Abstract
Functional MRI (fMRI) studies have demonstrated that a number of brain regions (cingulate, insula, prefrontal, and sensory/motor cortices) display blood oxygen level-dependent (BOLD) positive activity during swallow. Negative BOLD activations and reproducibility of these activations have not been systematically studied. The aim of our study was to investigate the reproducibility of swallow-related cortical positive and negative BOLD activity across different fMRI sessions. We studied 16 healthy volunteers utilizing an fMRI event-related analysis. Individual analysis using a general linear model was used to remove undesirable signal changes correlated with motion, white matter, and cerebrospinal fluid. The group analysis used a mixed-effects multilevel model to identify active cortical regions. The volume and magnitude of a BOLD signal within each cluster was compared between the two study sessions. All subjects showed significant clustered BOLD activity within the known areas of cortical swallowing network across both sessions. The cross-correlation coefficient of percent fMRI signal change and the number of activated voxels across both positive and negative BOLD networks were similar between the two studies (r ≥ 0.87, P < 0.0001). Swallow-associated negative BOLD activity was comparable to the well-defined “default-mode” network, and positive BOLD activity had noticeable overlap with the previously described “task-positive” network. Swallow activates two parallel cortical networks. These include a positive and a negative BOLD network, respectively, correlated and anticorrelated with swallow stimulus. Group cortical activity maps, as well as extent and amplitude of activity induced by volitional swallowing in the cortical swallowing network, are reproducible between study sessions.
Keywords: reliability, deglutition, test-retest, default-mode network
evidence has confirmed that cortical mechanisms play a crucial role not only in the initiation but also the integration of swallowing (4, 34, 40, 58). Functional neuroimaging and cortical stimulation studies have shown activation of bilateral regions of the human cerebral cortex, corroborating earlier findings obtained by electrophysiologic techniques in awake primates (2, 3, 21–23, 27, 28, 32, 33, 37, 38, 59). These studies reported mainly positive blood oxygenated level-dependent (BOLD) activity involving cortical regions such as sensorimotor, cingulate, insula, prefrontal, parietal, and pericentral sensorimotor cortices, and premotor area, as well as subcortical regions such as cerebellum, thalamus, and striatum.
The stability of cortical activation associated with a particular behavior is an important notion in functional imaging. Even though some cortically activated swallowing foci have consistently been identified, discrepancies have also emerged (57). For example, swallow-related activation of the insula and lateralization of sensorimotor region has been variable (12, 32, 59, 61, 68). Several studies have investigated reliability of the cortical activity in functional MRI (fMRI) studies during various cognitive, sensory, and motor functions such as finger opposition (46), wrist flexion (30), tactile palm stimulation (67), working memory (41), and visual stimulation (36, 49) showing variable results. Functional imaging of swallowing is inherently more challenging than other motor tasks because of the potential artifacts associated with magnetic susceptibility phenomena due to movements of swallowing-related components such as pharynx, palate, and larynx inside the field of view (2, 3, 66). Recently, a study employed a quantitative voxel-wise activation likelihood estimation meta-analysis technique, which pooled results from 12 studies to identify a shared distributed cortical network involved in control of swallowing (57), showing common topographic attributes of reported swallow-related activity. Despite these attempts, the fundamental question regarding reproducibility of the observed swallowing-related cortical activation has not been satisfactorily addressed.
Reliability in functional MRI can be evaluated by several methods, ranging from an evaluation of reproducibility of a particular region of activation to its particular characteristics such as signal amplitude (41). Since results ultimately depend on whether a voxel is considered active or inactive, perhaps the most important result of fMRI experiments is to identify the location of activation. Typically based on the notion that these are truly active regions, subsequent measurements of area and amplitude of the signal change are performed. We can measure the reliability of the location (e.g., centroid) of activation, the percentage or absolute signal change, and the area of activity that is deemed statistically significant (41).
Our aim in this study was to characterize systematically swallow-related positive and negative BOLD brain activity and evaluate their reproducibility across two sessions.
MATERIALS AND METHODS
Study Subjects
Sixteen healthy right-handed adult subjects recruited by advertisement (9 females, ages 20–34) were studied. The Human Research Review Committee of the Medical College of Wisconsin approved study protocol, and subjects gave written informed consent before the study. None of the subjects had any history of dysphagia or other gastrointestinal-related diseases based on a detailed health-related questionnaire and interview with a physician. The subjects were asked to fast for 6 h before the study.
Data Acquisition
Subjects were placed supine in a Signa LX (model 3.0T; General Electric Medical Systems, Waukesha, WI) equipped with an eight-channel receiver head coil and body quadrature transmit radiofrequency coil. Subjects were trained outside the scanner to maintain the lips and lower jaw relaxed and closed, and to swallow in a single motion of tongue pressing backward and upward against palate without rolling or a side-to-side motion resulting in a single swift laryngeal elevation. Subjects were instructed to avoid repetitive swallowing, and conscious nodding head motion was discouraged. Cardiorespiratory monitoring was performed at a sampling rate of 40 Hz with a pulse oximeter, and respiratory bellows equipment was provided as part of the MRI system. Paradigm-driven functional MRI data were acquired during two distinct 540-s runs while subjects performed 21 random single trial swallows across two sessions. A rear projection screen was placed at the head of the scanner bed to display visual cues to swallow and a fixation crosshair between swallows. Visual cue was a simple verbal command of “please swallow” shown on the screen replacing the fixation crosshair for 3 s. Timing of the visual cue was not locked to repetition times (TRs). These two scans were designed as a part of larger protocol and were 30 min apart across two different scanning sessions. Two anatomic scans were also acquired during each session using the high-resolution spoiled gradient recalled acquisition technique, consisting of 140 sagittal whole brain 1-mm thick slices over a 240-mm field of view and 256 × 224 within slice voxel resolution. These high-resolution anatomical images were used for subsequent superposition of cortical activity regions derived from the lower resolution echo planar BOLD contrast image data in each subject. Echo planar images (EPI) were acquired as 34 contiguous 4-mm thick sagittal slices over the whole brain volume in an interleaved fashion without any gap or overlap. EPI images were acquired with a slice-wise resolution of 64 × 64 voxels over a 240-mm field of view yielding a within-slice resolution of 3.75 × 3.75 mm, captured with an echo time (TE) of 23.4 ms and a TR of 2,000 ms in the first session, and TE = 17.5 ms, TR = 1,700 ms during the second session.
