a single neuron showing DMNV neuron response to nesfatin-1 in the presence and absence of extracellular Ca2+. DMNV neurons were exposed to nesfatin-1 for 30 s in the presence of extracellular Ca2+ and then allowed to return to baseline. Cells were subsequently superfused with Ca2+-free HBSS for 180 s and then with nesfatin-1 in HBSS without Ca2+ for 30 s. In the absence of extracellular Ca2+, nesfatin-1 had no effect on DMNV neuronal [Ca2+]i.
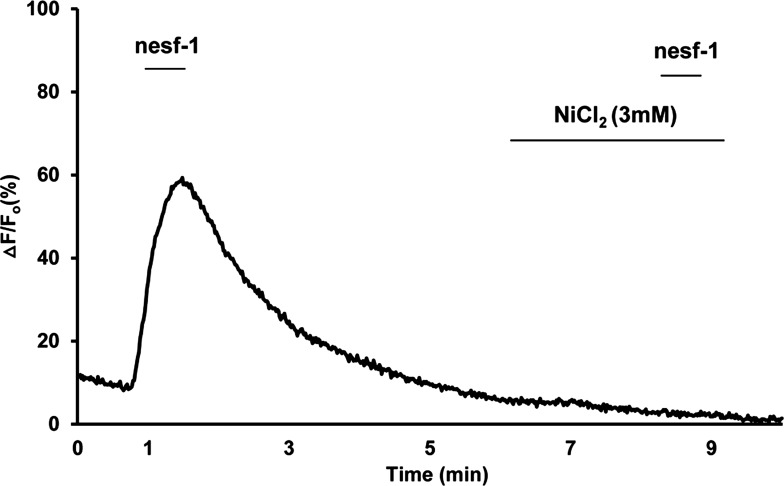
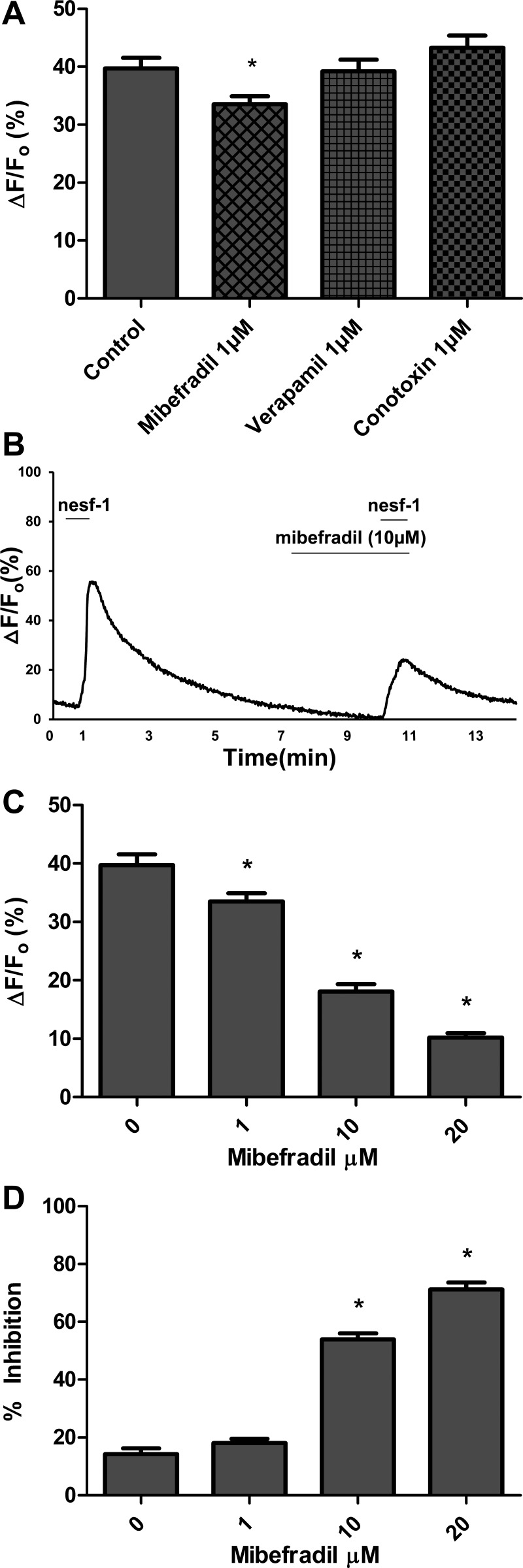
To determine which subtype of Ca2+ channel is involved in influx of Ca2+, DMNV neurons were exposed to nesfatin-1 in the presence of various Ca2+ channel blockers. NiCl2 (3 mM), a nonspecific Ca2+ channel blocker, abolished nesfatin-1-induced intracellular Ca2+ transients in all neurons (n = 93; Fig. 6). Antagonists specific to the three major Ca2+ channel subtypes were then utilized. Superfusion with the L-type Ca2+ channel blocker verapamil (1 μM) or the N-type Ca2+ channel blocker ω-conotoxin GVIA (1 μM) did not significantly alter nesfatin-1-induced peak ΔF/F0 (n = 73 and 86, respectively; Fig. 7A). Selective blockade of T-type Ca2+ channels with 1, 10, and 20 μM mibefradil caused dose-dependent decreases in average peak ΔF/F0 (33.5%, 18.0%, and 10.2%, respectively; Fig. 7, B–D). These observations suggest that nesfatin-1 increases cytoplasmic Ca2+ concentrations in DMNV neurons by influx of extracellular Ca2+, at least in part via a T-type Ca2+ channel.
Fig. 6.
Representative trace from a single cell showing that inhibition of nesfatin-1 induced Ca2+ transient by NiCl2. DMNV neurons were superfused with nesfatin-1 for 30 s and allowed to return to baseline fluorescence ratio. Cells were pretreated with 3 mM NiCl2 for 180 s and then with nesfatin-1 in the presence of NiCl2 for 30 s. NiCl2, a nonspecific Ca2+ channel blocker, abolished nesfatin-1-induced intracellular Ca2+ transients.
Fig. 7.
