Abstract
One way to link chronic inflammation with cancer is through the intrinsic inflammatory pathway, in which genetic alterations that induce malignant transformation also produce a cancer-promoting, inflammatory microenvironment. Signal transducer and activator of transcription 3 (STAT3) contributes to the intrinsic inflammatory pathway in Barrett's esophagus. In human tumors, honokiol (a polyphenol in herbal teas) has growth-inhibitory and proapoptotic effects associated with suppressed activation of STAT3. We used human Barrett's epithelial and esophageal adenocarcinoma cell lines to determine effects of honokiol on cell number, necrosis, apoptosis, and anchorage-independent growth and to explore STAT3's role in those effects. We determined Ras activity and expression of phosphorylated ERK1/2, phosphorylated Akt, and phosphorylated STAT3 in the presence or absence of honokiol. Cells were infected with constitutively active Stat3-C to assess effects of honokiol-induced STAT3 inhibition on apoptosis. Honokiol decreased cell number and increased necrosis and apoptosis in transformed Barrett's cells, but not in nontransformed cells. In adenocarcinoma cells, honokiol also increased necrosis and apoptosis and decreased anchorage-independent growth. Within 30 min of honokiol treatment, transformed Barrett's cells decreased expression of phosphorylated STAT3; decreases in Ras activity and phosphorylated ERK1/2 expression were detected at 24 h. Infection with Stat3-C significantly reduced apoptosis after honokiol treatment. Honokiol causes necrosis and apoptosis in transformed Barrett's and esophageal adenocarcinoma cells, but not in nontransformed Barrett's cells, and the proapoptotic effects of honokiol are mediated by its inhibition of STAT3 signaling. These findings suggest a potential role for targeting the intrinsic inflammatory pathways as a therapeutic strategy to prevent Barrett's carcinogenesis.
Keywords: Barrett's esophagus, esophageal adenocarcinoma, Ras, Akt, Stat3-C
despite a decline in the overall incidence of cancer in the United States in recent years, the incidence of esophageal adenocarcinoma continues to rise at an alarming rate (14). The major risk factors for esophageal adenocarcinoma are gastroesophageal reflux disease (GERD) and its sequela Barrett's esophagus, the condition in which the normal esophageal squamous epithelium is replaced by a metaplastic columnar epithelium that is predisposed to malignancy (24). GERD causes reflux esophagitis, a chronic inflammatory condition of the esophageal mucosa, that is thought to play a role in the development and neoplastic progression of Barrett's esophagus.
Chronic inflammation can be linked to carcinogenesis through two pathways: 1) the extrinsic pathway, in which nonneoplastic clinical conditions cause local tissue inflammation that promotes cancer development, and 2) the intrinsic pathway, in which precancerous cells acquire a series of growth-promoting genetic abnormalities, some of which also induce an inflammatory microenvironment that contributes to cancer development (21). GERD is widely regarded as the extrinsic inflammatory pathway for promoting carcinogenesis in Barrett's metaplasia. However, little is known about the role of the intrinsic inflammatory pathway in Barrett's esophagus. We recently reported activation of the IL-6/STAT3 pathway, which contributes to the intrinsic inflammatory pathway, during the in vitro malignant transformation of benign Barrett's epithelial cells (26). Moreover, activation of the IL-6/STAT3 signaling pathway in transformed Barrett's cells enabled them to resist apoptosis induced by bile acid exposure, suggesting that molecules of the intrinsic inflammatory pathway might play an important role in Barrett's carcinogenesis (26).
There has been intense interest in the salutary effects of dietary polyphenols, which have a number of anti-inflammatory, antioxidative, and anticarcinogenic properties (16). In epidemiological studies, diets rich in polyphenols have been linked with lower risks of cancer development, and polyphenols have been proposed as potential chemopreventive and chemotherapuetic agents (16). Honokiol, a polyphenol found in Asian herbal teas, has been shown to have substantial antitumor effects (8). In a number of solid-tumor cell lines (e.g., head and neck, breast, lung, colon, stomach), honokiol has been found to have growth-inhibitory actions and to induce apoptosis (reviewed in Ref. 8). Honokiol also has been shown to inhibit tumor growth in animal xenograft models (4, 10). In other animal studies, oral administration of honokiol has resulted in plasma concentration levels exceeding those that produced antitumor effects in vitro, a finding that supports the potential clinical use of honokiol as a therapeutic agent (4).
Honokiol appears to exert antitumor effects through two different pathways that are influenced by the p53 status of the neoplasm (8). In tumors with wild-type p53, honokiol causes mitochondrial dysfunction with the generation of reactive oxygen species and cell death due to necrosis (8). In tumors with defective p53 function, in contrast, honokiol induces apoptosis through mechanisms that are not well established (8). Moreover, the antitumor effects of honokiol appear to be more pronounced in cells that have Ras activation in addition to p53 defects, and in these cells, honokiol has been found to target a number of major cell survival signaling pathways, including STAT3 (8, 9, 18). Abnormalities in p53 and activation of Ras and STAT3 are found frequently as metaplastic Barrett's mucosa progresses to esophageal adenocarcinoma (1, 7, 20, 23). These data suggest that honokiol might be especially useful as a chemopreventive or chemotherapeutic agent for patients with Barrett's esophagus and cancer.
Treatment with acid-suppressing medications, such as proton pump inhibitors to control GERD, the extrinsic inflammatory pathway, has been recommended as a chemoprevention strategy for patients with Barrett's esophagus (25). Since little is known about the intrinsic inflammatory pathway in Barrett's esophagus, there are no clinical strategies aimed at this potentially important therapeutic target. Using our series of nontransformed and transformed Barrett's epithelial cell lines (generated by knockdown of p53 and expression of oncogenic H-Ras) and esophageal adenocarcinoma cell lines, we studied the effects of honokiol on cell growth, necrosis, apoptosis, and anchorage-independent growth and explored the contribution of the STAT3 pathway in mediating those effects.