Regions of Interest Identification
The studied regions of interest (ROI) were all defined anatomically a priori on individual original space reference high-resolution anatomic scan according to the literature and statistical probabilistic maps included in Analysis of Functional Neuroimages (AFNI) software suite (9). We marked ROIs manually and consistently by one investigator (S. Ahmad) on both hemispheres. These areas were chosen based on earlier reports of swallowing fMRI activity as well as negative BOLD response to nonswallow motor tasks reported in the literature.
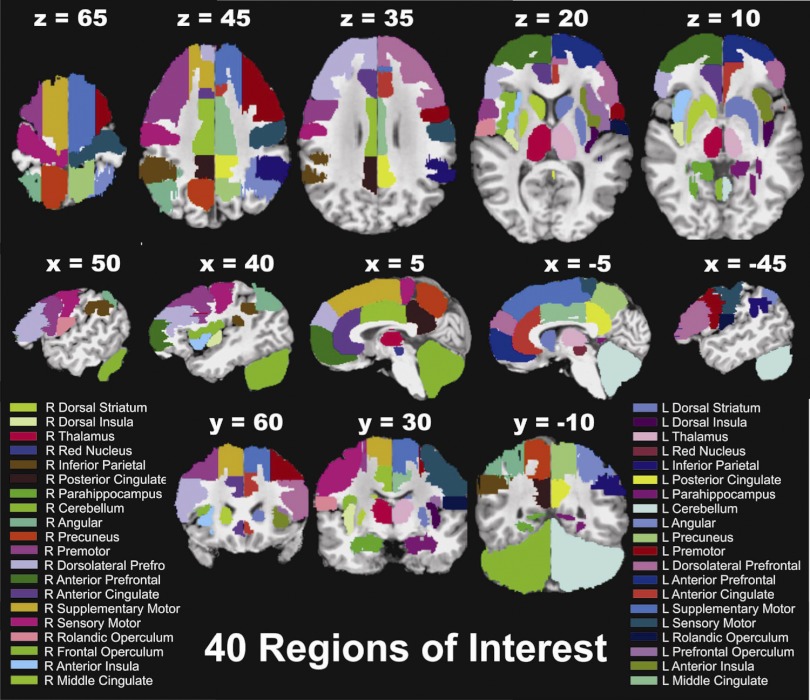
The cingulate cortex was defined as the portion of the cortex on the medial surface of cerebral hemispheres extending above corpus callosum and confined by the cingulate sulcus (15, 63). Cingulate sulcus is the most prominent sulcus on the medial surface and runs in parallel to corpus callosum. Paracingulate sulcus, if present, is noted to be superior and parallel to anterior cingulate sulcus and was included in cingulate cortex (14). Two imaginary vertical lines passing through anterior and posterior commissure divided cingulate gyrus to anterior, middle, and posterior regions. Anterior cingulate cortex primarily consisted of Brodmann areas (BAs) 24, 25, and 32; middle cingulate cortex mostly covered BAs 23, 24, and 33; posterior cingulate cortex mainly contained BAs 23, 29, 30, and 31 (63). The prefrontal area is the granular cortex, comprising majority of the frontal lobe (50), which, with electrical stimulation, does not evoke any visible bodily movement (13). Prefrontal cortex traditionally is divided into anterior, dorsolateral, and ventrolateral areas. Anterior (or frontopolar) prefrontal cortex extends from the most anterior (rostral) end of frontal lobe on the medial surface to cingulate sulcus posteriorly and superiorly to supplementary motor area. In axial sections, as we move inferiorly, the prefrontal cortex extends laterally and inferiorly on to the orbital surface of frontal lobe. It contains mostly BA 10 with minimal overlapping of BAs 11 and 12, inferiorly (45). The dorsolateral prefrontal cortex laterally occupies most of the middle frontal gyrus containing BAs 8, 9, and 46 extending posteriorly to premotor cortex. It includes small areas of inferior gyrus laterally and superior frontal gyrus medially just anterior to supplementary motor area. Ventrolateral prefrontal cortex mainly includes BAs 44 and 45 within inferior frontal gyrus, lateral orbital gyrus, and frontal operculum adjacent to insular lobe (43). Insula was defined as the region folded deep within the lateral sulcus buried underneath frontal and temporal lobes. Insula on sagittal view is divided by a prominent oblique central sulcus into anterior and posterior compartments. Insula covered BA 13, and short 3–5 gyri located rostral/anterior and long 2–3 gyri located caudal/ posterior to the central sulcus comprise the anterior and posterior insula, respectively (39). On axial section, insula was surrounded laterally by claustrum and capsule extrema and medially by opercular region. The sensory motor cortex consisted of BAs 1, 2, 3, and 4 and is defined as the immediate band of the precentral and postcentral gyrus adjacent to central sulcus on the lateral surface of the cortex, extending medially into the longitudinal cerebral fissure and laterally above axial plane of intersection of lateral and central sulcus (65, 69). Stimulation of the most caudolateral segment of motor cortex that has been associated with oropharyngeal deglutitive muscles (47, 48). We identified and marked the most caudolateral section of precentral and postcentral gyrus as rolandic operculum that included BAs 4, 40, and 43. Rolandic operculum extended 15 mm above the axial plane of intersection of lateral and central sulcus superiorly (to border sensory motor cortex) and 15 mm medially. This ROI also included areas commonly referred to as secondary and ventral somatosensory area. We marked supplementary motor and premotor areas with a more inclusive definition that mainly covered BA 6 and overlapped with BA 8. On sagittal section of the medial surface of hemisphere we marked all grey matter between prefrontal cortex anteriorly to sensory motor cortex posteriorly extending 15 mm from longitudinal hemispheric fissure as supplementary motor area. The remaining lateral section of frontal lobe anterior to sensory motor cortex and precentral sulcus covering BAs 6 and 8 was marked as secondary motor cortex bordering anteriorly with dorsolateral and ventrolateral prefrontal cortex. The precuneus region was defined as the medial surface (15 mm) of superior parietal cortex bordering sensory motor and posterior cingulate cortex ventrally. Precuneus was inferiorly demarcated by parietooccipital and anteriorly by marginal ramus of the cingulate sulcus and primarily contained BA 7 with overlap of BA 31 (5, 31). The remainder of the lateral parietal cortex including superior and inferior parietal lobule covering BAs 7, 39, and 40 was subdivided into two parts. First, we marked rostrolaterally and just posterior to the sensory motor and rolandic operculum region supramarginal gyrus and anterior section of inferior parietal lobule covering a small section of parietal operculum. This area covered primarily BAs 40 and 5 and was inferiorly limited by superior temporal gyrus/sylvian fissure and superiorly by intraparietal sulcus and was identified as inferior parietal lobule ROI. Two, further dorsolaterally angular gyrus and posterior section of inferior and superior parietal lobule that covered BAs 40, 39, and 7 was identified as angular ROI. Parahippocampal gyrus was identified on sagittal and coronal sections as the convoluted grey matter just superior and lateral to hippocampus deep in temporal lobe. It was located above fusiform gyrus and anterior to lingual gyrus of the occipital lobe and mainly covered BA 30. Subcortical regions with known strong cortical connections were also identified and masked. Dorsal striatum was easily identified on axial and sagittal sections and included putamen and caudate nucleus that were marked as a single ROI. Putamen (the outermost portion of basal ganglia) was identified as triangular-shaped grey matter nucleus just medial to claustrum and external capsule and lateral to globus pallidus posterior to internal capsule. Caudate nucleus was identified as the C-shaped grey matter nucleus adjacent to the lateral ventricles. Head of caudate nucleus forms part of the floor of anterior horn of the lateral ventricle and is located anterior to internal capsule that separates it from the putamen. Body and tail of caudate nucleus travels on the floor of lateral ventricle backward and then curves anteriorly to form the roof of inferior horn of lateral ventricle. Thalamus was identified as the midline large ovoid grey matter structure that is situated medial/superior to internal capsule and lateral to third ventricle. Red nucleus was a small region marked inferior to the thalamus and dorsorostral to substantia nigra. Cerebellum was identified and marked as the most inferior and posterior large mass of heavily folded grey matter infratentorial structure located posterior to the pons and brain stem. None of the ROIs had any overlapping areas (Fig. 1). Total volume of all ROIs bilaterally covered 177 of 193 cm3 of the group brain.