Dose-dependent inhibition of nesfatin-1 induced Ca2+ transients by mibefradil, but not by verapamil or ω-conotoxin GVIA. DMNV cells were superfused with 10−7 M nesfatin-1 for 30 s and allowed to return to baseline. Cells were pretreated with a Ca2+ channel blocker for 180 s and then superfused for 30 s with nesfatin-1 in the presence of that Ca2+ channel blocker. A: blockade of T-type Ca2+ channels with 1 μM mibefradil diminishes cytoplasmic Ca2+ response to nesfatin-1 (n = 58). *P < 0.05. Blockade of L- and N-type Ca2+ channels with 1 μM verapamil and 1 μM ω-conotoxin GVIA, respectively, did not significantly inhibit DMV neuron response to nesfatin-1 (n = 73, P = 0.85 for verapamil; n = 86, P = 0.22 for ω-conotoxin GVIA). B: representative trace of a single neuron demonstrating inhibition of nesfatin-induced Ca2+ transients in the presence of 10 μM mibefradil. C and D: dose-inhibition relationship. As concentration of mibefradil was increased, average ΔF/F0 decreased and inhibition of nesfatin-1-induced [Ca2+]i increased. *P < 0.05 vs. lower dose.
DISCUSSION
The present study represents the first report that nesfatin-1 inhibits gastric acid secretion stimulated by a central vagal mechanism. This conclusion is supported by the following observations: 1) intracerebroventricular nesfatin-1 decreased 2-DG-stimulated gastric acid output in a dose-dependent manner in urethane-anesthetized rats, with no effect on basal or pentagastrin-stimulated gastric acid production; 2) in conscious rats, 4V nesfatin-1 activates DMNV neurons; and 3) in primary cultured DMNV neurons, nesfatin-1 causes dose-dependent increments in cytosolic Ca2+ that can be abolished by the absence of extracellular Ca2+ or blockade of T-type Ca2+ channels. Taken together, these observations indicate that nesfatin-1 is able to activate vagal neurons in vitro and in vivo and that central exposure to nesfatin-1 inhibits vagally stimulated gastric acid secretion.
