Abstract
Cyclic AMP stimulates translocation of Na+/taurocholate cotransporting polypeptide (NTCP) from the cytosol to the sinusoidal membrane and multidrug resistance-associated protein 2 (MRP2) to the canalicular membrane. A recent study suggested that protein kinase Cδ (PKCδ) may mediate cAMP-induced translocation of Ntcp and Mrp2. In addition, cAMP has been shown to stimulate NTCP translocation in part via Rab4. The aim of this study was to determine whether cAMP-induced translocation of NTCP and MRP2 require kinase activity of PKCδ and to test the hypothesis that cAMP-induced activation of Rab4 is mediated via PKCδ. Studies were conducted in HuH-NTCP cells (HuH-7 cells stably transfected with NTCP). Transfection of cells with wild-type PKCδ increased plasma membrane PKCδ and NTCP and increased Rab4 activity. Paradoxically, overexpression of kinase-dead dominant-negative PKCδ also increased plasma membrane PKCδ and NTCP as well as Rab4 activity. Similar results were obtained in PKCδ knockdown experiments, despite a decrease in total PKCδ. These results raised the possibility that plasma membrane localization rather than kinase activity of PKCδ is necessary for NTCP translocation and Rab4 activity. This hypothesis was supported by results showing that rottlerin, which has previously been shown to inhibit cAMP-induced membrane translocation of PKCδ and NTCP, inhibited cAMP-induced Rab4 activity. In addition, LY294002 (a phosphoinositide-3-kinase inhibitor), which has been shown to inhibit cAMP-induced NTCP translocation, also inhibited cAMP-induced PKCδ translocation. In contrast to the results with NTCP, cAMP-induced MRP2 translocation was inhibited in cells transfected with DN-PKCδ and small interfering RNA PKCδ. Taken together, these results suggest that the plasma membrane localization rather than kinase activity of PKCδ plays an important role in cAMP-induced NTCP translocation and Rab4 activity, whereas the kinase activity of PKCδ is necessary for cAMP-induced MRP2 translocation.
Keywords: Rab4, HuH-NTCP cells, DN-PKCδ, siRNA-PKCδ
vectorial transport of solutes from blood to bile is accomplished by a number of transporters located on the sinusoidal and canalicular membrane of hepatocytes (1, 38). Cholestasis results when the vectorial transport of solutes destined for bile is compromised. Among these transporters, Na+/taurocholate cotransporting polypeptide (NTCP) is responsible for uptake of bile acid into hepatocytes across the sinusoidal membrane and multidrug resistant-associated protein 2 (MRP2) mediates secretion of organic anions across the canalicular membrane. Membrane localizations of these transporters and hence their physiological functions are regulated by choleretic and cholestatic agents. Hormones that increase intracellular cAMP are choleretic. Studies have shown that cAMP induces translocation of Mrp2 and Ntcp from an endosomal compartment to the plasma membrane (26, 30). The cellular mechanisms by which cAMP increases the plasma membrane location of these transporters are still incompletely understood.
Cyclic AMP (cAMP) increases taurocholate uptake by translocating NTCP in hepatocytes from an intracellular compartment to the plasma membrane (1, 26). Likewise, cAMP can also increase translocation of MRP2 to the canalicular membrane. These effects are mediated via phosphoinositide-3-kinase (PI3K) and PI3K-dependent activation of PKCδ (1, 33). The role of PKCδ was studied by using a chemical inhibitor and activator of PKCδ, namely rottlerin and bistratene A (33). Since the specificity of chemical inhibitors and activators is a potential concern, studies with more specific molecular probes are necessary to confirm whether kinase activity of PKCδ is required for cAMP-induced translocation of NTCP and MRP2. Other studies showed that cAMP-mediated activation of Rab4 facilitates NTCP translocation (34). It is, however, not known whether cAMP-induced Ntcp translocation involves a sequential pathway or two independent pathways mediated via Rab4 and PKCδ.
PKCs appear to be involved in vesicular pathways of transporters, receptors, and plasma membrane proteins (4, 5, 21) by affecting signaling pathways (3). There are several studies that support a role for PKCs in membrane transport. Previous studies in hepatocytes showed that PKCζ is involved in cAMP-induced Ntcp translocation (24) and microtubule-based motility of vesicles containing Ntcp (31). PKCλ and Rab4 have been shown to mediate insulin-induced GLUT4 translocation (8, 18). Of interest to the present study is the finding that insulin-induced activation of Rab4 is inhibited by dominant negative PKCλ (8, 18). Thus it is feasible that cAMP-induced translocation of NTCP involves activation of PKCδ followed by activation of Rab4.
The aim of the present study was to test the hypothesis that cAMP-induced activation of Rab4 is mediated via activation of PKCδ and to further confirm that kinase activity of PKCδ is required for cAMP-stimulated translocations of NTCP and MRP2. Our studies resulted in the unexpected findings of increased Rab4 activation and NTCP translocation by wild-type (WT) as well as kinase-dead dominant-negative (DN) PKCδ. Further studies suggested that plasma membrane localization, not the kinase activity of PKCδ, is important for cAMP-induced activation of Rab4 and NTCP translocation, whereas cAMP-induced MRP2 translocation is dependent on PKCδ kinase activity.
MATERIALS AND METHODS
Materials.
Rottlerin and LY294002 were obtained from Calbiochem (San Diego, CA). 8-Chlorophenylthio cAMP (CPT-cAMP), aprotinin, leupeptin, and okadaic acid were purchased from Sigma Chemical (St. Louis, MO). [α-32P]GTP was purchased from Perkin-Elmer (Boston, MA). Polyclonal Rab4 antibody was obtained from Millipore (Billerica, MA). WT- and kinase-dead DN-PKCδ plasmids were purchased from Addgene (Cambridge, MA). Each DNA mutation was confirmed by DNA sequencing. A small interfering RNA (siRNA) against PKCδ isoform (S102660539) and Allstar negative control siRNA (1027281) were purchased from Qiagen (Valencia, CA). Lipofectamine 2000 and Lipofectamine RNAiMAX were obtained from Invitrogen (Carlsbad, CA). HuH-NTCP cells (HuH-7 cells stably transfected with NTCP) were generously provided by Dr. Gores (Rochester, MN). Monoclonal Rab4 antibody, E-cadherin antibody and PKCδ antibody were purchased from BD Transduction Laboratories (San Jose, CA). Sulfo-NHS-LC-Biotin was purchased from Pierce (Rockford, IL). Total Akt and phospho-Akt antibodies were purchased from Cell Signaling (Danvers, MA).
Cell culture.
HuH-NTCP cells were grown in Eagle's minimum essential medium supplemented with 10% FCS, 1.2 g/l G418 100,000 units/l penicillin, 100 mg/l streptomycin, and 25 μg/ml amphotericin B at 37°C with 5% CO2. After culturing, the medium was changed to serum-free DMEM for 3 h and cells were then treated with or without CPT-cAMP, a cell-permeable analog of cAMP.
Rab4 activation.
Activation of Rab4 was determined by measuring GTP binding to Rab4 in immunoprecipitated Rab4 with use of a blot overlay assay, as previously described (34). The blot overlay assay has been used by others to determine GTP binding state of Rab proteins (6, 14, 23, 25, 32). Briefly, cells were incubated in serum-free media for 3 h and serum starved cells were stimulated with or without 5 μM rottlerin for 30 min, followed by treatment with or without 100 μM CPT-cAMP for 10 min. Cells were washed with PBS and then lysed with cell lysis buffer (20 mM Tris, 150 mM NaCl, 1% Triton, 1 mM phenylmethylsulfonyl fluoride, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 500 nM okadaic acid, and 1 mM orthovanadate, pH 7.5). The soluble fractions were immunoprecipitated with anti-rabbit polyclonal Rab4 antibody (Millipore) overnight at 4°C, followed by incubation with protein A-Sepharose beads for 2 h. The immunoprecipitated proteins resolved by SDS-PAGE and gels were transferred to nitrocellulose membrane. After transfer, the blot was incubated in binding buffer (50 mM sodium phosphate pH 7.5, 10 μM MgCl2, 2 mM DTT, 0.2% Tween 20, 4 μM ATP) for 30 min at room temperature, followed by incubation for 1 h in binding buffer with 1 μCi/ml [α-32P]GTP. Blots were washed in binding buffer twice for 15 min and autoradiographed by using Kodak XO-MAT AR films at −80°C to determine GTP-bound Rab4 (Rab4-GTP). GTP binding blots were stripped by washing in a stripping buffer (20 mM Tris·HCl pH 7.5, 1 mM EDTA, 1 mM DTT, 250 mM ammonium sulfate) followed by incubation for 1 h in another buffer (20 mM Tris·HCl pH 7.5, 5 mM MgCl2, 1 mM EDTA, 50 mM NaCl, 1 mM DTT, 200 μM GDP). Stripping was confirmed by autoradiography. Blots were blocked and then probed with primary antibody, anti-mouse monoclonal Rab4 antibody (BD Transduction), to determine total Rab4. After washing, blots were incubated with horseradish peroxidase-linked secondary antibody followed by chemiluminescence detection. The blots were routinely subjected to multiple exposure times, and those blots appearing to show possible saturation were analyzed for different exposure time for concordance. Rab4 activity was expressed as a ratio of Rab4-GTP to total Rab4 to minimize variability associated with immunoprecipitation.