Fig. 1.
A priori manual masking of the 40 regions of interest (ROI) on a reference high-resolution anatomical scan is displayed. This example is from subject 14 and all ROIs are color coded. All ROIs were drawn based on standard stereotaxic statistical probabilistic maps available in the Analysis of Functional Neuroimages (AFNI) suite and were modified based on individualized known gyral and sulcal patterns described in the literature.
Image Analysis
All fMRI signal analyses were carried out using the AFNI package (9). We processed the data according to accepted fMRI analysis pipeline and took some additional steps to minimize inherent noise associated with oropharyngeal movement and performed confirmatory analysis to verify subjects' compliance to swallow commands. All participants maintained a peak-to-peak global (EPI volume) head motion of < 2 mm, and only two subjects showed > 1 mm global head motion that was swallow related. Physiologic signal (cardiac and respiratory)-related signal changes were retrospectively corrected (17). Brain in a structural anatomical data set was extracted from surrounding tissue (55). We then computed an alignment matrix to a mean anatomical dataset from the two acquired anatomic scans in each of the sessions and registered both anatomic scans to this mean dataset. We used the resultant dataset as the reference anatomic scan for alignment of all EPI datasets to remove the bias of either session. Reference anatomical scan was spatially normalized to match the standard Talaraich-Tournoux stereotaxic template (60). We performed slice timing correction and modeled head motion using 12 degrees of freedom indexed by time, which was used to interpolate the time series back to the original acquisition grid (51). We computed the alignment matrix between EPI datasets and reference anatomical scan, and applied that to register and align all datasets to the reference anatomic scan grid. The data was transformed and resampled into standard Talaraich-Tounoux space with voxel size of 3.75 × 3.75 × 3.75 mm in one interpolation within AFNI package. Voxel-wise extreme fluctuations of the signal were replaced by a fitted smooth curve to the time series. fMRI BOLD signal was then normalized voxel-wise to the average signal over the course of time series to yield percent signal change at the end of analysis pipeline. White matter and cerebrospinal fluid containing voxels were identified automatically based on their signal intensity and manually verified within each subject. The average time course within the white matter and cerebrospinal fluid voxels were extracted. General linear modeling techniques were used to remove undesirable signal contamination correlated with 12 motion parameters, white matter, and cerebrospinal fluid temporal components out of the BOLD signal as covariates of no interest. We utilized functionally acquired pharyngeal signal and identified each swallow with its associated susceptibility changes to extract timing of actual swallows. We used actual swallow timing as our reference time course and the main signal of interest. Analysis was performed with multiple linear regression using generalized least squares time series fit with partially nonlinear restricted maximum likelihood estimation of serial temporal autocorrelation structure. Results were then spatially smoothed to a full width at half maximum of 5 mm. A second-level group, random, two-sample, mixed-effects, multilevel analysis was then performed to identify active cortical regions (6). Clusters with P < 0.05 corrected for multiple comparisons (using Monte Carlo simulation) were considered to be significant. A clustered activity map exceeding the significance threshold was overlapped by a priori identified group mask of each ROI to determine activated clusters within each ROI.
Statistical Analysis
Reproducibility testing compared stereotaxic location, extent, and intensity of the cortical activity within each ROI across both sessions. The number of activated voxels along with coordinates of center of the clustered activity, and the average and peak percent signal change within each ROI were compared between study sessions. To further determine reproducibility we performed a linear regression analysis across both sessions for the number of activated voxels and percent signal change within each ROI and report the corresponding correlation coefficients.
RESULTS
The adherence to visual cues and accuracy of swallow timing was determined using acquired functional data. Two adjacent midline slices were chosen, and the time series of an ideal voxel in pharynx behind the uvula was carefully inspected (Fig. 2). The nasopharyngeal passage closed at the time of each swallow, and the EPI time series of the identified voxel demonstrated a swallow-related signal spike in the exact TR that coincided with swallow. Swallow spike could be visualized well either in even or odd slices due to interleaved EPI acquisition and rapid rate of swallow-related pharyngeal closure. Visual inspection of the EPI image in motion was also used to confirm the occurrence of swallow in the identified TR.
Fig. 2.
Identification of swallow-related spike in midline sagittal echo planar image (EPI). EPI of nasopharyngeal passage at rest and during swallow are displayed. Blood oxygen level-dependent (BOLD) signal time series of the identified voxel in nasopharynx clearly demonstrates a swallow-related spike and associated repetition time could be recorded for stimulus timing analysis.