Nesfatin-1 was first described by Oh et al. (33) as a hypothalamic neuropeptide able to reduce dark-phase food intake. Subsequent studies, including this one, provide concordant evidence that nesfatin-1 injected into the brain elicits an anorexigenic response in rodents (3, 18, 26, 40, 41, 48). Nesfatin-1 immunoreactivity has been identified in areas of the brain relevant to feeding behavior, including the paraventricular nucleus, supraoptic nucleus, lateral hypothalamic area, arcuate nucleus, dorsomedial hypothalamic nucleus, periventricular nucleus, Edinger-Westphal nucleus, NTS, and DMNV (7, 15–17, 21, 24). As with other anorexigenic neuropeptides, nesfatin-1 has been demonstrated to affect digestive processes as well as feeding behavior. Centrally administered nesfatin-1 delays gastric emptying in rats (41) and mice (17) and also suppresses gastroduodenal motility (3). This inhibition of gastrointestinal motility by nesfatin-1 is hypothesized to contribute to the induction of satiety.
In this study, we microinjected nesfatin-1 into the 4V to investigate its effects on gastrointestinal secretory function. Our results confirm that nesfatin-1 causes dose-dependent, short-term inhibition of dark-phase food intake. At doses sufficient to inhibit food intake, nesfatin-1 exhibited no appreciable effect on basal or pentagastrin-stimulated acid secretion. In contrast, 2-DG-stimulated acid production was inhibited by nesfatin-1 in a dose-dependent manner. As 2-DG is known to trigger gastric acid secretion by activation of the vagal center (10, 19, 32), our study indicates that nesfatin-1 suppresses vagally stimulated gastric acid production.
The selective inhibition of vagally mediated gastric acid production is rare, but not unique. Nitric oxide donors, for example, have been observed to inhibit vagally stimulated gastric acid secretion without affecting basal or pentagastrin- or histamine-stimulated acid production (4). Nitric oxide is hypothesized to serve as an intermediary in the gastric acid suppression induced by endotoxin, cytokines, or stress (13, 14, 27). Interestingly, while several orexigenic hormones have been implicated in vagal stimulation of gastric acid production, nesfatin is the only anorexigenic hormone confirmed to inhibit vagally mediated gastric acid production (11, 28, 46). Vagovagal circuits relayed through the DVC regulate a variety of gastric functions, including the secretion of gastric acid (8, 9). The DVC is a paired structure located in the dorsal caudal medulla alongside the central canal, which is contiguous with the 4V. It comprises two adjacent and functionally integrated nuclei: the NTS and the DMNV. Afferent vagal fibers from the gastrointestinal tract synapse on interneurons located in the NTS. In turn, these neurons synapse on efferent vagal motor neurons in the DMNV. These preganglionic, efferent vagal fibers project to ganglia in the upper gastrointestinal tract (5, 44, 50), with the highest density terminating in the stomach (6). The majority of the associated postganglionic neurons project onto interstitial cells of Cajal, providing parasympathetic control of gastrointestinal motility (9, 44). A minority of these postganglionic neurons directly innervate parietal cells and gastric paracrine cells, resulting in cholinergic stimulation of gastric acid production.