METHODS
Cell culture.
In earlier reports, we described the development of a series of transformed and nontransformed Barrett's epithelial cell lines (13, 27). Briefly, we started with a nontransformed, telomerase-immortalized, p16-deficient cell line (BAR-T) that we established from biopsy specimens of nondysplastic Barrett's metaplasia. To knock down p53, we infected BAR-T cells with the retroviral vector pSUPER-RNAi-p53 (OligoEngine, Seattle, WA). To express human oncogenic H-Ras, we infected BAR-T cells with the retroviral vector pBabe-H-RasG12V (obtained from Dr. Robert Weinberg, Whitehead Institute, Cambridge, MA) (19, 27). The BAR-T cells containing both pSUPER-p53RNAi and pBabe-H-RasG12V were transformed (27). The nontransformed BAR-T cells were cocultured with a fibroblast feeder layer, as previously described (13, 27); the transformed BAR-T p53RNAi H-RasG12V-expressing cell lines (clones R1 and R2) did not require a fibroblast feeder layer. The OE33 esophageal adenocarcinoma cell line was purchased from Sigma; the JH-EsoAd1 esophageal adenocarcionoma cell line was a generous gift of Drs. James Eshleman and Anirban Maitra (Johns Hopkins University) (2). All cell lines were maintained in monolayer culture at 37°C in humidified air with 5% CO2 in growth medium, as previously described (2, 6, 27). For individual experiments, the nontransformed cell line was equally seeded into collagen IV-coated wells (BD Biosciences, San Jose, CA), whereas transformed and esophageal adenocarcinoma cell lines were equally seeded onto standard culture dishes. Cellular morphology was documented using the MetaMorph imaging system (Universal Imaging, Downingtown, PA).
Honokiol treatment.
Honokiol was dissolved in ethanol as a 50 mM stock solution, which was diluted to the desired concentration using cell culture medium. For individual experiments, cells were treated with honokiol for 24–48 h. Cells treated with vehicle only (ethanol) served as controls. To optimize activation of oncogenic H-RasG12V, cells were cultured in reduced-serum (0.5%) medium lacking additional growth factors for all experiments (9). In preliminary experiments, we treated nontransformed BAR-T cells with 10, 20, 40, and 80 μM honokiol for 24 and 48 h and found that 40 and 80 μM honokiol significantly decreased cell viability. Therefore, we selected to use <40 μM honokiol for all further experiments.
Stat3-C expression vector.
We obtained a murine Stat3-C plasmid [James E. Darnell, Jr., Rockefeller University (plasmid 8722, Addgene, Cambridge, MA)] containing substitutions in cysteine residues (A661 and N663 of Stat3) that enable the protein to dimerize spontaneously [in the absence of tyrosine phosphorylation (Y705)] (3). As a result of this spontaneous dimerization, Stat3-C can constitutively bind to DNA and enhance transcription; nevertheless, the DNA binding and transcriptional activity of this “constitutively active” construct can be further increased by tyrosine phosphorylation (Y705) (3). Stat3-C cDNA was PCR-amplified from the plasmid to incorporate new EcoRI and SalI cloning sites using the primer sequences Stat3-C-EcoRI (forward) 5′-AAA GAA TTC ATG GCT CAG TGG AAC CAG CTG CA and Stat3-C-SalI (reverse) 5′-AAA GTC GAC TCA CAT GGG GGA GGT AGC ACA CT. PCR was performed using the iCycler (Bio-Rad, Hercules, CA). PCR conditions consisted of 95°C for 5 min followed by 42 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 2.5 min. PCR products were electrophoresed on 1.5% agarose gels and stained with ethidium bromide. The cDNA was purified using a QIAquick gel extraction kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The Stat3-C cDNA fragment was inserted into the pCR2.1-TOPO vector (Invitrogen, Carsbad, CA) and then digested with EcoRI/SalI (Roche, Indianapolis, IN) and cloned into the EcoRI/SalI cloning site of the pWZL-hygromycin B retroviral mammalian expression vector (obtained from Dr. Scott Lowe, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) to generate the retroviral vector pWZL-Stat3-C-hygromycin B; the retroviral vector pWZL-hygromycin B without the insert served as a control. The pWZL-Stat3C-hygromycin B vector and control were transformed into competent bacterial cells (Subcloning Efficiency DH5a, Invitrogen) according to the manufacturer's instructions. Ampicillin-resistant colonies were selected, and the plasmid was isolated. The presence of the insert was confirmed by EcoRI and SalI digestion and DNA sequencing. Retroviral particles were generated as previously described (13). The transformed BAR-T p53RNAi H-RasG12V-expressing clone R1 cells were infected at ∼50% confluence in the presence of Polybrene (4 mg/ml; Sigma, St. Louis, MO) for 10–12 h. After recovery for 72 h, cells were selected in hygromycin B (80 μg/ml) for 10 days. Thus we generated transformed BAR-T p53RNAi H-RasG12V-expressing cells containing pWZL-vector or pWZL-Stat3-C.
Cell proliferation, apoptosis, necrosis, and anchorage-independent growth.