Transfection of cells with mutant isoforms of PKCδ.
HuH-NTCP cells were transfected with an empty vector (EV) or HA-tagged DN-PKCδ or WT-PKCδ, by using Lipofectamine 2000 according to the manufacturer's instructions. Briefly, the culture medium was changed to OptiMem containing Lipofectamine and EV, DN-PKCδ, or WT-PKCδ and incubated at 37°C for 24 h. The ratio of DNA and Lipofectamine was 1 μg DNA to 3 μl Lipofectamine. Expression of DN and WT PKCδ in HuH-NTCP cells was confirmed by immunoblotting with anti-HA antibodies and anti-PKCδ antibodies. The amount of PKCδ was increased overall by ninefold after transfection.
Transfection of cells with siRNA PKCδ.
HuH-NTCP cells were transfected with scrambled siRNA and siRNA against PKCδ by using Lipofectamine RNAiMAX according to the manufacturer's instructions. Briefly, after cell culturing, the medium was changed to antibiotics-free regular DMEM media, followed by addition of OptiMEM containing Lipofectamine RNAiMAX and scrambled siRNA or siRNA PKCδ mixture and incubation at 37°C for 48 h.
Translocation of NTCP and MRP2 and PKCδ.
A cell surface biotinylation method was used to quantitate NTCP and MRP2 translocation (33). Briefly, after various treatments, cells were incubated with sulfo-NHS-LC-Biotin (0.5 mg/ml) at 4°C to achieve the selective labeling of cell surface proteins. Biotinylated proteins were isolated with streptavidin-agarose beads followed by immunoblot analysis. Immunoblotting with E-cadherin, a plasma membrane protein, was used for loading control.
Other methods.
The Lowry method was used to determine cell protein (22).
Data analysis.
Results were expressed as means ± SE. Data were analyzed by one-way ANOVA, followed by Holm-Sidak test for multiple comparisons or by paired t-test. P < 0.05 was considered statistically significant.
RESULTS
Rottlerin inhibits cAMP-stimulated Rab4 activity.
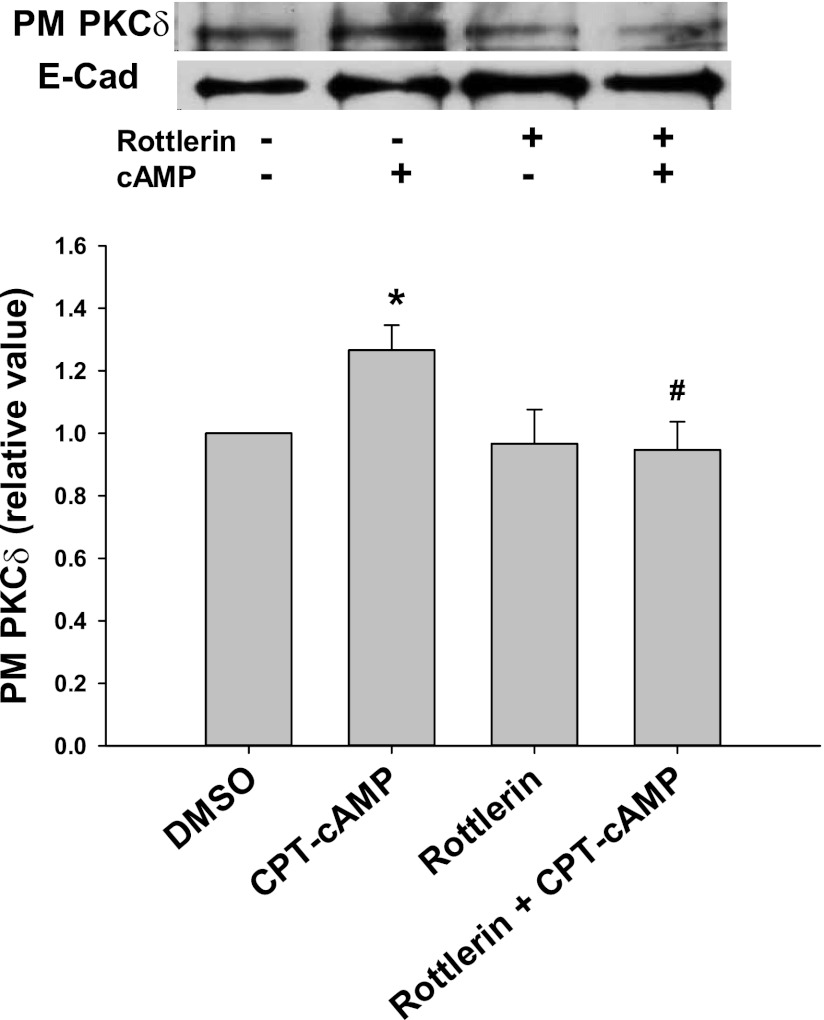
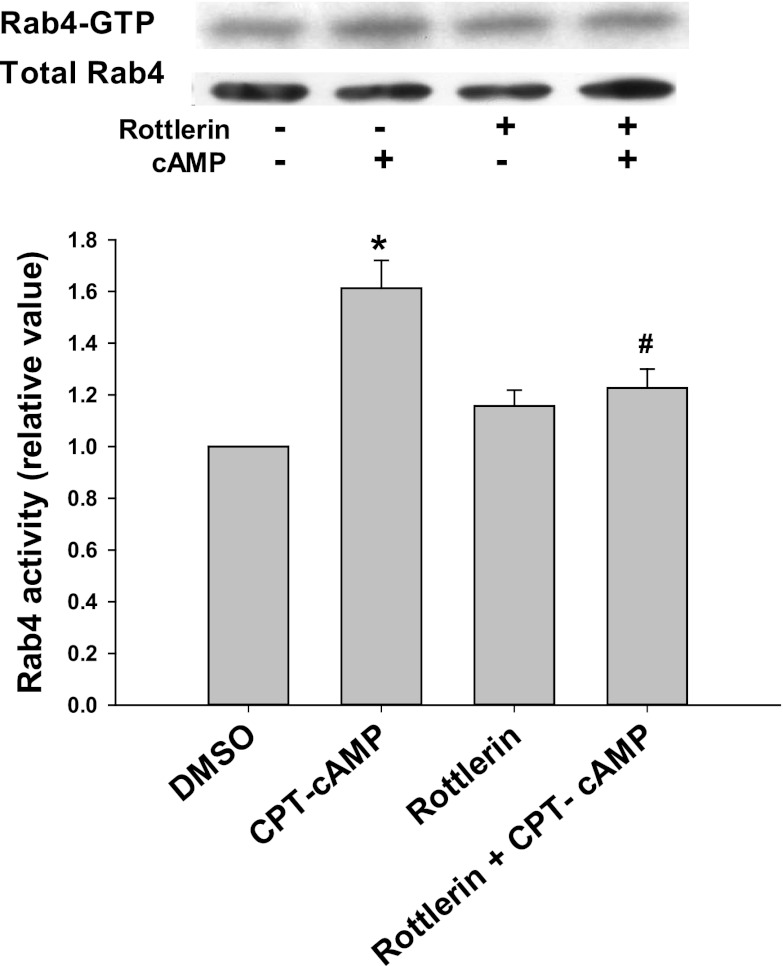
We hypothesized that cAMP-induced activation of Rab4 may be mediated via PKCδ. To test this hypothesis, we determined the effect of rottlerin, an inhibitor of PKCδ (16), on cAMP-induced Rab4 activation. Rottlerin has previously been shown to inhibit cAMP-induced membrane translocation of PKCδ in rat hepatocytes (33). In our study using HuH-NTCP cells, CPT-cAMP significantly increased the plasma membrane translocation of PKCδ by 1.3-fold and pretreatment with rottlerin prevented this (Fig. 1). Rottlerin alone did not change the localization of plasma membrane PKCδ compared with the control. As seen in Fig. 2, CPT-cAMP increased Rab4 activity by 1.6-fold in HuH-NTCP cells compared with the control, and the pretreatment with rottlerin significantly decreased the ability of cAMP to increase Rab4 activity.
Fig. 1.