Study subjects swallowed 21.3 ± 0.5 times throughout first scan session and 21.2 ± 0.6 times throughout the second session, displaying compliance to the swallow commands. Subjects predominantly swallowed at the TR corresponding to the visual command or one TR after the visual cue. Subjects primarily swallowed 100 to 300 ms after projection of the visual cue. Subjects swallowed 15.2 ± 0.5 times during the TR corresponding to the visual command during the first session and 14.7 ± 0.5 during the second session, which was not statistically significant.
Positive BOLD Network
Group analysis showed significant clustered-positive BOLD activity within the targeted ROIs of the deglutition network during both scan sessions: sensorimotor cortex, premotor area, rolandic operculum, middle cingulate, insula and adjacent prefrontal operculum, and inferior parietal lobule (Fig. 3). Total volume of significant clustered-positive BOLD activity within our ROIs was 7.2 and 5.9 cm3 across first and second sessions, respectively, which covered 7.4 and 6.1% of their respective ROIs. There was significant bilateral activity within cuneus or occipital cortex (BAs 18 and 19) and superior and transverse temporal (Heschl's) cortex (BAs 38, 41, and 42) that we did not further analyze. This activity is presumably related to the processing of visual commands that triggered the swallow (28).
Fig. 3.
Positive BOLD cortical swallow-related activity displayed across 2 sessions. Sagittal, axial, and coronal images are displayed to demonstrate the extent and location of activity throughout the whole brain.
Sensory motor region activity was the largest cluster of activity that was located in lateral segment of the pericentral sulcus. During the first session, left and right sensory motor clusters' volumes of activity were 1.5 and 1.8 cm3, which remained the largest clusters during the second session with volume of 1.3 and 0.9 cm3, respectively. Rolandic operculum bilaterally showed activity that compared with all of other ROIs and occupied the largest percentage of a priori identified ROI (55 and 31% of the ROI for left hemisphere, 79 and 52% of the ROI for right hemisphere, during the first and second sessions, respectively). Rolandic operculum also carried the highest magnitude of percent signal increase (1.04 and 1.08% for the left and right hemispheres, respectively) during the first session, and (0.80 and 0.82% for the left and right hemisphere, respectively) during the second session.
Insular region clustered activity was almost located in middle of the insula crossing the superior insular sulcus into adjacent frontal operculum and was divided by oblique middle insular sulcus into anterior and posterior clusters, respectively. Right precuneus and right supplementary motor clusters did not reach the statistically significant threshold in one of the sessions. They were both midline structures, and the clustered activity was divided into hemispheric subclusters that may have not reached statistical significance on one side with family-wise error correction. Subcortical activity appeared to be unilateral in our study involving right dorsal striatum, right thalamus, and left cerebellum. Thalamic activity was located in medial dorsal nucleus, and striatal activity was centered at the junction of posterior part of right putamen to lateral globus pallidus. Red nucleus activity was not observed in the first session of scanning; however, it showed significant clustered activity during the second session. Details of volume, magnitude, and coordinates of peak and centroid (geometric center of the mass) of positive BOLD activity are shown in Table 1.
Table 1.
Details of swallow-related bilateral positive blood oxygen level-dependent (BOLD) cortical activity across 2 sessions
| Positive BOLD Activation |
Session 1 |
Session 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RPI | BA | ROI Volume, cm3 | % Signal Change | Activity Volume, mm3 | Center of Activity, x,y,z | Peak Activity, x,y,z | % Signal Change | Activity Volume, mm3 | Center of Activity, x,y,z | Peak Activity, x,y,z |
| L Supplementary motor | 6, 8 | 22.9 | 1.03 ± 0.24 | 232 | −2, −12, 60 | −2, −14, 58 | 0.73 ± 0.05 | 216 | −4, −10, 53 | −2, −10, 54 |
| L Premotor | 6, 8 | 18.8 | 0.64 ± 0.23 | 456 | −53, −1, 26 | −56, −4, 30 | 0.60 ± 0.10 | 56 | −50, 1, 15 | −48, 2, 16 |
| L Sensory motor | 1, 2, 3, 4 | 18.6 | 0.97 ± 0.37 | 1,544 | −50, −13,35 | −56, −8, 32 | 0.68 ± 0.08 | 1,288 | −48, −15, 37 | −56, −8, 26 |
| L Rolandic operculum | 43, 4 | 3.1 | 1.04 ± 0.27 | 632 | −54, −8, 19 | −56, −4, 16 | 0.82 ± 0.09 | 360 | −53, −9, 20 | −56, −10, 22 |