Equally seeded wells of cells were cultured in full growth medium for 24 h; then full growth medium was replaced with reduced-serum (0.5% or 1%) medium lacking growth factors. Honokiol (0–20 μM) was added to the reduced-serum medium for 24 and 48 h. Cells were trypsinized, and cell numbers were determined using a Z1 particle counter (Beckman Coulter, Fullerton, CA). Apoptosis rates were assessed qualitatively by optic morphology and by terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL) using the TUNEL Apoptosis Detection Kit (Millipore, Billerica, MA) and quantitatively using Cell Death Detection ELISAPLUS assay (Roche Applied Science, Indianapolis, IN) according to the manufacturers' instructions (12). For TUNEL staining, cells were cultured in a four-well chamber slide (Fisher Scientific, Waltham, MA) with and without 20 μM honokiol in 0.5% or 1% fetal bovine serum and KBM-2 or RPMI 1640 low-serum medium containing 10 μM CaCl2 for 24 h. Cells were then fixed with 4% paraformaldehyde for 15 min, washed, and incubated with 50 μl of TdT end-label cocktail (containing TdT and biotin-dUTP) per well for 1 h at room temperature. After incubation, avidin-FITC solution (50 μl) was added to the wells for 30 min. Cell were washed, counterstained with 4′,6-diamidino-2-phenylindole, and analyzed by a confocal microscope (model TCS SP5, Leica Microsystem, Buffalo Grove, IL). For necrosis determinations, the Cell Death Detection ELISAPLUS assay was used according to the manufacturer's instructions. Using monoclonal antibodies against DNA and histones, this assay detects mono- and oligonucleosomes in the cell cytoplasm as an index of apoptosis and in the cell culture supernatants as an index of cell necrosis. Apoptosis and necrosis rates were expressed as a percentage of those in vehicle-only-containing control cells. All analyses were performed in duplicate. Anchorage-independent growth was assessed for OE33 cells. Briefly, 3 × 103 cells were plated in triplicate in soft agar, as previously described (27). Each well of the cell-agar mixture was treated immediately with 100 μl of control medium or medium containing 20 μM honokiol, which was repleted twice a week for 3 wk. Plates were examined daily for 3 wk. Cells were imaged and colonies were counted using a Bio-Rad Molecular Imager.
Ras activity assays.
Ras is active when bound to GTP, and active Ras binds specifically to the Ras-binding domain (RBD) of Raf1. To perform Ras activity assays, we used an Active Ras Pull-Down kit (Pierce, Rockford, IL) according to the manufacturer's instructions. This assay uses a GST-fusion protein of the Ras-binding domain RBD of Raf1 along with a glutathione agarose resin to specifically pull down active Ras from the protein lysate. For analysis of GTP-bound Ras, equally seeded plates of cells were cultured in growth medium for 24 h; then full growth medium was replaced with reduced-serum (0.5%) medium lacking growth factors. Honokiol (0–20 μM) was added to the reduced-serum medium over a 24-h time course. Immediately after the treatments, cells were washed in cold 1× Tris-buffered saline and then lysed in 0.3–0.5 ml of lysis/binding/wash buffer according to the manufacturer's instructions. Supernatants were collected for protein quantification using the BCA-200 Protein Assay kit (Pierce). Supernatants (containing equal amounts of protein) were incubated at 4°C for 1 h with 80 μg of GST-Raf1-RBD and glutathione resin. Precipitated proteins bound to the resin were washed three times with lysis buffer, eluted in Laemmli sample buffer, and subjected to immunoblot analyses. All analyses were performed in duplicate.
Western blotting.
Cells were washed in cold PBS and then lysed in 1× cell lysis buffer (Cell Signaling Technology, Beverly, MA). Protein concentrations were determined using the BCA-200 Protein Assay kit (Pierce); equal amounts of protein were separated by SDS-PAGE. After separation and transfer to nitrocellulose membranes, the membranes were incubated with primary antibodies (Cell Signaling Technology) to total STAT3 (1:1,000 dilution); phosphorylated STAT3 (Y705, 1:500 dilution), phosphorylated ERK1/2 (1:1,000 dilution), total ERK1/2 (1:1,000 dilution), phosphorylated Akt (1:1,000 dilution), total Akt (1:1,000 dilution), and total H-Ras (1:1,000 dilution; BD Science Technology); Mcl-1 (1:300 dilution; Santa Cruz Biotechnology); and anti-β-tubulin or anti-β-actin (1:5,000 dilution; Sigma-Aldrich). Horseradish peroxidase secondary antibodies were used, and chemiluminescence was determined using the Super Signal West Dura detection system (Pierce); β-tubulin or β-actin was used to confirm equal loading. All Western blots were performed in duplicate.
Statistical analyses.
Quantitative data are means ± SE. Statistical analyses were performed using an unpaired Student's t-test with the Instat for Windows statistical software package (GraphPad Software, San Diego, CA). For multiple comparisons, ANOVA and Student-Newman-Keuls multiple-comparisons test were performed. P ≤ 0.05 was considered significant for all analyses.
RESULTS
Honokiol inhibits growth in transformed Barrett's cells, but not in nontransformed cells with intact p53.
We treated nontransformed BAR-T and transformed BAR-T p53 RNAi/RasG12V cells (clones R1 and R2) with 0–20 μM honokiol and determined effects on cell number at 24 and 48 h. In nontransformed BAR-T cells, 10 and 20 μM honokiol had no significant effects on cell numbers (Fig. 1A). In transformed BAR-T p53 RNAi/RasG12V cells (clones R1 and R2), in contrast, honokiol significantly decreased cell numbers in a dose-dependent manner at 24 h (Fig. 1, B and C). By 48 h, cell numbers had increased in transformed cells treated with 10 μM honokiol, whereas cell numbers continued to decrease significantly in cells treated with 20 μM honokiol (Fig. 1, B and C).
Fig. 1.