Rottlerin inhibits cAMP-induced PKCδ translocation to the plasma membrane. HuH-NTCP cells were treated with or without 5 μM rottlerin for 30 min, followed by treatment with or without 100 μM 8-chlorophenylthio cAMP (CPT-cAMP) for 15 min. PKCδ translocation was measured by a biotinylation method. Top: representative immunoblot of the amount of PKCδ in the plasma membrane (PM). Bottom: densitometric analysis is shown. E-cadherin (E-cad) was used as a loading control. The relative values of PKCδ in the PM are expressed as means ± SE (n = 4). Data were analyzed by paired t-test. *Significantly different (P < 0.05) from control values in the absence of cAMP and rottlerin; #significantly different (P < 0.05) from cAMP treatment.
Fig. 2.
Rottlerin inhibits cAMP-induced activation of Rab4. HuH-NTCP cells were treated with or without 5 μM rottlerin for 30 min, followed by treatment with or without 100 μM CPT-cAMP for 10 min. Rab4 activation was determined by GTP overlay assay as described in materials and methods. A representative immunoblot of Rab4-GTP and total Rab4 is shown at top with the densitometric analysis shown at bottom. Rab4 activity (means ± SE, n = 3) was expressed as a ratio of Rab4-GTP to total Rab4 to correct for variations due to loading and immunoprecipitation. Data were analyzed by 1-way ANOVA. *Significantly different (P < 0.05) from control values in the absence of cAMP and rottlerin; #significantly different (P < 0.05) from cAMP treatment.
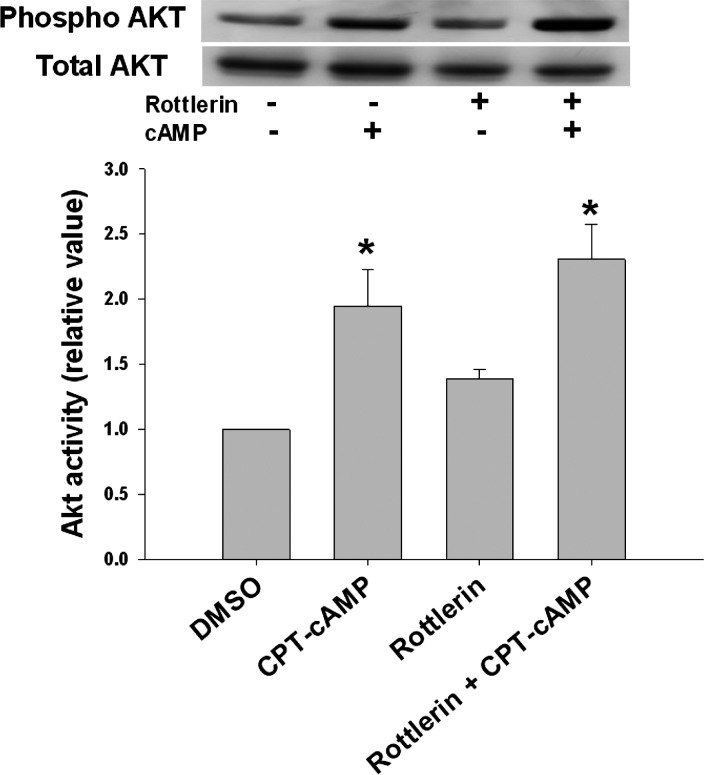
cAMP has been shown to stimulate Ntcp translocation via the PI3K/Akt signaling pathway (41). Thus it is possible that the inhibitory effect of rottlerin is caused by blocking PI3K pathway. To test this, cells were treated with rottlerin for 30 min, followed by treatment with or without cAMP for 10 min. CPT-cAMP increased Akt phosphorylation by 1.9-fold compared with the control (Fig. 3). Rottlerin treatment neither affected basal Akt activity nor the effect of cAMP on Akt significantly. Collectively, these results indicate that rottlerin does not affect cAMP effect on PI3K signaling pathway but inhibits cAMP-mediated PKCδ activation and Rab4 activation.
Fig. 3.
Rottlerin does not inhibit cAMP-induced Akt phosphorylation. HuH-NTCP cells were treated with or without 5 μM rottlerin for 30 min, followed by treatment with or without 100 μM CPT-cAMP for 10 min. Cell lysates from treated cells were subjected to immunoblot analysis. A representative immunoblot is shown at top with the densitometric analysis shown at bottom. Akt activity was determined from the ratio of phosphorylated Akt to total Akt and is expressed as means ± SE (n = 3). Data were analyzed by 1-way ANOVA. *Significantly different (P < 0.05) from control values in the absence of cAMP and rottlerin.
Although the results with rottlerin suggest that cAMP-induced activation of Rab4 is dependent on PKCδ activity, the specificity of pharmacological inhibitors is always a concern (19, 35). Thus, to further define the role of PKCδ in cAMP-induced Rab4 activation, we studied the effect of kinase-dead DN- and WT-PKCδ as well as siRNA against PKCδ on cAMP-induced Rab4 activity.
DN- and WT-PKCδ increase Rab4 activity and NTCP translocation.
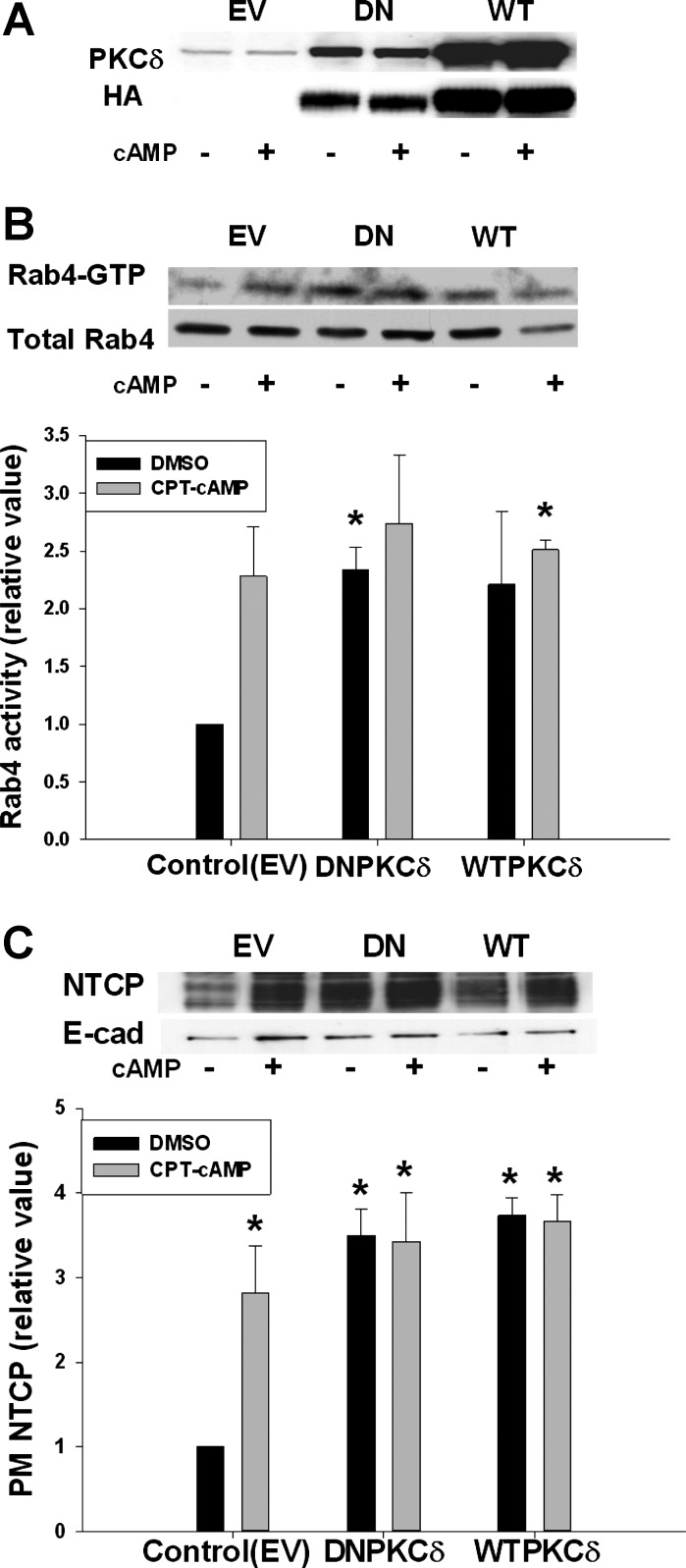
To test whether kinase activity of PKCδ is necessary for cAMP-induced activation of Rab4 and NTCP translocation, HuH-NTCP cells were transfected with DN- or WT-PKCδ followed by CPT-cAMP treatment. Expressions of WT- and DN-PKCδ were confirmed by immunoblots for PKCδ and HA tag (Fig. 4A). HA-tagged PKCδ expression was on an average ninefold higher than the basal level of PKCδ. CPT-cAMP increased Rab4 activity in cells transfected with EV compared with the control (Fig. 4B). Transfection of cells with WT-PKCδ increased the basal level of Rab4 activity in the absence of cAMP. Treatment with cAMP did not further increase the Rab4 activity and this may indicate that the system is already maximally stimulated by the overexpression of WT-PKCδ. Unexpectedly, DN-PKCδ transfection itself increased the basal level of Rab4 activity, and did not block the effect of cAMP on Rab4 activity.