Given our observation that nesfatin-1 administered in close proximity to the DVC inhibited vagally stimulated gastric acid production, we hypothesized that nesfatin-1 might have a direct effect on neurons involved in vagovagal circuits. In our in vivo study, DMNV, but not NTS, neurons were activated by 4V nesfatin-1, as evidenced by c-Fos immunofluorescence. Confirmation that DMNV neurons can be stimulated by nesfatin-1 was obtained through the examination of intracellular Ca2+ signaling in vitro. Nesfatin-1 exhibited the capacity to evoke Ca2+ transients in 95% of primary cultured rat DMNV neurons. Ca2+ ions are critically important to many functions of the nervous system, from neurotransmitter release to intracellular signal transduction. The large gradient between intra- and extracellular Ca2+ concentration highlights the importance of the mechanisms controlling its influx and efflux (12, 37). Nesfatin-1 has been reported to evoke Ca2+ signaling in isolated vagal afferent neurons via N-type Ca2+ channels (22) and in cultured hypothalamic neurons via L- and P/Q-type Ca2+ channels (7). Our study indicates that nesfatin-1 evokes Ca2+ transients in vagal neurons via modulation of T-type Ca2+ channels. T-type channels are low-voltage-activated channels found throughout the central nervous system in areas such as the hippocampus, amygdala, frontal cortex pyramids, and dorsal horn (20, 37). The significance of T-type Ca2+ channel activation in vagal neuron response to nesfatin-1 and the ultimate impact of this particular signaling pathway on gastric acid secretion are unknown.
In addition to vagal, endocrine, and paracrine influences, gastric acid secretion is influenced by a variety of factors, including blood pressure and local blood flow (1, 31, 43). Additionally, all common general anesthetics have some impact on gastrointestinal secretory function (31). In these studies, care was taken to curtail the impact of these other factors. Urethane is the anesthetic agent classically utilized in physiology studies of gastric acid secretion. It has depressive effects on gastric acid production, with augmentation of somatostatin secretion (31, 47). While urethane anesthesia does influence gastric acid secretion, the effects are well-characterized in the literature, do not preclude stimulation of acid secretion with 2-DG, and are assumed to be similar in the treatment and control groups. Moreover, a single intraperitoneal urethane injection can produce >6 h of general anesthesia, thus minimizing fluctuations in blood pressure associated with varying depth of sedation and analgesia. Care was taken to preserve the neurovascular supply to the gastrointestinal tract and to maintain normothermia.
The studies presented here provide further evidence to support the role of nesfatin-1 as an anorexigenic neuropeptide involved in the central regulation of feeding behavior. Nesfatin-1 has been previously reported to influence digestion via inhibition of gastroduodenal motility; this study represents the first report that nesfatin-1 affects signaling in efferent vagal neurons and inhibits vagally stimulated gastric acid production.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-043225.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.-F.X. performed the experiments; Z.-F.X., J.-Y.L., B.C., C.Z., W.Z., and M.W.M. analyzed the data; Z.-F.X., D.M.F., J.-Y.L., B.C., C.Z., W.Z., and M.W.M. interpreted the results of the experiments; Z.-F.X. and D.M.F. prepared the figures; Z.-F.X. and D.M.F. drafted the manuscript; Z.-F.X., D.M.F., W.Z., and M.W.M. edited and revised the manuscript; Z.-F.X., D.M.F., J.-Y.L., B.C., C.Z., W.Z., and M.W.M. approved the final version of the manuscript; W.Z. and M.W.M. are responsible for conception and design of the research.