Honokiol (HNK) inhibits growth in transformed Barrett's cells, but not in nontransformed cells. Cell counts were performed 24 and 48 h after treatment with 0–20 μM honokiol in nontransformed BAR-T cells (A), which have intact p53 protein and do not express H-Ras, transformed BAR-T p53RNAi/H-RasG12V clone R1 cells (B), and transformed BAR-T p53RNAi/H-RasG12V clone R2 cells (C; R1 and R2 have p53 knockdown and express H-Ras). Values are means ± SE of ≥2 individual experiments. **P < 0.01 and ***P < 0.001 vs. nontreated controls. ++P < 0.01 vs. 10 μM honokiol at 24 h. @P < 0.05 vs. 20 μM honokiol at 24 h.
Honokiol induces necrosis and apoptosis in transformed Barrett's epithelial cells, but not in nontransformed cells with intact p53.
We determined rates of cell necrosis using cell culture supernatants in the Cell Death ELISA from nontransformed BAR-T and transformed Barrett's cells treated with 10 and 20 μM honokiol for 24 h. Neither dose of honokiol had a significant effect on cell necrosis in the nontransformed BAR-T cells (Fig. 2A). In transformed BAR-T p53 RNAi/RasG12V clones R1 and R2, in contrast, 20 μM honokiol significantly increased rates of cell necrosis; 10 μM honokiol had no significant effect on necrosis rates (Fig. 2, B and C).
Fig. 2.
Honokiol induces necrosis in transformed Barrett's cells, but not in nontransformed cells. Necrosis rates were determined 24 h after treatment with 0–20 μM honokiol in nontransformed BAR-T cells (A), transformed BAR-T p53RNAi/H-RasG12V clone R1 cells (B), and transformed BAR-T p53RNAi/H-RasG12V clone R2 cells (C). Values are means ± SE of ≥2 individual experiments. ***P < 0.001 vs. nontreated controls. +++P < 0.001 vs. 10 μM honokiol at 24 h.
Using optic morphology, TUNEL staining, and Cell Death ELISA (performed on cell lysates) in nontransformed BAR-T and transformed Barrett's cells, we also evaluated the effects of honokiol for 24 h on apoptosis. In nontransformed BAR-T cells, we found no difference in morphology or in staining for TUNEL after treatment with 10 or 20 μM honokiol compared with nontreated controls (Fig. 3). In contrast, transformed BAR-T p53 RNAi/RasG12V cells (clones R1 and R2) treated with 20 μM honokiol were small and shrunken, a morphology that suggests an apoptotic phenotype, and they demonstrated marked staining for TUNEL (Fig. 3). Using the Cell Death ELISA, we confirmed that there was no increase in apoptosis with either dose of honokiol in nontransformed BAR-T cells (Fig. 4A). In contrast, transformed BAR-T p53 RNAi/RasG12V cells (clones R1 and R2) treated with 20 μM honokiol exhibited significantly increased rates of apoptosis compared with nontreated control cells and those treated with 10 μM honokiol; treatment with 10 μM honokiol had no significant effects on apoptosis rates in either transformed cell line (Fig. 4, B and C).
Fig. 3.
Honokiol induces features of apoptosis in transformed Barrett's cells, but not in nontransformed cells. Representative experiment showing results of optic morphology and terminal deoxynucleotidyl transferase nick end labeling (TUNEL) staining 24 h after treatment with 0–20 μM honokiol in nontransformed BAR-T cells, transformed BAR-T p53RNAi/H-RasG12V clone R1 cells, and transformed BAR-T p53RNAi/H-RasG12V clone R2 cells. Magnification ×4 for optic morphology and ×20 for TUNEL.
Fig. 4.
Honokiol induces apoptosis in transformed Barrett's cells, but not in nontransformed Barrett's cells. Apoptosis rates were determined 24 h after treatment with 0–20 μM honokiol in nontransformed BAR-T cells (A), transformed BAR-T p53RNAi/H-RasG12V clone R1 cells (B), and transformed BAR-T p53RNAi/H-RasG12V clone R2 cells (C). Values are means ± SE of ≥2 individual experiments. ***P < 0.001 vs. nontreated controls. +++P < 0.001 vs. 10 μM honokiol at 24 h.
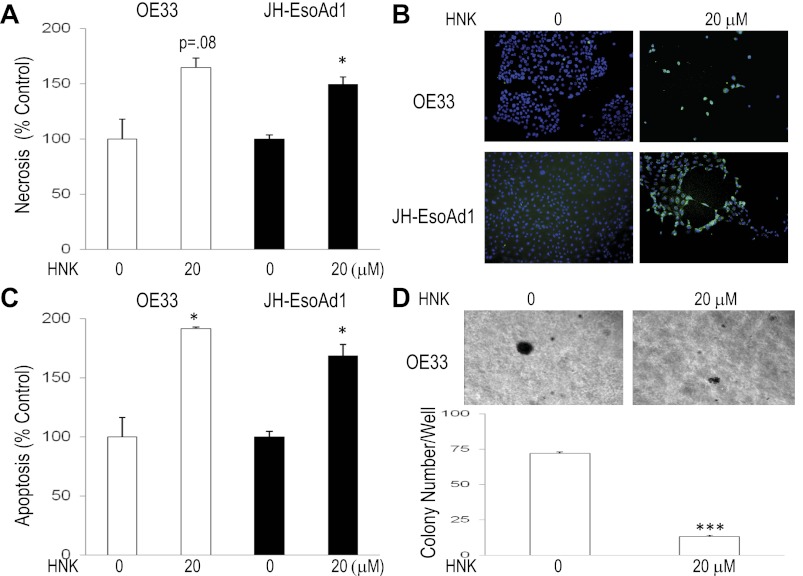
Honokiol induces necrosis and apoptosis and decreases anchorage-independent growth in Barrett's-associated esophageal adenocarcinoma cell lines OE33 and JH-EsoAd1.