| L Posterior insula | 13 | 2.9 | 0.48 ± 0.11 | 112 | −39, −7, 7 | −40, −6, 6 | 0.40 ± 0.03 | 16 | −32, −15, 18 | −32, −16, 18 |
| L Anterior insula | 13 | 5.3 | 0.48 ± 0.11 | 184 | −39, −2, 9 | −40, −2, 6 | 0.44 ± 0.02 | 88 | −36, −4, 13 | −34, −4, 16 |
| L Ventrolateral prefrontal | 44, 45 | 4.7 | 0.44 ± 0.06 | 88 | −39, −5, 16 | −32, 8, 16 | 0.44 ± 0.02 | 320 | −38, −9, 17 | −42, −4, 14 |
| L Middle cingulate | 24 | 10.0 | 0.56 ± 0.17 | 40 | −2, 7, 34 | 0, 8, 34 | 0.60 ± 0.05 | 144 | −2, 0, 40 | 0, 0, 40 |
| L Inferior parietal lobe | 40 | 9.0 | 0.69 ± 0.11 | 344 | −57, −29, 25 | −58, −24, 24 | 0.58 ± 0.06 | 408 | −54, −30, 24 | −60, −24, 26 |
| R Precuneus | 7 | 16.7 | 0.33 ± 0.01 | 24 | 8, −43, 47 | 8, −44, 48 | 0.00 | 0 | NA | NA |
| R Supplementary motor | 6 | 21.8 | 0.82 ± 0.20 | 168 | 8, −6, 60 | 4, −12, 62 | 0.63 ± 0.03 | 16 | 19, −8, 63 | 20, −8, 62 |
| R Premotor | 6 | 18.1 | 0.75 ± 0.16 | 168 | 57, 0, 23 | 58, −2, 22 | 0.48 ± 0.03 | 136 | 52, −2, 23 | 54, 0, 10 |
| R Sensory motor | 4, 3 | 17.6 | 0.95 ± 0.28 | 1,816 | 49, −14, 34 | 52, −12, 24 | 0.72 ± 0.07 | 984 | 49, −14, 34 | 54, −12, 22 |
| R Rolandic operculum | 43, 4 | 2.6 | 1.08 ± 0.31 | 752 | 55, −11, 16 | 56, −8, 14 | 0.80 ± 0.07 | 496 | 54, −8, 15 | 58, −6, 14 |
| R Posterior insula | 13 | 3.3 | 0.44 ± 0.05 | 32 | 37, −11, 7 | 36, −12, 8 | 0.39 ± 0.03 | 56 | 37, −17, 5 | 36, −20, 6 |
| R Anterior insula | 13 | 5.3 | 0.37 ± 0.03 | 88 | 36, 8, 6 | 36, 8, 6 | 0.50 ± 0.03 | 248 | 39, 1, 8 | 42, 0, 8 |
| R Ventrolateral prefrontal | 44, 45 | 5.3 | 0.52 ± 0.13 | 128 | 42, −12, 15 | 42, −12, 14 | 0.55 ± 0.05 | 504 | 43, 4, 8 | 50, 8, 4 |
| R Middle cingulate | 24 | 9.9 | 0.85 ± 0.02 | 16 | 1, 9, 33 | 1, 9, 33 | 0.65 ± 0.06 | 192 | 5, 0, 40 | 4, 0, 40 |
| R Inferior parietal lobe | 40 | 8.6 | 0.65 ± 0.12 | 224 | 57, −37, 25 | 56, −34, 24 | 0.51 ± 0.03 | 88 | 57, −36, 24 | 58, −30, 22 |
| R Dorsal striatum | 5.7 | 0.34 ± 0.03 | 24 | 22, −6, 1 | 22, −6, 1 | 0.48 ± 0.03 | 56 | 26, −4, 2 | 26, −2, 0 | |
| R Thalamus | 7.6 | 0.51 ± 0.08 | 56 | 11, −21, 11 | 12, −22, 8 | 0.59 ± 0.03 | 24 | 9, −20, 12 | 10, −20, 12 | |
| R Red nucleus | 0.5 | 0.00 | 0 | NA | NA | 0.33 ± 0.03 | 104 | 5, −23, −4 | 6, −24, −2 | |
| L Cerebellum | 37.3 | 0.33 ± 0.03 | 48 | −10, −55, −13 | −10, −56, −14 | 0.30 ± 0.01 | 64 | −9, −55, −11 | −10, −56, −10 | |
Volume of the corresponding a priori identified regions of interest (ROI) and associated Brodmann area (BA) are displayed. Volume and magnitude of the cortical activity in each cluster are presented. Magnitude of activity is displayed as mean ± SD of activity of total activated cluster of voxels within an ROI. Stereotaxic coordinates x,y,z of the peak activated voxel, and the voxel at the geometric center of each activated cluster within an ROI are presented in SPM order (left-right, posterior-anterior, inferior-superior). L, left; R, right; NA, not applicable.
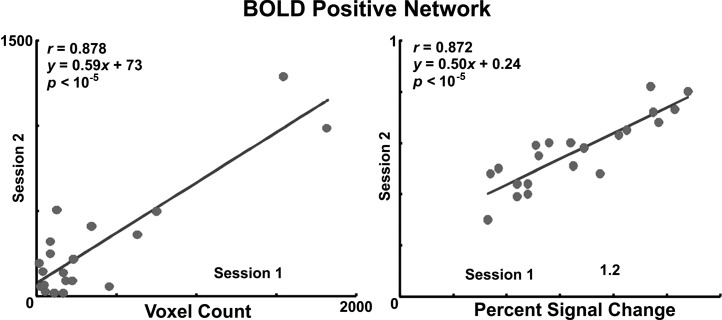
Reproducibility of positive BOLD activity.
Scatter plot and linear regression analysis of volume of activity (y = 0.59x + 73) and magnitude of percent signal change (y = 0.50x + 0.24) across both sessions demonstrated significant correlation across sessions (Fig. 4). As demonstrated, the first session revealed higher volume and magnitude of clustered activity compared with the second session. The decrease in activation volume and percent change in the second session, considering reduced echo and repetition times, is consistent with a true BOLD response instead of motion artifact. Overlap of activity maps showed that coordinates of the centeroid of the activated clusters were within 6 ± 5 mm across sessions.
Fig. 4.
Reproducibility of the volume and magnitude of swallow-related positive BOLD signal change across two functional MRI sessions.
Negative BOLD Network
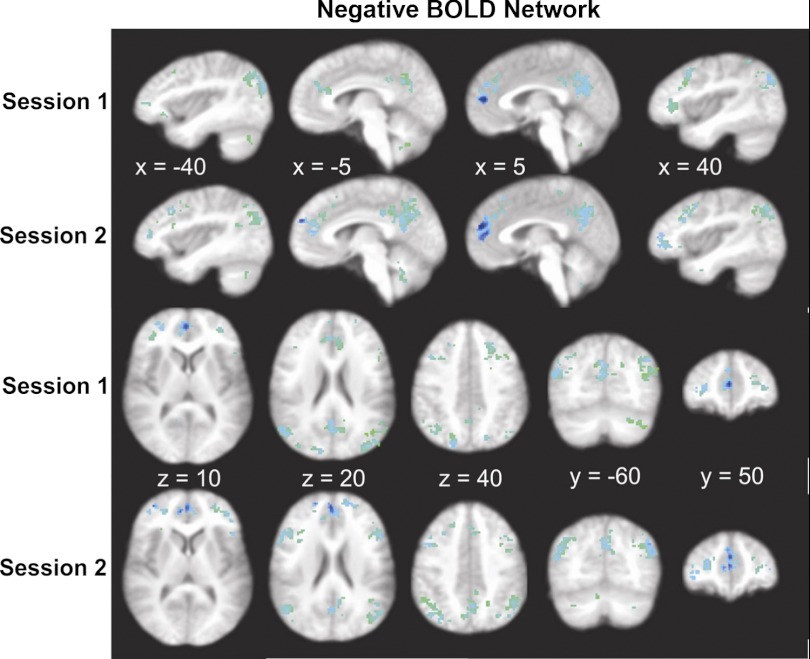
Group analysis showed significant clustered negative BOLD activity within the targeted ROIs: anterior medial prefrontal and dorsolateral prefrontal cortices, anterior and posterior cingulate cortices, precuneus and dorsolateral parietal cortices, parahippocampal gyrus, and premotor areas (Fig. 5). Overall volume of negative BOLD activity was 23.5 and 27.3 cm3 across first and second sessions, respectively. This covered 21.4 and 24.9% of their relevant ROI mask regions. Volume of clustered negative BOLD activity was significantly larger than positive BOLD activity (P < 0.001), but the magnitude of negative BOLD activity (%signal change) was significantly less compared with positive BOLD activity in either of two sessions (P < 0.001).