Fig. 4.
Dominant-negative (DN)- and wild-type (WT)-PKCδ increased Rab4 activity and PM NTCP. HuH-NTCP cells were transfected with empty vector (EV), DN-PKCδ, and WT-PKCδ, followed by treatment with or without 100 μM CPT-cAMP for 15 min. Rab4 activation was determined using a GTP overlay assay (B). NTCP translocation was measured by a biotinylation method (C). A: transfection was confirmed with PKCδ and HA antibodies. B: representative blot of Rab4-GTP and total Rab4 (top) and densitometric analysis (bottom). Rab4 activity (means ± SE, n = 3) was expressed as a ratio of Rab4-GTP to total Rab4. Data were analyzed by paired t-test. C: representative immunoblot of PM NTCP and E-cadherin (top) and densitometric analysis (bottom). Relative values of NTCP in the PM are expressed as means ± SE (n = 3). Data were analyzed by 1-way ANOVA. *Significantly different (P < 0.05) from respective control values in the absence of cAMP in cells transfected with EV.
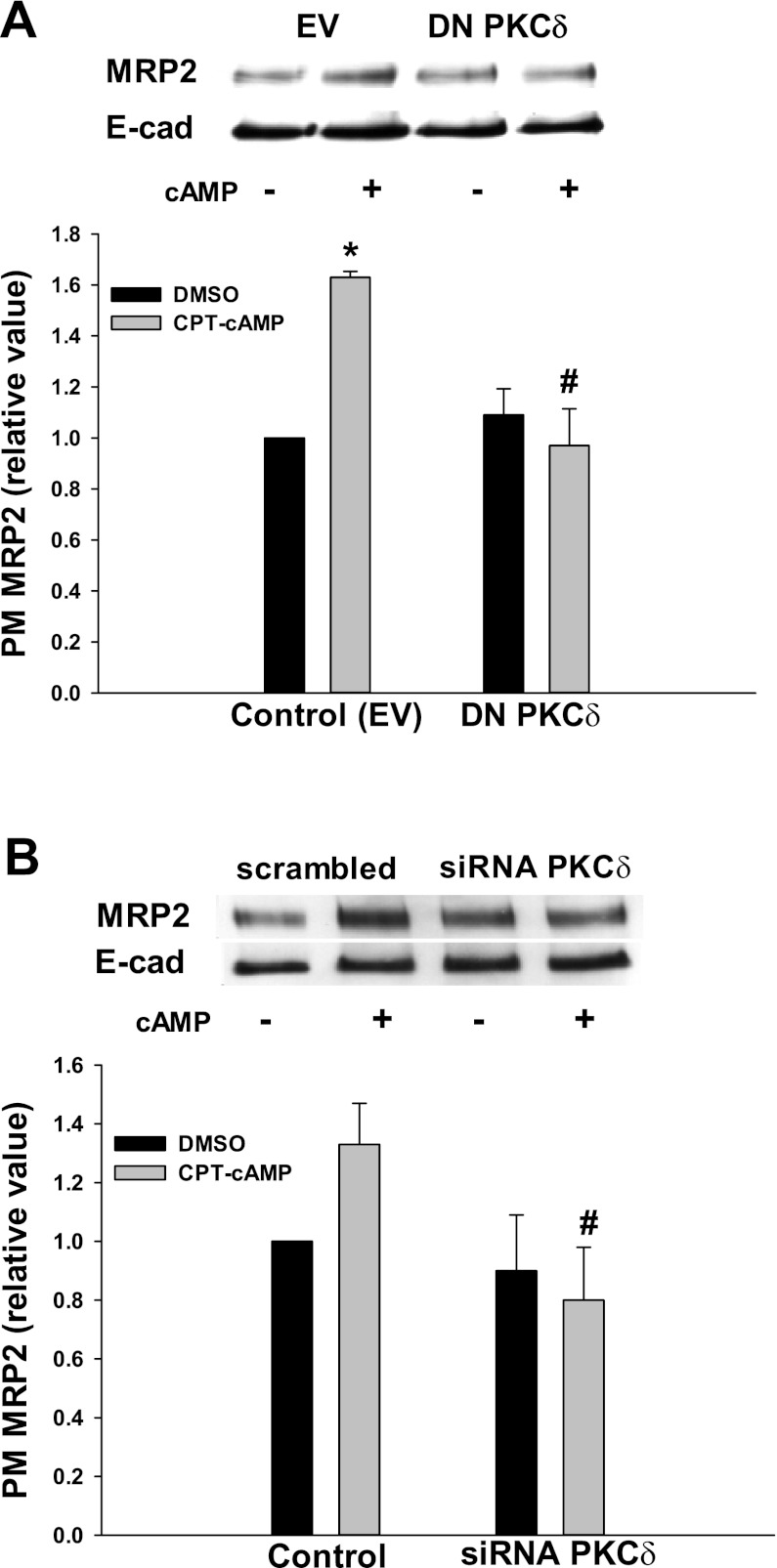
Similar effects of WT- and DN-PKCδ were also observed for NTCP translocation. The amount of plasma membrane NTCP in cells transfected with EV was significantly increased by cAMP compared with the control (Fig. 4C). WT-PKCδ significantly increased the amount of NTCP in the plasma membrane compared with the control, and this effect was not further altered by cAMP treatment. As in the case of Rab4 activation, DN-PKCδ significantly increased the amount of NTCP in plasma membrane in the absence of cAMP. There was no difference in plasma membrane NTCP level in the presence and absence of cAMP in cells transfected with DN-PKCδ. Note that DN-PKCδ did inhibit cAMP-induced MRP2 translocation (see Fig. 10A). These results may suggest that the kinase activity of PKCδ may not be involved in cAMP-induced activation of Rab4 and NTCP translocation.
Fig. 10.
DN-PKCδ and siRNA PKCδ inhibited cAMP-induced MRP2 translocation. HuH-NTCP cells were transfected with EV and DN-PKCδ (A), or transfected with scrambled siRNA (control) and siRNA PKCδ (B) followed by treatment with or without 100 μM CPT-cAMP for 15 min. A biotinylation method was used to determine PM MRP2. A: representative immunoblot of MRP2 and E-cadherin (top); densitometric analysis (bottom). Relative values of MRP2 in the PM are expressed as means ± SE (n = 3). Data were analyzed by 1-way ANOVA. B: representative blot of PM MRP2 and E-cadherin (top); densitometric analysis (bottom). Relative values of MRP2 in the PM are expressed as means ± SE (n = 3). Data were analyzed by paired t-test. *Significantly different (P < 0.05) from control values in the absence of cAMP; #significantly different (P < 0.05) from control values in the presence of cAMP.
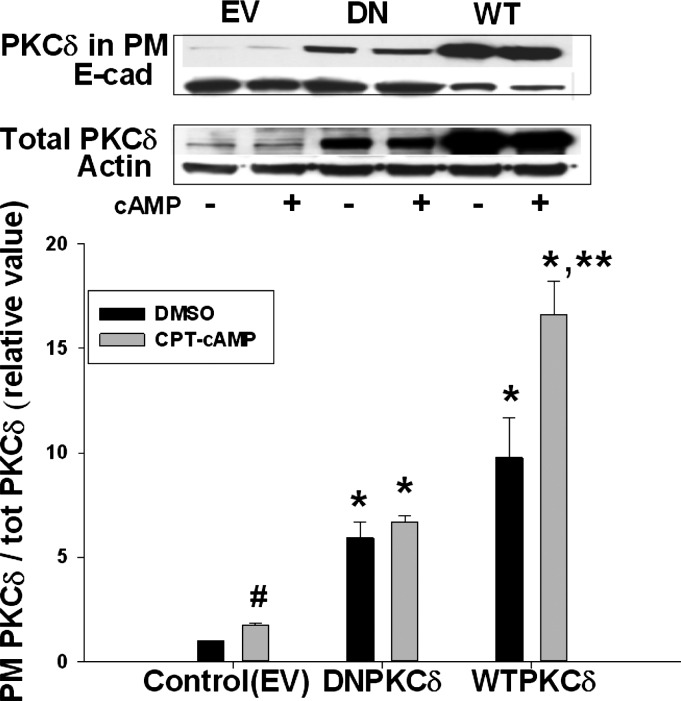
An alternate possibility may be that plasma membrane localization rather than kinase activity of PKCδ is involved. Indeed, a previous study showed that the DN-PKCδ (PKCδ-K376R) construct we used was exclusively localized in membrane fraction in transfected control cells (20). Thus we determined the plasma membrane localization of PKCδ in cells transfected with WT- and DN-PKCδ. Transfection with either WT- or DN-PKCδ resulted in significantly higher plasma membrane levels of PKCδ compared with cells transfected with EV in the absence of cAMP (Fig. 5). The plasma membrane level of PKCδ was increased further by cAMP in WT-PKCδ transfected cells, but not in DN-PKCδ transfected cells. Taken together, these results raise the possibility that cAMP-induced increases in Rab4 activity or NTCP translocation might be due to plasma membrane localization of PKCδ and not the kinase activity of PKCδ.