We next determined the effect of honokiol on rates of necrosis, apoptosis, and anchorage-independent growth in soft agar in Barrett's-associated esophageal adenocarcinoma cell lines OE33 and JH-EsoAd1. Honokiol (20 μM) significantly increased rates of necrosis in both cancer cell lines (Fig. 5A). Honokiol-treated Barrett's cancer cells also demonstrated marked staining for TUNEL and significantly elevated rates of apoptosis (Fig. 5, B and C). Unlike OE33 cells, JH-EsoAd1 cells did not exhibit growth in soft agar after 3 wk (data not shown). Therefore, effects on anchorage-independent growth could be assessed only in OE33 cells, in which honokiol caused a decrease in colony size and number. This suggests that honokiol limits anchorage-independent growth of Barrett's-associated esophageal adenocarcinoma cells (Fig. 5D).
Fig. 5.
Honokiol induces necrosis and apoptosis and decreases anchorage-independent growth in Barrett's-associated esophageal adenocarcinoma cell lines. A and C: necrosis and apoptosis rates 24 h after treatment with 0 and 20 μM honokiol. Values are means ± SE of ≥2 individual experiments. B: confirmation of honokiol-induced apoptosis by TUNEL staining. Magnification ×10. D: soft agar plates demonstrating decreased colony size and bar graph demonstrating decreased colony number for honokiol-treated OE33 cells after 3 wk of growth in soft agar. Values are means ± SE of ≥3 individual experiments. *P < 0.05 and ***P < 0.001 vs. nontreated controls.
Honokiol inhibits the Ras and STAT3 signaling pathways in transformed Barrett's epithelial cells.
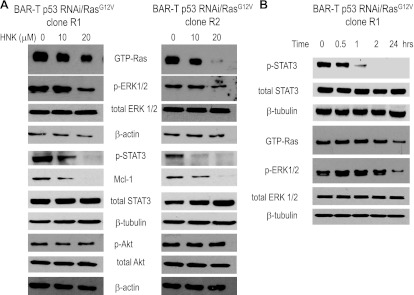
In transformed Barrett's cells, we explored whether honokiol inhibited 1) Ras/ERK signaling (by performing Western blotting for active, GTP-bound Ras and its downstream kinase phosphorylated ERK1/2), 2) JAK/STAT3 signaling (by performing Western blotting for phosphorylated STAT3 and its downstream target survival protein Mcl-1), and 3) phosphoinositide 3-kinase (PI3K)/Akt signaling (by performing Western blotting for phosphorylated Akt). Treatment with 20 μM honokiol markedly decreased expression levels of GTP-bound Ras and phosphorylated ERK1/2 and completely abolished expression of phosphorylated STAT3 and Mcl-1; treatment with 10 μM honokiol had lesser effects on these proteins (Fig. 6A). Neither dose of honokiol affected expression levels of phosphorylated Akt (Fig. 6A). These findings demonstrate that honokiol inhibits Ras and STAT3 signaling, but not PI3K/Akt in transformed Barrett's cells.
Fig. 6.
Honokiol inhibits Ras and STAT3 signaling pathways in transformed Barrett's cell lines. A: representative Western blots 24 h after treatment with 0–20 μM honokiol in BAR-T p53RNAi/RasG12V clones R1 and R2. β-Actin served as a loading control. B: representative Western blots 0–24 h after treatment with 20 μM honokiol in transformed BAR-T p53RNAi/H-RasG12V clone R1 cells. β-Tubulin served as a loading control.
Honokiol rapidly decreases phosphorylation of STAT3 in transformed Barrett's epithelial cells.
Having found that honokiol inhibits Ras and STAT3 signaling in transformed Barrett's cells, we studied a time course for expression levels of GTP-bound Ras, phosphorylated ERK1/2, total STAT3, and phosphorylated STAT3 in BAR-T p53 RNAi+ H-RasG12V+ clone R1 after treatment with honokiol. We found that honokiol slightly decreased expression levels of phosphorylated STAT3 (but not total STAT3) by 30 min, which further decreased by 1 h and were absent from 2 to 24 h (Fig. 6B). Although expression levels of GTP-bound Ras and phosphorylated ERK1/2 were decreased at 24 h, honokiol did not affect levels of either protein at earlier time points (Fig. 6B). These findings show that honokiol rapidly decreases phosphorylation of STAT3, but not Ras activation, in transformed Barrett's cells. Moreover, these data suggest that the STAT3 signaling pathway may be more sensitive to honokiol-mediated inhibition.
Expression of constitutively active Stat3-C attenuates the proapoptotic effects of honokiol in transformed Barrett's epithelial cells.