Fig. 5.
Negative BOLD cortical swallow-related activity displayed across 2 sessions. Sagittal, axial, and coronal images are displayed to demonstrate extent and location of activity throughout the whole brain.
The largest cluster of negative BOLD activity was located in dorsolateral parietal cortex covering angular gyrus and inferior and superior parietal lobules. During the first session, left and right dorsolateral parietal clusters' volumes of activity were 4.5 and 3.2 cm3, respectively, and during the second session, they remained largest clusters with volumes of 5.7 and 3.7 cm3, respectively. Two large clusters of negative BOLD activity were on the medial surface of both hemispheres, posterior/dorsal and anterior/rostral to corpus collosum. The posterior clusters on either side were divided by subparietal sulcus into precuneus and posterior cingulate clusters. The anterior clusters on either side were divided by cingulate sulcus into anterior prefrontal cortex and anterior cingulate clusters. Anterior cingulate cortex bilaterally showed a remarkable activity that compared with all of other ROIs occupied the largest percentage of a priori identified ROI (41 and 74% of the ROI for left hemisphere and 46 and 41% of the ROI for right hemisphere, during first and second sessions, respectively). The anterior medial prefrontal cortex carried the highest magnitude of percent signal change (−0.62 and −0.65% for the left and right hemisphere, respectively) during the first session and (−0.41 and −0.49% for the left and right hemisphere, respectively) during the second session. In contrast to positive BOLD, subcortical negative BOLD activity was bilateral. The only ROIs that contained both positive and negative BOLD active clusters were premotor area and cerebellum. Details of volume, magnitude, and coordinates of peak and centroid of clustered negative BOLD activity are shown in Table 2.
Table 2.
Details of swallow-related bilateral negative BOLD cortical activity across 2 sessions
| Negative BOLD Activation |
Session 1 |
Session 2 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | BA | ROI Volume, cm3 | % Signal Change | Activity Volume, mm3 | Center of Activity, x,y,z | Peak of Activity, x,y,z | % Signal Change | Activity Volume, mm3 | Center of Activity, x,y,z | Peak of Activity, x,y,z |
| L Anterior prefrontal | 10 | 6.3 | −0.62 ± 0.11 | 888 | −25, 48, 16 | −22, 52, 18 | −0.41 ± 0.06 | 640 | −23, 49, 17 | −18, 50, 22 |
| L Dorsolateral prefrontal | 9, 46 | 18.2 | −0.36 ± 0.11 | 712 | −49, 17, 18 | −50, 16, 18 | −0.28 ± 0.04 | 1,760 | −47, 18, 20 | −50, 18, 22 |
| L Anterior cingulate | 24, 32 | 6.9 | −0.44 ± 0.14 | 1,048 | −5, 41, 23 | −5, 42, 24 | −0.30 ± 0.05 | 1,920 | −4, 39, 22 | −6, 52, 26 |
| L Posterior cingulate | 23, 31 | 10.4 | −0.38 ± 0.11 | 1,408 | −7, −48, 29 | −8, −56, 18 | −0.27 ± 0.03 | 2,296 | −5, −47, 28 | −6, −54, 16 |
| L Premotor | 6, 8 | 18.8 | −0.36 ± 0.09 | 1,432 | −27, 8, 46 | −24, 8, 52 | −0.25 ± 0.03 | 1,120 | −39, 4, 45 | −36, 10, 48 |
| L Precuneus | 7 | 15.9 | −0.42 ± 0.10 | 784 | −4, −59, 32 | 0, −62, 32 | −0.25 ± 0.03 | 2,016 | −4, −60, 36 | 0, −62, 36 |
| L Dorsolateral parietal | 39, 40 | 25 | −0.42 ± 0.11 | 4,528 | −40, −60, 38 | −46, −64, 36 | −0.27 ± 0.08 | 5,664 | −45, −58, 35 | −46, −64, 34 |
| L Parahippocampus | 24 | 10.3 | −0.27 ± 0.11 | 776 | −25, −39, −5 | −26, −42, −4 | −0.23 ± 0.01 | 96 | −17, −36, −6 | −16, −36, −6 |
| L Cerebellum | NA | 37.3 | −0.25 ± 0.04 | 880 | −33, −64, −24 | −24, −64, −24 | −0.25 ± 0.03 | 688 | −33, −64, −32 | −42, −60, −32 |
| R Anterior prefrontal | 10 | 5.9 | −0.65 ± 0.12 | 520 | 31, 47, 8 | 34, 48, 8 | −0.49 ± 0.09 | 760 | 31, 46, 12 | 36, 48, 8 |
| R Dorsolateral prefrontal | 9, 46 | 15.7 | −0.42 ± 0.10 | 1,304 | 43, 18, 30 | 43, 24, 21 | −0.30 ± 0.05 | 2,272 | 45, 17, 25 | 50, 22, 22 |
| R Anterior cingulate | 24, 32 | 7.4 | −0.41 ± 0.19 | 1,288 | 5, 43, 12 | 2, 46, 12 | −0.28 ± 0.06 | 1,144 | 5, 43, 16 | 2, 48, 18 |
| R Posterior cingulate | 23, 31 | 10.8 | −0.41 ± 0.10 | 1,528 | 6, −54, 24 | 2, −52, 18 | −0.25 ± 0.03 | 704 | 5, −54, 25 | 2, −60, 22 |
| R Premotor | 6, 8 | 18.1 | −0.40 ± 0.08 | 1,064 | 32, 13, 44 | 30, 14, 48 | −0.30 ± 0.04 | 1,016 | 35, 9, 45 | 24, 8, 54 |
| R Precuneus | 7 | 16.7 | −0.38 ± 0.12 | 824 | 5, −60, 34 | 4, −52, 36 | −0.30 ± 0.04 | 840 | 4, −62, 35 | 2, −60, 36 |
| R Dorsolateral parietal | 39, 40 | 22.8 | −0.45 ± 0.11 | 3,200 | 41, −66, 34 | 38, −74, 32 | −0.28 ± 0.07 | 3,656 | 42, −60, 36 | 46, −68, 32 |
| R Parahippocampus | 10.6 | −0.28 ± 0.10 | 568 | 27, −38, −6 | 24, −38, −4 | −0.26 ± 0.01 | 128 | 18, −37, −10 | 18, −36, −10 | |
| R Cerebellum | NA | 36 | −0.23 ± 0.05 | 736 | 29, −61, −31 | 30, −62, −30 | −0.22 ± 0.04 | 616 | 33, −61, −29 | 30, −66, −30 |
Volume of the corresponding a priori identified ROI and associated BA are displayed. Volume and magnitude of the cortical activity in each cluster are presented. Magnitude of activity is displayed as mean ± SD of activity of total activated cluster of voxels within a ROI. Stereotaxic coordinates x,y,z of the peak activated voxel and the voxel at the geometric center of each activated cluster within an ROI are presented in SPM order (left-right, posterior-anterior, inferior-superior).