Fig. 5.
DN- as well as WT-PKCδ increased PM PKCδ. HuH-NTCP cells were transfected with EV, DN-PKCδ, or WT-PKCδ, followed by treatment with or without 100 μM CPT-cAMP for 15 min. A biotinylation method was used to determine PM PKCδ. Representative immunoblots of PM PKCδ (top) and total PKCδ (middle) and the densitometric analysis (bottom). Amount of PKCδ localization in the PM was expressed as a ratio of PM PKCδ to total PKCδ. Relative values of PKCδ in the PM are expressed as means ± SE (n = 3). Data were analyzed by 1-way ANOVA. *Significantly different (P < 0.05) from control values in the absence of cAMP; **significantly different (P < 0.05) from values in the absence of cAMP in cells transfected with WT-PKCδ. Control values in the absence and presence of cAMP are #significantly different (P < 0.05) by paired t-test.
Localization of PKCδ in the plasma membrane elevates Rab4 activity and NTCP translocation.
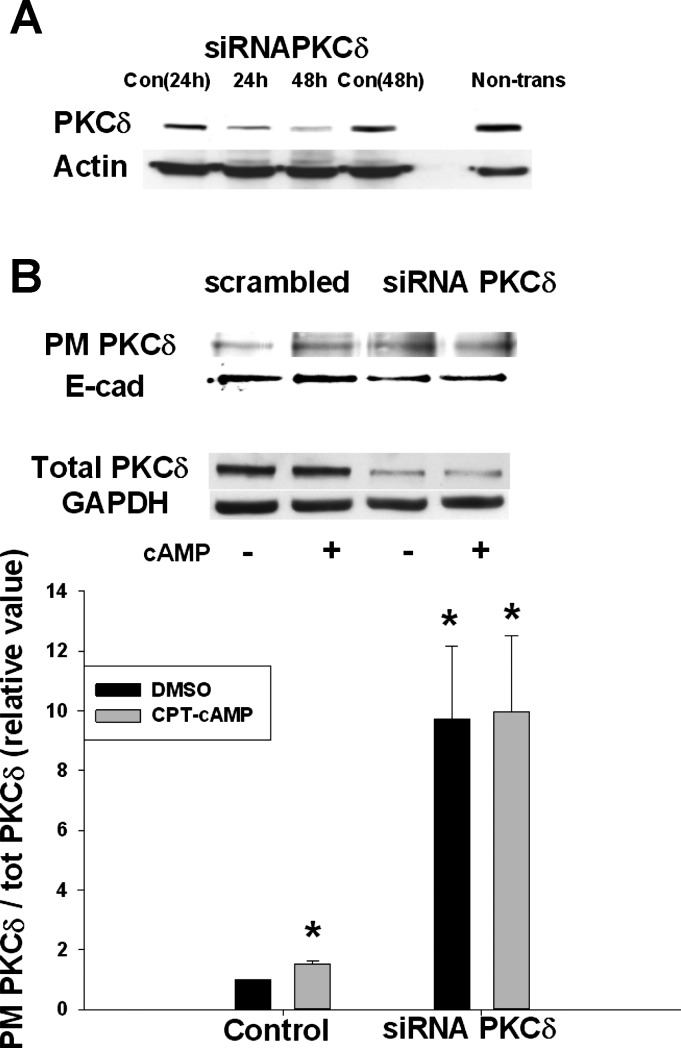
To further clarify the role of PKCδ on Rab4 activity and NTCP translocation, PKCδ was knocked down by using siRNA against PKCδ. The expectation was that the knockdown of PKCδ would result in a decrease in plasma membrane PKCδ. To optimize the knockdown conditions, cells were transfected with siRNA PKCδ for 24 or 48 h. When cells were transfected with siRNA PKCδ for 48 h, PKCδ protein level decreased to 20–30% of the control level (Fig. 6A). Thus further studies were conducted in cells transfected for 48 h to determine the effect of PKCδ knockdown on Rab4 activity, NTCP translocation, and plasma membrane distribution of PKCδ. As expected, cAMP increased plasma membrane PKCδ in cells transfected with scrambled siRNA and total PKCδ level decreased by 70% in cells treated with siRNA PKCδ (Fig. 6B). However, despite a decrease in total PKCδ, plasma membrane PKCδ increased by approximately twofold and cAMP treatment did not affect already increased plasma membrane PKCδ level in siRNA PKCδ transfected cells. Thus plasma membrane level of NTCP and Rab4 activity appears to be regulated by the plasma membrane level of PKCδ.
Fig. 6.
Small interfering RNA (siRNA) PKCδ increased PM PKCδ. A: level of PKCδ protein was downregulated up to 80% in cells transfected with siRNA PKCδ for 48 h compared with cells transfected with scrambled siRNA (Con) or nontransfected cells (Non-trans) B: HuH-NTCP cells were transfected with scrambled siRNA and siRNA PKCδ, followed by treatment with or without 100 μM CPT-cAMP for 15 min. A biotinylation method was used to determine PM PKCδ. Representative immunoblots of PM PKCδ (top), total PKCδ (middle), and the densitometric analysis (bottom) are shown. The relative values of PKCδ in the PM are expressed as means ± SE (n = 4). Data were analyzed by paired t-test. *Significantly different (P < 0.05) from control values in the absence of cAMP.
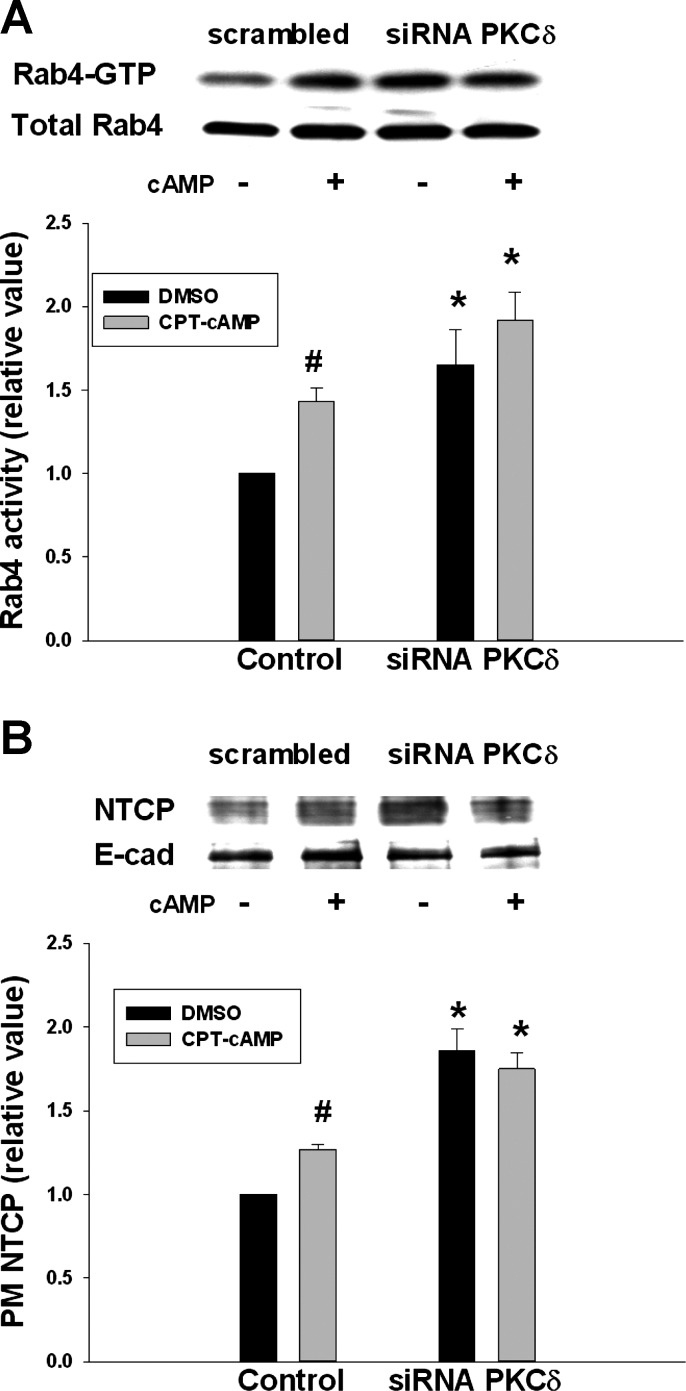
As shown in Fig. 7A, CPT-cAMP increased Rab4 activity by 1.4-fold in cells transfected with scrambled siRNA (control). However, in cells transfected with siRNA PKCδ, Rab4 activity was increased significantly in the absence and presence of cAMP. Similarly, PKCδ knockdown resulted in significantly increased amount of NTCP in the plasma membrane compared with the control (Fig. 7B). Treatment with CPT-cAMP in cells transfected with siRNA PKCδ did not affect already increased plasma membrane NTCP level.