We previously reported that the STAT3 pathway is active in transformed, but not in nontransformed, Barrett's epithelial cells and that STAT3 activation contributes to their resistance to bile acid-induced apoptosis (26). Having found that STAT3 signaling may be more sensitive to honokiol-mediated inhibition in transformed Barrett's cells, we sought to determine whether STAT3 inhibition contributes to honokiol-induced apoptosis. Transformed Barrett's cells (BAR-T p53 RNAi+ H-RasG12V+ clone R1) were stably infected with the retroviral vector pWZL-Stat3-C, which contains a constitutively active form of STAT3, or with the empty pWZL vector (3). As expected, BAR-T p53 RNAi+ H-RasG12V+ cells infected with the Stat3-C vector showed a marked increase in expression of total and phosphorylated STAT3 (Fig. 7A). Empty vector and Stat3-C-expressing cells were treated with honokiol for 2 h, a time point at which STAT3 inhibition is maximal and Ras signaling is not affected (Fig. 6B), and expression levels of phosphorylated STAT3 and Mcl-1 were determined. Treatment with honokiol decreased expression levels of phosphorylated STAT3 and Mcl-1 in cells containing the empty vector and the Stat3-C vector, but the decreases were much less pronounced in the cells with the Stat3-C vector (Fig. 7B). This suggests that expression of Stat3-C attenuates inhibitory effects of honokiol on the STAT3 signaling pathway. Next, we assessed rates of apoptosis in empty vector and Stat3-C-expressing BAR-T p53RNAi/RasG12V clone R1 cells following treatment with honokiol for 2 h. As in noninfected control cells, honokiol caused a significant increase in apoptosis in BAR-T p53RNAi/RasG12V clone R1 cells containing the empty vector and the Stat3-C vector (Fig. 7C). However, the increase in apoptosis was significantly lower in cells with the Stat3-C vector. These observations suggest that proapoptotic effects of honokiol in transformed Barrett's cells are due in part to inhibition of the STAT3 signaling pathway.
Fig. 7.
Expression of constitutively active STAT3 (Stat3-C) decreases honokiol-induced apoptosis in transformed Barrett's cells. A: representative Western blots for total STAT3 in cells stably infected with empty vector and cells stably infected with Stat3-C. β-Actin served as a loading control. B: representative Western blots for phosphorylated STAT3 and Mcl-1 in empty vector-containing and Stat3-C-expressing cells with (+) and without (−) treatment with 20 μM honokiol for 2 h. C: rates of apoptosis in noninfected control cells, cells stably infected with empty vector, and cells stably infected with Stat3-C at baseline and after treatment with 20 μM honokiol for 2 h. Values are means ± SE from 2 individual experiments. ***P < 0.001 vs. noninfected control cells. +++P < 0.001 vs. honokiol-treated noninfected and empty vector-containing cells.
DISCUSSION
In earlier experiments, we found activation of the IL-6/STAT3 pathway, which is involved in the intrinsic inflammatory pathway, during the in vitro malignant transformation of benign Barrett's epithelial cells (26). We also found that STAT3 signaling contributed to the apoptotic resistance phenotype of transformed Barrett's cells (26). In the present experiments, we used honokiol, a dietary polyphenol that has been shown to have antitumor effects associated with inhibition of STAT3 phosphorylation, to target the intrinsic inflammatory pathway in Barrett's carcinogenesis. We have shown that honokiol induces necrosis and apoptosis in transformed Barrett's epithelial cells and in Barrett's-associated esophageal adenocarcinoma cells, but not in nontransformed Barrett's cells, which have intact p53 protein. In the transformed cells, we found that honokiol decreases STAT3 and Ras pathway signaling and induces apoptosis in part by inhibiting STAT3 signaling.
Chen et al. (5) reported that honokiol, in concentrations and exposure durations similar to those used in the present study, caused mitochondrial dysfunction and extensive cell necrosis in esophageal adenocarcinoma cell lines and in a cell line from a patient with high-grade dysplasia in Barrett's esophagus (CP-C). Using our cell lines with well-characterized genetic abnormalities, we have confirmed the finding of Chen et al. that honokiol causes necrosis in transformed Barrett's cells and in esophageal adenocarcinoma cells. In addition, we found that honokiol did not cause necrosis in benign, nonneoplastic Barrett's epithelial cells. However, Chen et al. focused on honokiol effects on mitochondrial function, reactive oxygen species production, and cell necrosis and did not explore effects on apoptosis, the other major pathway whereby honokiol exerts antitumor activity.
We sought evidence of honokiol-induced apoptosis by optic morphology and TUNEL staining and found that our transformed Barrett's cell lines treated with 20 μM honokiol appeared small and shrunken, a morphology that suggests an apoptotic phenotype, and demonstrated marked staining for TUNEL. We confirmed this finding using the Cell Death ELISA, demonstrating that exposure to 20 μM honokiol for 24 h induces substantial levels of apoptosis in transformed Barrett's cells, but not in benign, nonneoplastic BAR-T cells, which have an intact and functional p53 protein (13). We also explored effects of honokiol on two Barrett's-associated esophageal adenocarcinoma cell lines, OE33 and Jh-EsoAd1, and found that honokiol caused a significant increase in necrosis and apoptosis in both lines. We assessed the effect of honokiol on the anchorage-independent growth of OE33 cells in soft agar, a marker of tumorigenicity. Honokiol treatment decreased colony size and number, suggesting that it may be an antitumorigenic agent for Barrett's-associated esophageal adenocarcinoma. Whether honokiol causes necrosis or apoptosis depends in part on whether the tumor contains wild-type or mutant p53 and whether there is loss of p16 (8). Honokiol-induced cell necrosis has been found to predominate in tumors that maintain wild-type p53 expression but have loss of functional p16, whereas the proapoptotic effect occurs primarily in tumors with mutant p53 and functional p16 (8). In Barrett's metaplasia, inactivation of p16 appears to be the earliest and most common genetic alteration, affecting >80% of patients (17, 20). The limited data available on the order in which genetic alterations accumulate as metaplastic Barrett's esophagus progresses to cancer suggest that inactivation of p53 follows inactivation of p16, and activation of oncogenic Ras occurs later in dysplastic or cancerous Barrett's cells (1, 20, 22, 23). Our benign, nonneoplastic BAR-T cells exhibit inactivation of p16 but maintain an intact and functional p53 pathway (13). Subsequent sequencing of p53 exons 3–8 has identified no mutations in BAR-T cells (R. Souza, unpublished data). However, we did not observe necrosis or apoptosis in BAR-T cells treated with honokiol, suggesting that inactivation of p16 alone does not render cells susceptible to the agent's antitumor effects.