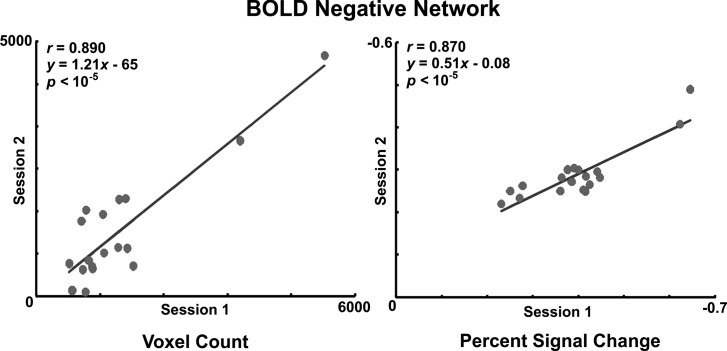
Reproducibility of negative BOLD activity.
Scatter plot and linear regression analysis of volume of activity (y = 1.21x − 68) and magnitude of percent signal change (y = 0.51x − 0.08) demonstrated significant correlation across both sessions (Fig. 6). Overlap of activity maps showed that coordinates of the centeroid (geometric center of the mass) of the activated negative BOLD clusters were within 5 ± 3 mm across sessions.
Fig. 6.
Reproducibility of the volume and magnitude of swallow-related negative BOLD signal change across 2 functional MRI sessions.
DISCUSSION
In this study, we determined the degree of reproducibility of fMRI swallow-associated activity from one session to the other in the same group of healthy individuals. We utilized the nasopharyngeal susceptibility changes to identify exact timing of the swallow task and employed an event-related analysis technique to determine location, spatial extent, and signal amplitude of BOLD activations in a priori identified brain regions. Swallow-induced positive and negative BOLD cortical activations showed notable overlap to task-positive and task-negative (or default mode) networks, respectively. Study findings indicate that although the findings are significantly reproducible in terms of volume and magnitude of activity, the reproducibility is not perfect. These findings have clinical and investigational ramifications and need to be taken into consideration when evaluating the effect of any intervention on cortical swallow-related activity.
Swallowing and Negative BOLD fMRI Activity
Functional brain mapping operates on the assumption that regions of cortex showing a positive temporal correlation between the signal and a particular stimulus are involved in processing that stimulus. However, both PET and fMRI studies have shown certain areas of the cortex with responses that are anticorrelated with stimulus. The ambiguous origin of observed anticorrelated cortical responses (negative BOLD, in case of fMRI) with stimuli is poorly understood. Three theoretical mechanisms have been proposed for negative BOLD response (64): 1) vascular steal that is a hemodynamic effect of vascular diversion of oxygenated hemoglobin resulting in negative BOLD signal of neighboring areas (10, 24); 2) active neuronal suppression suggesting reduced functional neuronal activity of the regions below baseline due to active neuronal inhibition (1, 20, 54); and 3) extended initial dip that actually represents an increased level of neuronal activity during the stimulus period that was not matched by a corresponding boost in blood flow, depleting local oxygen levels. (25). There is accumulating evidence to suggest that decreased neuronal activity is the underlying origin of the negative BOLD signal (18, 52, 53); however, nature of this neuronal suppression is still debatable.
The present study documents, for the first time, the extent and magnitude of negative BOLD activity during swallowing. These negative BOLD-activated regions were comparable to the regions that comprise the default-mode network described in the literature (44). The default-mode network has been identified in other functional brain studies, including motor tasks by reduced activity compared with baseline resting state. Distinct regions of the brain have been regularly observed to decrease their activity during cognitively engaging tasks and are thought to mediate brain processes during the resting state that is suspended during goal-directed behaviors (16, 44). These regions have been called task-negative or default-mode network (19, 44). Studies have also shown that attention-demanding tasks, regardless of the nature of the task (sensory, motor, cognitive, visual, or auditory), consistently activate a group of brain regions (8). These regions have been collectively called task-positive or task-control networks (8, 11). The results of our study are in accordance with these previous reports, in that, among 40 distinct regions involved in swallowing, nearly half showed negative BOLD activity quite similar to regions of default-mode network (19), and the other half that showed positive BOLD activity displayed overlap to the task-positive network previously described in literature (11).
Swallowing and Reproducibility of fMRI Activity
fMRI detects local task-related changes in cerebral blood oxygenation, closely reflecting the underlying neural activity (29). However, fMRI activity changes may arise from either random or systematic processes in different scanning sessions (30). Random processes may be nonphysiologic, such as changes in the position of the subject, field inhomogeneities, shimming differences, variability of the scanner signal, data processing and analysis, statistical errors, or physiologic, such as random cognitive processes and level of arousal, among many other factors (35, 62). All of these factors can change from one session to another and from one subject to another at random. Systematic nonrandom processes are related to the repeated performance of a specified task, such as attention, habituation, and learning, and could lead to alteration of activation from one session to the other (26).