REFERENCES
- 1. Adami M, Coruzzi G. Measurement of gastric acid secretion in the anaesthetized rat. Curr Protoc Toxicol chapt 21: unit 21.5, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Ammori JB, Zhang W, Newman EA, Mulholland MW. Glutamate-induced calcium transients in rat neurons of the dorsal motor nucleus of the vagus. J Gastrointest Surg 11: 1016–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Atsuchi K, Asakawa A, Ushikai M, Ataka K, Tsai M, Koyama K, Sato Y, Kato I, Fujimiya M, Inui A. Centrally administered nesfatin-1 inhibits feeding behaviour and gastroduodenal motility in mice. Neuroreport 21: 1008–1011, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Barrachina D, Calatayud S, Esplugues J, Whittle BJ, Moncada S, Esplugues JV. Nitric oxide donors preferentially inhibit neuronally mediated rat gastric acid secretion. Eur J Pharmacol 262: 181–183, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Beckett EA, McGeough CA, Sanders KM, Ward SM. Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation. J Physiol 553: 545–559, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berthoud HR, Carlson NR, Powley TL. Topography of efferent vagal innervation of the rat gastrointestinal tract. Am J Physiol Regul Integr Comp Physiol 260: R200–R207, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148: 5088–5094, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastric function. Neurogastroenterol Motil 22: 1154–1163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Browning KN, Travagli RA. Plasticity of vagal brainstem circuits in the control of gastrointestinal function. Auton Neurosci 161: 6–13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colin-Jones DG, Himsworth RL. The location of the chemoreceptor controlling gastric acid secretion during hypoglycaemia. J Physiol 206: 397–409, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 280: 904–907, 2001 [DOI] [PubMed] [Google Scholar]
- 12. DeCoster MA. Calcium dynamics in the central nervous system. Adv Neuroimmunol 5: 233–239, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Esplugues JV, Barrachina MD, Beltran B, Calatayud S, Whittle BJ, Moncada S. Inhibition of gastric acid secretion by stress: a protective reflex mediated by cerebral nitric oxide. Proc Natl Acad Sci USA 93: 14839–14844, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esplugues JV, Barrachina MD, Calatayud S, Pique JM, Whittle BJ. Nitric oxide mediates the inhibition by interleukin-1β of pentagastrin-stimulated rat gastric acid secretion. Br J Pharmacol 108: 9–10, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Foo KS, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156: 563–579, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, Mori M, Luppi PH. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience 155: 174–181, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Goebel-Stengel M, Wang L, Stengel A, Tache Y. Localization of nesfatin-1 neurons in the mouse brain and functional implication. Brain Res 1396: 20–34, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goebel M, Stengel A, Wang L, Tache Y. Central nesfatin-1 reduces the nocturnal food intake in mice by reducing meal size and increasing inter-meal intervals. Peptides 32: 36–43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hirschowitz BI, Sachs G. Vagal gastric secretory stimulation by 2-deoxy-d-glucose. Am J Physiol 209: 452–460, 1965 [DOI] [PubMed] [Google Scholar]
- 20. Iftinca MC. Neuronal T-type calcium channels: what's new? J Med Life 4: 126–138, 2011 [PMC free article] [PubMed] [Google Scholar]
- 21. Inhoff T, Stengel A, Peter L, Goebel M, Tache Y, Bannert N, Wiedenmann B, Klapp BF, Monnikes H, Kobelt P. Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides 31: 257–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iwasaki Y, Nakabayashi H, Kakei M, Shimizu H, Mori M, Yada T. Nesfatin-1 evokes Ca2+ signaling in isolated vagal afferent neurons via Ca2+ influx through N-type channels. Biochem Biophys Res Commun 390: 958–962, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Kanai S, Hosoya H, Akimoto S, Ohta M, Matsui T, Takiguchi S, Funakoshi A, Miyasaka K. Gastric acid secretion in cholecystokinin-1 receptor, -2 receptor, and -1, -2 receptor gene knockout mice. J Physiol Sci 59: 23–29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149: 1295–1301, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Konczol K, Pinter O, Ferenczi S, Varga J, Kovacs K, Palkovits M, Zelena D, Toth ZE. Nesfatin-1 exerts long-term effect on food intake and body temperature. Int J Obes (Lond). In press [DOI] [PubMed] [Google Scholar]