Human cancer cell lines that have p53 defects and activating mutations of Ras appear to be the most susceptible to the proapoptotic effects of honokiol (9). To optimize activation of wild-type and oncogenic H-RasG12V, our experiments were conducted on Barrett's cells cultured in reduced-serum (0.5%) medium, which lacks additional growth factors. In agreement with earlier studies on breast, lung, and bladder carcinoma cell lines, we found that transformed Barrett's epithelial cells that have p53 knockdown and oncogenic Ras overexpression were susceptible to the proapoptotic effects of honokiol (reviewed in Ref. 8).
Honokiol is known to target a number of major cell survival signaling pathways, including Ras/ERK, PI3K/Akt, and JAK/STAT3 (9, 18). We studied honokiol's effects on these pathways in transformed Barrett's cells to explore the mechanism underlying the agent's proapoptotic actions. We found that honokiol markedly decreased signaling via the Ras/ERK and JAK/STAT3 pathways but did not affect the PI3K/Akt pathway. Using time course studies, we found that honokiol caused a reduction in phosphorylated STAT3 expression levels as early as 30 min after treatment, whereas decreased Ras/ERK activity was not detected until 24 h. Our data also suggest that transformed Barrett's cells may be more sensitive to honokiol-mediated inhibition of STAT3 signaling than Ras signaling.
In earlier studies, we found that bile acid exposure did not trigger apoptosis in transformed Barrett's cells unless the STAT3 signaling pathway was inhibited. This suggests that STAT3 is an important mediator of apoptotic resistance in transformed Barrett's cells (26). To demonstrate that honokiol-mediated inhibition of STAT3 phosphorylation is physiologically relevant, we stably infected our transformed cells with Stat3-C, a constitutively active form of STAT3, and determined the ability of honokiol to induce apoptosis. We found that expression of Stat3-C significantly decreased, but did not abolish, honokiol-induced apoptosis. This is likely due to the fact that expression of Stat3-C attenuates, but does not eliminate, the inhibitory effects of honokiol on the STAT3 signaling pathway, as shown in Fig. 6B. However, it is also possible that honokiol has proapoptotic effects in transformed Barrett's cells that are independent of STAT3 inhibition.
Antisecretory agents, such as proton pump inhibitors, have been recommended to control GERD, the extrinsic inflammatory pathway in Barrett's esophagus, as a means to prevent cancer development (25). Our findings suggest that agents targeting molecules of the intrinsic inflammatory pathway (e.g., STAT3) also might be used as a chemopreventive strategy for patients with Barrett's metaplasia and for the treatment of Barrett's-associated esophageal adenocarcinoma. We know of no STAT3 inhibitors that have been approved for clinical use. We have shown that honokiol induces necrosis and apoptosis in transformed Barrett's cells by inhibiting STAT3 signaling. Honokiol is a naturally occurring polyphenol that has a number of highly desirable features for a chemopreventive agent, including oral bioavailability, the absence of side effects, and the lack of toxicity in nonneoplastic cells (8). For patients with Barrett's-associated esophageal adenocarcinoma, furthermore, our finding that honokiol limits anchorage-independent growth in cancer cells suggests that this agent might be a useful cancer treatment. Other studies have shown that honokiol enhances the tumor-killing effects of a number of chemotherapeutic agents, including cisplatin, a drug used frequently in the treatment of esophageal adenocarcinoma (11, 15).
In conclusion, we have shown that honokiol exerts growth-inhibitory effects via the induction of necrosis and apoptosis in transformed Barrett's epithelial cells and Barrett's-associated esophageal adenocarcinoma cells, but not in benign, nontransformed Barrett's epithelial cells that have intact p53. Moreover, we have found that honokiol exerts antitumorigenic effects by limiting anchorage-independent growth of esophageal cancer cells. In transformed Barrett's epithelial cell lines, we have found that honokiol decreases STAT3 and Ras pathway activation. We have also demonstrated that expression of a constitutively active form of STAT3 can attenuate inhibitory effects of honokiol on the STAT3 signaling pathway and significantly decrease the rates of honokiol-induced apoptosis. These findings suggest that molecules of the intrinsic inflammatory pathway may be useful therapeutic targets for preventing cancer in Barrett's esophagus. Moreover, our data have elucidated molecular pathways by which honokiol exerts proapoptotic effects in transformed Barrett's epithelial cells and suggest that honokiol may have a potential role as an antitumorigenic agent for patients with Barrett's-associated esophageal adenocarcinoma.
GRANTS
This work was supported by the Office of Medical Research, Department of Veterans Affairs (D. H. Wang, R. F. Souza, and S. J. Spechler); National Institutes of Health Grants R01-DK-63621 (R. F. Souza), R01-CA-134571 (R. F. Souza and S. J. Spechler), RO1-AR-47901 (J. L. Arbiser), T32 DK-007745-14 (E. Cheng), and K12 HD-068369-01 (E. Cheng); the Margolis Foundation (J. L. Arbiser); the Jamie Rabinowitch-Davis Foundation (J. L. Arbiser); and an American Gastroenterological Association Institute Fellow-to-Faculty Transition Award (E. Cheng).