Fig. 7.
siRNA PKCδ increased Rab4 activity and the amount of PM NTCP. HuH-NTCP cells were transfected with scrambled siRNA (control) or siRNA PKCδ, followed by treatment with or without 100 μM CPT-cAMP for 15 min and then determination of Rab4 activity (A). A biotinylation method was used to determine PM NTCP (B). A: representative blot of Rab4-GTP and total Rab4 (top); densitometric analysis (bottom). Rab4 activity (means ± SE, n = 3) was expressed as a ratio of Rab4-GTP to total Rab4. Data were analyzed by 1-way ANOVA. *Significantly (P < 0.05) different from control values in the absence of cAMP in cells transfected with scrambled siRNA. Control values in the absence and presence of cAMP are #significantly different (P < 0.05) by paired t-test. B: representative immunoblot of PM NTCP and E-cadherin (top); densitometric analysis (bottom). Relative values of NTCP in the PM are expressed as means ± SE (n = 3). Data were analyzed by 1-way ANOVA. *Significantly (P < 0.01) different from control values in the absence of cAMP. Control values in the absence and presence of cAMP are #significantly different (P < 0.05) by paired t-test.
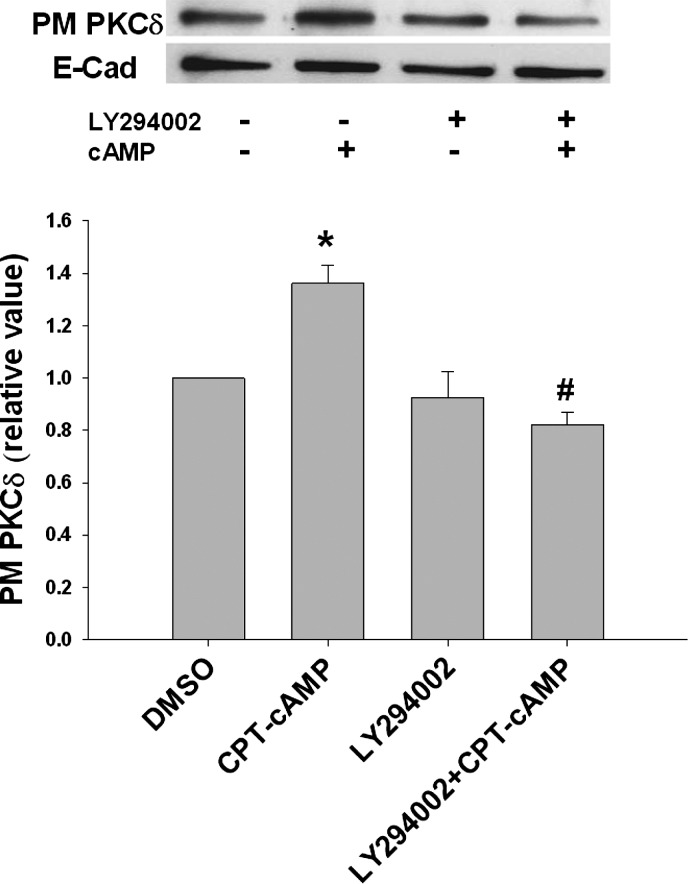
Our previous study showed that cAMP stimulates NTCP translocation in a PI3K-dependent manner (33). If the localization of PKCδ is essential for cAMP-induced NTCP translocation, one would expect inhibition of cAMP-induced PKCδ translocation by PI3K inhibitor. To test this possibility, we determined the effect of LY294002, a specific PI3K inhibitor, on the plasma membrane localization of PKCδ. CPT-cAMP significantly increased the plasma level of PKCδ (Fig. 8). LY294002 treatment itself did not change the basal level of PKCδ compared with the control, but inhibited the effect of cAMP on plasma level of PKCδ. This result suggests that cAMP-induced PKCδ translocation is PI3K dependent and further supports a role for PKCδ translocation in cAMP-induced NTCP translocation.
Fig. 8.
LY294002 inhibited cAMP-induced PKCδ translocation to the PM. HuH-NTCP cells were treated with or without 20 μM LY294002 for 30 min, followed by treatment with or without 100 μM CPT-cAMP for 15 min. PKCδ translocation was measured by a biotinylation method. Top: representative immunoblot of the amount of PKCδ in the PM. Bottom: densitometric analysis is shown. E-cadherin was used as a loading control. The relative values of PKCδ in the PM are expressed as means ± SE (n = 3). Data were analyzed by 1-way ANOVA. *Significantly (P < 0.05) different from control values in the absence of cAMP and LY294002; #significantly (P < 0.05) different from cAMP treatment.
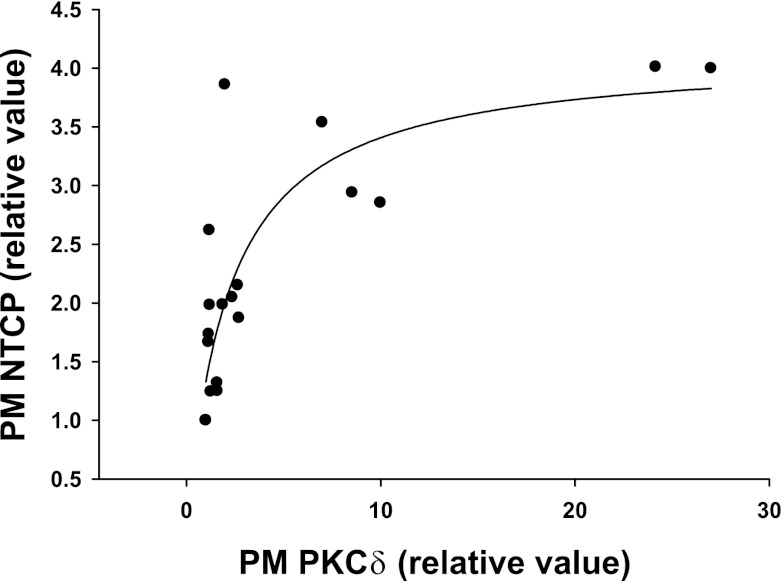
If plasma membrane localization of PKCδ is important for NTCP translocation, then one would expect a positive correlation between plasma membrane levels of NTCP and PKCδ. To verify this, plasma membrane NTCP values from individual experiments were plotted against corresponding plasma membrane PKCδ values. The result showed a curvilinear relationship best fitted by a rectangular hyperbola (Fig. 9), indicating that plasma membrane NTCP increases with plasma membrane PKCδ until a plateau is reached around 10-fold increase in plasma membrane PKCδ. Although this does not indicate a cause-effect relationship, it is nevertheless consistent with the hypothesis that plasma membrane localization rather than kinase activity of PKCδ may be important for NTCP translocation (see discussion).
Fig. 9.
Significant correlation between PM PKCδ and NTCP. Values for PM PKCδ and PM NTCP were from cells transfected with DN-PKCδ or siRNA against PKCδ. Solid line represents best-fit line for PM NTCP = 4.12*PM PKCδ/(2.10+PM PKCδ); R = 0.833, P < 0.0001, n = 18.
Kinase activity of PKCδ is required for cAMP-induced MRP2 translocation.
We studied the effect of DN-PKCδ and siRNA PKCδ on cAMP-induced MRP2 translocation to determine whether plasma membrane level or kinase activity of PKCδ is involved. In contrast to the above results with NTCP and Rab4, DN-PKCδ had no effect on the basal level of MRP2 localization in the plasma membrane. Moreover, the effect of cAMP on MRP2 translocation was significantly inhibited by DN-PKCδ (Fig. 10A). As expected, the amount of MRP2 in the plasma membrane was significantly increased by cAMP in cells transfected with EV. This result suggests that the kinase activity rather than plasma membrane level of PKCδ is required for cAMP-induced MRP2 translocation. To further determine the role of plasma membrane PKCδ on cAMP-stimulated MRP2 translocation, cells were transfected with scrambled siRNA and siRNA against PKCδ and then treated with cAMP 48 h after siRNA transfection. PKCδ knockdown did not increase plasma membrane level of MRP2 in the absence of cAMP (Fig. 10B). Moreover, cAMP treatment did not increase MRP2 level in the plasma membrane in siRNA PKCδ transfected cells. cAMP did increase plasma membrane MRP2 in cells transfected with scrambled siRNA. Taken together, these results would suggest that the kinase activity rather than plasma membrane level of PKCδ is necessary for cAMP-induced MRP2 translocation.