- 26. Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, Yoshida N, Koike M, Uchiyama Y, Fujiwara K, Yashiro T, Horvath TL, Dietrich MO, Tanaka S, Dezaki K, Oh IS, Hashimoto K, Shimizu H, Nakata M, Mori M, Yada T. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab 10: 355–365, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Martinez-Cuesta MA, Esplugues JV, Whittle BJ. Modulation by nitric oxide of spontaneous motility of the rat isolated duodenum: role of tachykinins. Br J Pharmacol 118: 1335–1340, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuda M, Aono M, Moriga M, Okuma M. Centrally administered NPY stimulated gastric acid and pepsin secretion by a vagally mediated mechanism. Regul Pept 35: 31–41, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Mulholland MW, Debas HT. Cholecystokinin receptor antagonism of stimulated pancreatic and gastric secretion. J Surg Res 47: 460–464, 1989 [DOI] [PubMed] [Google Scholar]
- 30. Nagata M, Okuma Y, Osumi Y. Nicotine applied into the dorsal motor nucleus of the vagus inhibits enhanced gastric acid output and mucosal blood flow in rats. Eur J Pharmacol 121: 313–318, 1986 [DOI] [PubMed] [Google Scholar]
- 31. Norlen P, Kitano M, Lindstrom E, Hakanson R. Anaesthetic agents inhibit gastrin-stimulated but not basal histamine release from rat stomach ECL cells. Br J Pharmacol 130: 725–730, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nozu T, Tuchiya Y, Kumei S, Takakusaki K, Ataka K, Fujimiya M, Okumura T. Endogenous orexin-A in the brain mediates 2-deoxy-d-glucose-induced stimulation of gastric motility in freely moving conscious rats. J Gastroenterol 47: 404–411, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Oh IS, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Palasz A, Krzystanek M, Worthington J, Czajkowska B, Kostro K, Wiaderkiewicz R, Bajor G. Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides 46: 105–112, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Bowen Hills, Australia: Academic, 1998 [Google Scholar]
- 36. Ralser M, Wamelink MM, Struys EA, Joppich C, Krobitsch S, Jakobs C, Lehrach H. A catabolic block does not sufficiently explain how 2-deoxy-d-glucose inhibits cell growth. Proc Natl Acad Sci USA 105: 17807–17811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ross WN. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci 13: 157–168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schubert ML. Gastric secretion. Curr Opin Gastroenterol 26: 598–603, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology 134: 1842–1860, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Stengel A, Goebel M, Tache Y. Nesfatin-1: a novel inhibitory regulator of food intake and body weight. Obes Rev 12: 261–271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Monnikes H, Lambrecht NW, Tache Y. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor-2 receptor. Endocrinology 150: 4911–4919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stengel A, Tache Y. Nesfatin-1—an emerging new player in the brain-gut, endocrine, and metabolic axis. Endocrinology 152: 4033–4038, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takeuchi K, Ohuchi T, Miyake H, Niki S, Okabe S. Effects of nitric oxide synthase inhibitors on duodenal alkaline secretion in anesthetized rats. Eur J Pharmacol 231: 135–138, 1993 [DOI] [PubMed] [Google Scholar]
- 44. Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 68: 279–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wren AM, Bloom SR. Gut hormones and appetite control. Gastroenterology 132: 2116–2130, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Yamada H, Takahashi N, Tanno S, Nagamine M, Takakusaki K, Okumura T. A selective orexin-1 receptor antagonist, SB334867, blocks 2-DG-induced gastric acid secretion in rats. Neurosci Lett 376: 137–142, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Yang H, Wong H, Wu V, Walsh JH, Tache Y. Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anesthetized rats. Gastroenterology 99: 659–665, 1990 [DOI] [PubMed] [Google Scholar]
- 48. Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 298: R1642–R1647, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang W, Hu Y, Newman EA, Mulholland MW. Serum-free culture of rat postnatal neurons derived from the dorsal motor nucleus of the vagus. J Neurosci Methods 150: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Zheng H, Berthoud HR. Functional vagal input to gastric myenteric plexus as assessed by vagal stimulation-induced Fos expression. Am J Physiol Gastrointest Liver Physiol 279: G73–G81, 2000 [DOI] [PubMed] [Google Scholar]