DISCLOSURES
J. L. Arbiser is listed as the inventor for a patent filed by Emory University on honokiol and honokiol derivatives. Emory University has also licensed intraperitoneal honokiol to Naturopathic Pharmacies. The remaining authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
C.Y., Q.Z., H.Y.Z., E.C., D.H.W., J.L.A., S.J.S., and R.F.S. are responsible for conception and design of the research; C.Y., Q.Z., H.Y.Z., X.Z., and X.H. performed the experiments; C.Y., Q.Z., H.Y.Z., X.Z., X.H., J.L.A., and R.F.S. analyzed the data; C.Y., Q.Z., H.Y.Z., X.Z., X.H., E.C., D.H.W., J.L.A., S.J.S., and R.F.S. interpreted the results of the experiments; C.Y. and R.F.S. drafted the manuscript; C.Y., S.J.S., and R.F.S. edited and revised the manuscript; C.Y., Q.Z., H.Y.Z., X.Z., X.H., E.C., D.H.W., J.L.A., S.J.S., and R.F.S. approved the final version of the manuscript; R.F.S. prepared the figures.
REFERENCES
- 1. Abdelatif OM, Chandler FW, Mills LR, McGuire BS, Pantazis CG, Barrett JM. Differential expression of c-myc and H-ras oncogenes in Barrett's epithelium. A study using colorimetric in situ hybridization. Arch Pathol Lab Med 115: 880–885, 1991 [PubMed] [Google Scholar]
- 2. Alvarez H, Koorstra JB, Hong SM, Boonstra JJ, Dinjens WN, Foratiere AA, Wu TT, Montgomery E, Eshleman JR, Maitra A. Establishment and characterization of a bona fide Barrett esophagus-associated adenocarcinoma cell line. Cancer Biol Ther 7: 1753–1755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell 98: 295–303, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Chen F, Wang T, Wu YF, Gu Y, Xu XL, Zheng S, Hu X. Honokiol: a potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol 10: 3459–3463, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen G, Izzo J, Demizu Y, Wang F, Guha S, Wu X, Hung MC, Ajani JA, Huang P. Different redox states in malignant and nonmalignant esophageal epithelial cells and differential cytotoxic responses to bile acid and honokiol. Antioxid Redox Signal 11: 1083–1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dvorak K, Chavarria M, Payne CM, Ramsey L, Crowley-Weber C, Dvorakova B, Dvorak B, Bernstein H, Holubec H, Sampliner RE, Bernstein C, Prasad A, Green SB, Garewal H. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to Barrett's esophagus. Clin Cancer Res 13: 5305–5313, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid Redox Signal 11: 1139–1148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia A, Zheng Y, Zhao C, Toschi A, Fan J, Shraibman N, Brown HA, Bar-Sagi D, Foster DA, Arbiser JL. Honokiol suppresses survival signals mediated by Ras-dependent phospholipase D activity in human cancer cells. Clin Cancer Res 14: 4267–4274, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of oriental medicinal herb Magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res 14: 1248–1257, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Herskovic A, Martz K, Al Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 326: 1593–1598, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Huo X, Juergens S, Zhang X, Rezaei D, Yu C, Strauch ED, Wang JY, Cheng E, Meyer F, Wang DH, Zhang Q, Spechler SJ, Souza RF. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-κB activation in benign Barrett's epithelial cells. Am J Physiol Gastrointest Liver Physiol 301: G278–G286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, Hormi-Carver K, Shen Y, Elder F, Ramirez RD, Sarosi GA, Jr, Spechler SJ, Souza RF. Characterization of telomerase-immortalized, non-neoplastic, human Barrett's cell line (BAR-T). Dis Esophagus 20: 256–264, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100: 1672–1694, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang QQ, Fan LY, Yang GL, Guo WH, Hou WL, Chen LJ, Wei YQ. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer 8: 242, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang NJ, Shin SH, Lee HJ, Lee KW. Polyphenols as small molecular inhibitors of signaling cascades in carcinogenesis. Pharmacol Ther 130: 310–324, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Klump B, Hsieh CJ, Holzmann K, Gregor M, Porschen R. Hypermethylation of the CDKN2/p16 promoter during neoplastic progression in Barrett's esophagus. Gastroenterology 115: 1381–1386, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Leeman-Neill RJ, Cai Q, Joyce SC, Thomas SM, Bhola NE, Neill DB, Arbiser JL, Grandis JR. Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin Cancer Res 16: 2571–2579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, Fleming MD, Olsen JC, Miller SW, Weinberg RA, Hahn WC. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene 21: 4577–4586, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Maley CC, Galipeau PC, Li X, Sanchez CA, Paulson TG, Blount PL, Reid BJ. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res 64: 7629–7633, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 454: 436–444, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Meltzer SJ, Mane SM, Wood PK, Resau JH, Newkirk C, Terzakis JA, Korelitz BI, Weinstein WM, Needleman SW. Activation of c-Ki-ras in human gastrointestinal dysplasias determined by direct sequencing of polymerase chain reaction products. Cancer Res 50: 3627–3630, 1990 [PubMed] [Google Scholar]
- 23. Sommerer F, Vieth M, Markwarth A, Rohrich K, Vomschloss S, May A, Ell C, Stolte M, Hengge UR, Wittekind C, Tannapfel A. Mutations of BRAF and KRAS2 in the development of Barrett's adenocarcinoma. Oncogene 23: 554–558, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Souza RF, Spechler SJ. Concepts in the prevention of adenocarcinoma of the distal esophagus and proximal stomach. CA Cancer J Clin 55: 334–351, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 140: 1084–1091, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Zhang HY, Zhang Q, Zhang X, Yu C, Huo X, Cheng E, Wang DH, Spechler SJ, Souza RF. Cancer-related inflammation and Barrett's carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett's cells. Am J Physiol Gastrointest Liver Physiol 300: G454–G460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang X, Yu C, Wilson K, Zhang H, Melton SD, Huo X, Wang DH, Genta RM, Spechler SJ, Souza RF. Malignant transformation of non-neoplastic Barrett's epithelial cells through well-defined genetic manipulations. PLos One 5: e13093, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]