DISCUSSION
The aim of this study was twofold: 1) to test the hypothesis that cAMP-induced activation of Rab4 is mediated by PKCδ and 2) to obtain further evidence for PKCδ kinase activity in cAMP-induced translocation of NTCP and MRP2. Results of the present study showed that 1) rottlerin, an inhibitor of PKCδ, decreased plasma membrane PKCδ and inhibited cAMP-induced activation of Rab4 and NTCP translocation, 2) WT-PKCδ, DN-PKCδ, and siRNA PKCδ increased plasma membrane NTCP and activated Rab4, and these effects were associated with increased plasma membrane PKCδ, 3) cAMP-induced translocation of PKCδ was inhibited by LY294002, and 4) DN-PKCδ and siRNA PKCδ decreased cAMP-induced MRP2 translocation. On the basis of these results we propose that membrane localization instead of kinase activity of PKCδ is involved in cAMP-induced Rab4 activation and NTCP translocation and that cAMP-induced MRP2 translocation is dependent on PKCδ kinase activity.
The present study revealed both kinase-dependent and independent effects of PKCδ in the regulation of NTCP and MRP2 translocation. Rottlerin inhibits the kinase activity of PKCδ by competing with ATP binding and it has been used to investigate the role of PKCδ in many studies (16). Thus the inhibition of cAMP-induced activation of Rab4 and NTCP translocation by rottlerin would have suggested that the kinase activity of PKCδ is required for these effects. Since rottlerin did not affect cAMP-induced activation of Akt, it is unlikely that the effect of rottlerin was mediated via inhibition of PI3K pathway (33, 41). However, kinase-dead DN-PKCδ failed to inhibit cAMP-induced activation of Rab4 and NTCP translocation. Moreover, DN-PKCδ alone activated Rab4 and induced NTCP translocation. These results would argue against a role for kinase activity of PKCδ. Instead, results of the present study suggest a role for plasma membrane localization of PKCδ for activation of Rab4 and NTCP translocation as discussed below.
Both rottlerin and DN-PKCδ have been shown to inhibit the kinase activity of PKCδ (16, 36). However, the effect on plasma membrane PKCδ was different; rottlerin decreased cAMP-induced increases in plasma membrane PKCδ, whereas there was an increase in plasma membrane PKCδ in cells transfected with DN-PKCδ. Moreover, despite a decrease in total PKCδ in cells transfected with siRNA PKCδ, there was an increase in plasma membrane PKCδ associated with an increase in plasma membrane NTCP and Rab4 activation. In addition, the level of plasma membrane NTCP increased with increasing level of plasma membrane PKCδ before reaching a plateau. Taken together, these results would suggest that plasma membrane localization instead of kinase activity of PKCδ is involved in Rab4 activation and NTCP translocation.
Recent studies suggest that PKC-mediated effects do not always require the kinase activity. Goerke et al. (12) showed that kinase inactive PKCδ (PKCδ-K376R) mimicked the effect of WT-PKCδ and induced apoptosis in vascular smooth muscle cells and that PKCδ translocation preceded apoptosis in all experiments. In addition, Oka et al. (28) reported that the introduction of PKCδ and kinase-dead PKCδ (PKCδ-K376R) showed identical effects on phospholipase D (PLD) activity and suppressed PKCα-mediated activation of PLD1. A study in cardiomyocytes showed that the catalytic activation of PKCδ is not required for the H2O2-induced physical interaction of PKCδ with Shc (17). Results of the present study in hepatic cell line are consistent with the general finding in other cells and support the conclusion that the effect of PKCδ on Rab4 activation and NTCP translocation is independent of kinase activity. Although translocation to membranes has been considered to indicate activation of PKCs, recent studies suggest that PKC activity and membrane translocation are not interdependent. For example, kinase inactive PKCα still translocates to the membrane in response to phorbol ester (27) and DN-PKCδ was found to be exclusively localized in the membrane fraction in 32D cells even prior to TPA stimulation (20). In the present study, the amount of PKCδ in the plasma membrane in cells transfected with DN-PKCδ was increased as much as in cells transfected with EV and treated with cAMP (Fig. 6). Thus it would seem likely that localization in the plasma membrane rather than kinase activity of PKCδ plays an important role in Rab4 activation and NTCP translocation.
The mechanism by which plasma membrane localization of PKCδ may lead to activation of Rab4 and NTCP translocation is speculative at this point. However, it can be suggested that PKCδ might exert its kinase-independent effect by binding to membrane proteins involved in Rab4 activation and NTCP retention, although identity of such binding proteins is yet to be determined. There is evidence that PKCδ might act as a signal-regulated scaffold. Oka et al. (28) showed that PKCδ suppresses PLD1 activation through the protein-protein interaction with PLD1. Additionally, Guo et al. (17) showed that overexpression of kinase-dead PKCδ increased H2O2-induced physical interaction of PKCδ with Shc. Whether PKCδ-mediated NTCP translocation requires binding to NTCP, Rab4, or other proteins in the membrane remains to be established.
In a previous study, we reported that cAMP-induced phosphorylation of PKCδ at Thr505 is dependent on PI3K (33). We speculated that cAMP-induced NTCP translocation may be mediated via cAMP-induced phosphorylation/activation of PKCδ. The present study showed that cAMP-induced PKCδ translocation is also dependent on PI3K. Since activation precedes translocation of novel PKCs from cytosol to membranes (7), it would appear that activation/phosphorylation as well as translocation of PKCδ is PI3K dependent. It should be noted that translocation may precede activation of PKCδ in pancreatic acinar cells (37). In that case, inhibition of cAMP-dependent phosphorylation/activation of PKCδ by PI3K inhibitor may be secondary to inhibition of cAMP-induced PKCδ translocation.
Our previous studies showed that cAMP activates PKCζ in a PI3K-dependent manner without inducing translocation of PKCζ to the plasma membrane in rat hepatocytes (24) and that PKCζ is required specifically for the intracellular movement of vesicles that contain the Ntcp (31). The present study suggest that cAMP-induced translocation of NTCP may involve membrane localization rather than kinase activity of PKCδ. Thus it would appear that cAMP-induced NTCP translocation may involve multiple pathways, one mediated via PKCδ and another via PKCζ. It is unknown whether these two pathways converge on a common mediator. One possibility may be that the common mediator is Rab4. However, whether Rab4 is involved in PKCζ-mediated NTCP translocation has not been established. Further studies will be needed to clarify the pathways used by these kinases.
The present study showed that rottlerin inhibited cAMP-induced GTP binding of Rab4. In a previous study we reported that cAMP increases Rab GTP binding in a time-dependent manner (34). Since these effects were observed in a GTP overlay assay, it is likely that Rab4 has undergone posttranslational modification in cells treated with rottlerin and cAMP. Although Rab-GTPases undergo posttranslational modifications that affect membrane association of Rab proteins (29), studies addressing posttranslational modification resulting in changes in GTP binding are limited. One study reported that PKC-mediated phosphorylation of Rab6 results in higher affinity for GTP than for GDP (9). Rab4 is phosphorylated by p34 (cdc2) kinase in CHO cells (11, 39) and HeLa cells (2). However, this phosphorylation did not affect GTP binding affinity of Rab4 (2). Interestingly, increased phosphorylation of Rab4 in mitosis is associated with increased amount of Rab4-GTP in cytoplasm, raising the possibility that phosphorylation may increase GTP binding of Rab4 (11). Thus the possibility that Rab4 phosphorylation may result in altered GTP binding to Rab4 in hepatic cells cannot be ruled out. It has also been suggested that transamidation of Rab4 with 5-hydroxytryptamine by transglutaminase stabilizes Rab4 in its active GTP-bound form by blocking Rab4-GTPase activity in platelets (40). Since cAMP increases intracellular calcium (15) and activation of transglutaminases requires calcium (13), it is possible that Rab4 GTP binding may be affected by transamidation in hepatic cells. Further studies are needed to determine whether phosphorylation and/or transamidation is involved in cAMP/rottlerin-induced changes in GTP binding to Rab4.
In contrast to the effect of PKCδ on Rab4 activation and NTCP translocation, the effect of PKCδ on cAMP-induced MRP2 translocation is clearly dependent on the kinase activity. Our previous studies have shown that rottlerin inhibits cAMP-induced MRP2 translocation in hepatocytes (33). In the present study, DN-PKCδ inhibited cAMP-induced MRP2 translocation, indicating requirement for kinase activity of PKCδ. Moreover, knockdown of PKCδ with siRNA also decreased cAMP-induced MRP2 translocation further supporting a role for PKCδ. Since these effects of DN-PKCδ and siRNA were observed despite an increase in plasma membrane PKCδ, it is unlikely that plasma membrane localization of PKCδ plays an independent role in cAMP-induced MRP2 translocation.
In summary, the present study showed for the first time that kinase-dependent and -independent effect of PKCδ are involved in cAMP-induced activation of MRP2 translocation and Rab4 activation/NTCP translocation, respectively.
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK33436 and DK90010 (M. S. Anwer) and DK65975 (C. R. L. Webster).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W.P. and M.S.A. conception and design of research; S.W.P. performed experiments; S.W.P. analyzed data; S.W.P., C.M.S., C.R.W., and M.S.A. interpreted results of experiments; S.W.P. prepared figures; S.W.P. drafted manuscript; S.W.P., C.M.S., C.R.W., and M.S.A. edited and revised manuscript; M.S.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Holly Jameson and Ariel Hobson for excellent technical assistance.
REFERENCES
- 1. Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology 39: 581–590, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bailly E, McCaffrey M, Touchot N, Zahraoui A, Goud B, Bornens M. Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature 350: 715–718, 1991 [DOI] [PubMed] [Google Scholar]
- 3. Alvi F, Idkowiak-Baldys J, Baldys A, Raymond JR, Hannun YA. Regulation of membrane trafficking and endocytosis by protein kinase C: emerging role of the pericentrion, a novel protein kinase C-dependent subset of recycling endosomes. Cell Mol Life Sci 64: 263–270, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Awayda MS. Specific and nonspecific effects of protein kinase C on the epithelial Na (+) channel. J Gen Physiol 115: 559–570, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker KP, Hannun YA. cPKC-dependent sequestration of membrane-recycling components in a subset of recycling endosomes. J Biol Chem 278: 52747–52754, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Doucet JP, Tuana BS. Identification of low molecular weight GTP-binding proteins and their istes of interaction in subcellular fractions from skeletal muscle. J Biol Chem 266: 17613–17620, 1991 [PubMed] [Google Scholar]
- 7. Duquesnes N, Lezoualc'h F, Crozatier B. PKC-delta and PKC-epsilon: foes of the same family or strangers? J Mol Cell Cardiol 51: 665, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Farese RV, Sajan MP, Standaert ML. Atypical protein kinase C in insulin action and insulin resistance. Biochem Soc Trans 33: 350–353, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Fitzerald ML, Guy L, Reed GL. Rab6 is phosphorylated in thrombin-activated platelets by a protein kinase C-dependent mechanism: effects on GTP/GDP binding and cellular distribution. Biochem J 342: 353–360, 1999 [PMC free article] [PubMed] [Google Scholar]
- 10. Formisano P, Oriente F, Fiory F, Caruso M, Miele C, Maitan MA, Andreozzi F, Vigliotta G, Condorelli G, Beguinot F. Insulin-activated protein kinase Cβ bypasses Ras and stimulates mitogen-activated protein kinase activity and cell proliferation in muscle cells. Mol Cell Biol 20: 6323–6333, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerez L, Mohrmann K, van Raak M, Jongeneelen M, Zhou XZ, Lu KP, van der Sluijs P. Accumulation of rab4GTP in the cytoplasm and association with the peptidyl-prolyl isomerase Pin1 during mitosis. Mol Biol Cell 11: 2201–2211, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goerke A, Sakai N, Gutjahr E, Schlapkohl WA, Mushinski JF, Haller H, Kolch W, Saito N, Mischak H. Induction of apoptosis by protein kinase C delta is independent of its kinase activity. J Biol Chem 277: 32054–32062, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J 368: 377–396, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gromov PS, Celis JE. Several small GTP-binding proteins are strongly down-regulated in simian virus 40 (SV40) transformed human keratinocytes and may be required for the maintenance of the normal phenotype. Eletrophoresis 15: 474–481, 1994 [DOI] [PubMed] [Google Scholar]
- 15. Grüne S, Engelking LR, Anwer MS. Role of intracellular calcium and protein kinase C in the activation of hepatic Na+/taurocholate cotransport by cyclic AMP. J Biol Chem 268: 17734–17741, 1993 [PubMed] [Google Scholar]
- 16. Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199: 93–98, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Guo J, Cong L, Rybin VO, Gertsberg Z, Steinberg SF. Protein kinase C-δ regulates that subcellular localization of Shc in H2O2-treated cardiomyocytes. Am J Physiol Cell Physiol 299: C770–C778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Imamura T, Huang J, Usui I, Satoh H, Bever J, Olefsky JM. Insulin-induced GLUT4 translocation involves protein kinase C-λ-mediated functional coupling between Rab4 and the motor protein kinesin. Mol Cell Biol 23: 4892–4900, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kayali AG, Austin DA, Webster NJ. Rottlerin inhibits insulin-stimulated glucose transport in 3T3–L1 adipocytes by uncoupling mitochondrial oxidative phosphorylation. Endocrinology 143: 3884–3896, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Li W, Yu JC, Shin DY, Piece JH. Characterization of a PKCδ ATP binding mutant. J Biol Chem 270: 8311–8318, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem 278: 22168–22174, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lowry DH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem 193: 265–275, 1951 [PubMed] [Google Scholar]
- 23. McCaffrey MW, Bielli A, Cantalupo G, Mora S, Roberti V, Santillo M, Drummond F, Bucci C. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett 495: 21–30, 2001 [DOI] [PubMed] [Google Scholar]
- 24. McConkey M, Gillin H, Webster CRL, Anwer MS. Cross-talk between protein kinases Cζ and B in cyclic AMP-mediated sodium taurocholate co-transporting polypeptide translocation in hepatocytes. J Biol Chem 279: 20882–20888, 2004 [DOI] [PubMed] [Google Scholar]
- 25. McGrath JP, Capon DJ, Goeddel DV, Levinson AD. Comparative biochemical properties of normal and activated human ras p21 protein. Nature 310: 644–649, 1984 [DOI] [PubMed] [Google Scholar]
- 26. Mukhopadhayay S, Ananthanarayanan M, Stieger B, Meier PJ, Suchy FJ, Anwer MS. cAMP increases liver Na+-taurocholate cotransport by translocating transporter to plasma membranes. Am J Physiol Gastrointest Liver Physiol 273: G842–G848, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Ohno S, Konno Y, Akita Y, Yno A, Suzuki K. A point mutation at the putative ATP-binding site of protein kinase C alpha abolishes the kinase activity and renders it down-regulation-insensitive. A molecular link between autophosphorylation and down-regulation. J Biol Chem 265: 6296–6300, 1990 [PubMed] [Google Scholar]
- 28. Oka M, Okada T, Nakamura S, Ohba M, Kuroki T, Kikkawa U, Nagai H, Ichihashi M, Nishigori C. PKCδ inhibits PKCα-mediated activation of phospholipase D1 in a manner independent of its protein kinase activity. FEBS Lett 554: 179–184, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Pylypenko O, Goud B. Posttranslational modifications of Rab GTPases help their insertion into membranes. Proc Natl Acad Sci USA 109: 5555–5556, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roelofsen H, Soroka CJ, Keppler D, Boyer JL. Cyclic AMP stimulates sorting of the canalicular organic anion transporter (Mrp2/cMoat) to the apical domain in hepatocyte couplets. J Cell Sci 111: 1137–1145, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Sarkar S, Bananis E, Nath S, Anwer MS, Wolkoff AW, Murray JW. PKCζ Is required for microtubule-based motility of vesicles containing the ntcp transporter. Traffic 7: 1078–1091, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Saxena S, Singh M, Shibata H, Kaur S, George C. Rab4 GTP/GDP modulates amiloride-sensitive sodium channel (EnaC) function in colonic epithelia. Biochem Biophys Res Commun 340: 726–733, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Schonhoff CM, Gillin H, Webster CRL, Anwer MS. Protein kinase Cδ mediates cyclic adenosine monophosphate- stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes. Hepatology 47: 1309–1316, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Schonhoff CM, Thankey K, Webster CRL, Wakabayashi Y, Wolkoff AW, Anwer MS. Rab4 facilitates cyclic adenosine monophosphate stimulated bile acid uptake and Na+-taurocholate cotrnasporting polypeptide translocation. Hepatology 48: 1665–1670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slotoff SP. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly block protein kinase C delta tyrosine phosphorylation. J Biol Chem 276: 37986–37992, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Soh JW, Lee EH, Prywes R, Weinstein IB. Novel roles of specific isoforms of protein kinase C in activation of the c-fos serum response element. Mol Cell Biol 19: 1313–1324, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tapia JA, Garcia-Marin LJ, Jensen RT. Cholecystokinin-stimulated protein kinase C δ kinase activation, tyrosine phosphorylation, and translocation are mediated by Src tyrosine kinases in pancreatic acinar cells. J Biol Chem 278: 35220–35230, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83: 633–671, 2003 [DOI] [PubMed] [Google Scholar]
- 39. van der Sluijs P, Hull M, Huber LA, Male P, Goud B, Mellman I. Reversible phosphorylation-dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J 11: 4379–89, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walther DJ, Peter JU, Winter S, ltje MH, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, Bader M. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet α-granule release. Cell 115: 851–862, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem 277: 28578–83, 2002 [DOI] [PubMed] [Google Scholar]