The present study used two main advancements in functional MRI scanning and analytical techniques to improve signal detection and alleviate some of random processes described above. First, investigators recently used a unique imaging sequence including anatomical (midsagittal only) and functional (whole brain) data acquisition simultaneously (SimulScan) (42). The sequence was developed to monitor oropharyngeal motions during swallowing. We monitored subjects' oropharyngeal motion using acquired functional data by focusing on pharyngeal region. The nasopharyngeal passage closes with each swallow with elevation of soft palate and pharyngeal contraction that results in a significant spurious change in fMRI signal due to their effect on the magnetic B field that is readily detectable as a spike in time series of preidentified voxels. Since functional data is acquired in an interleaved fashion and with sampling frequency of < 1 Hz, we used either an odd or even sagittal slice at the midline to harvest swallow-related signal change at its peak. Doing so, we were able to verify swallow timing based on the data itself rather than assuming adherence to the visual cue to swallow. We also did so without a complicated sequence that allocates half of the time of each TR to anatomical data acquisition (42), which has no further use in analysis other than detection of pharyngeal movements described above. Second, conventional group analytical techniques consider intrasubject variance of the signal of interest insignificant relative to intersubject variance of signal, and also presume a Gaussian distribution of data values without outliers per each voxel. We utilized a mixed-effects multilevel analysis (7) for group analysis that incorporates variance of the measured signal from individual subject analysis along with variability across subjects. Conventional variance assumptions in presence of outliers are imprecise, and a test that factors in intrasubject variability yields higher statistical power and more precise results. We performed conventional group analysis techniques that produced results that were less reproducible (data not shown). We propose that since swallow is associated with an inherent motion inside the field of view that is different among study subjects, within-subject variability plays an important role in better reproducibility of our results using mixed-effects multilevel analysis.
In our study, task-related motions were not specific to a particular functional area, and susceptibility changes were mostly in midline slices simultaneous with swallow-related oropharyngeal movements (sagittal acquisition). Motion effects yielding susceptibility changes are necessarily going to occur at the instant of motion which is temporally out of phase with the hemodynamic response that is modeled in the statistical analysis. Such temporal shifts are good at differentiating motion-induced signal changes from BOLD signal changes associated with oral tasks (56). Therefore, susceptibility effects associated with motion are likely not associated with the observed task-related activations. Additionally, the magnetic field perturbations associated with motion of structures in the oral cavity, although rapidly varying in the immediate proximity of the structures, vary more smoothly over the cortex. Thus, associated changes would be expected over large, contiguous regions of cortex and not specific functional cortical areas.
The two scan sessions included echo times of 23.4 and 17.5 ms, paired with repetition times of 2,000 and 1,700 ms, respectively. The selection of these timing parameters enables a cross-session comparison of BOLD results. As BOLD contrast is associated with changes in magnetic susceptibility, an increase in echo time results in an increase in functional contrast. This increase in contrast, thus yields increases in percent signal change and sensitivity, yielding increased activation volumes. The alteration of repetition time, conversely, increases sensitivity to changes in apparent longitudinal relaxation times. As such, the second session with a reduced repetition time features an increased sensitivity to subject motion (which brings spins from neighboring slices with differing longitudinal magnetization into the imaged volume) with the potential for increased activation resulting from subject motion. The observed results, with increased volume of activation and percent signal change in the first session, are thus consistent with BOLD activation more so than subject motion. On the other hand, we are unable to differentiate potential neuromodulatory effects of habituation, performing a voluntary swallow protocol twice from pure magnetic impact of changes in TR and TE on BOLD signal contrast. Therefore, the final activity observed during the second session reflects both the influence of TR and TE changes as well as habituation in two consecutive experimental sessions.
Considering all above challenges and using state of the art analytical techniques, we were able to demonstrate that reproducibility of swallow-related cortical activity is reasonable and resides in the range of 0.0 to 1.2 cm3 for volume and 0 to 0.3% signal change for magnitude of BOLD (positive and negative) activity. Furthermore, reproducibility of the exact location of the centroid of activated clusters is in the range of 0 to 11 mm from one study session to the other. In summary, the present study investigated swallow-associated cortical BOLD activity in a young healthy population across two sessions applying slightly different scanner settings. We applied novel fMRI analytical techniques and were able to demonstrate the reproducibility of the swallow-associated BOLD signal. Topographic location of negative BOLD activity was comparable to well defined default-mode network, and positive BOLD activity demonstrated overlap with task-positive network previously described in literature.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants 5R01-DK-025731-29 and 2T32-DK-061923-06.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B., B.D.W., A.N., and R.S. conception and design of research; A.B. and A.N. performed experiments; A.B., B.D.W., S.A., and A.P. analyzed data; A.B. and S.A. interpreted results of experiments; A.B. prepared figures; A.B. and R.S. drafted manuscript; A.B., B.D.W., S.-J.L., J.S.H., and R.S. edited and revised manuscript; A.B. and R.S. approved final version of manuscript.
REFERENCES
- 1. Allison JD, Meador KJ, Loring DW, Figueroa RE, Wright JC. Functional MRI cerebral activation and deactivation during finger movement. Neurology 54: 135–142, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Birn RM, Bandettini PA, Cox RW, Jesmanowicz A, Shaker R. Magnetic field changes in the human brain due to swallowing or speaking. Magn Reson Med 40: 55–60, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Birn RM, Bandettini PA, Cox RW, Shaker R. Event-related fMRI of tasks involving brief motion. Hum Brain Mapp 7: 106–114, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Car A. [Cortical control of the bulbar swallowing center]. J Physiol (Paris) 62: 361–386, 1970 [PubMed] [Google Scholar]
- 5. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain J Neurol 129: 564–583, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Chen G, Saad ZS, Cox R. Modeling multilevel variance components and outliers in group analysis Barcelona: 2010 [Google Scholar]
- 7. Chen G, Saad ZS, Nath AR, Beauchamp MS, Cox RW. FMRI group analysis combining effect estimates and their variances. Neuroimage 60: 747–765, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Rev Neurosci 3: 201–215, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Devor A, Ulbert I, Dunn AK, Narayanan SN, Jones SR, Andermann ML, Boas DA, Dale AM. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc Natl Acad Sci USA 102: 3822–3827, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron 50: 799–812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dziewas R, Soros P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage 20: 135–144, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Ferrier D. The Croonian Lectures on Cerebral Localisation. BMJ 2: 68–75, 1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fornito A, Whittle S, Wood SJ, Velakoulis D, Pantelis C, Yucel M. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. Neuroimage 33: 843–854, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Fornito A, Wood SJ, Whittle S, Fuller J, Adamson C, Saling MM, Velakoulis D, Pantelis C, Yucel M. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum Brain Mapp 29: 222–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44: 162–167, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Gold L, Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc Natl Acad Sci USA 99: 7699–7704, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Rev Neurosci 2: 685–694, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, Tallis RC, Thompson DG. The cortical topography of human swallowing musculature in health and disease. Nat Med 2: 1217–1224, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Hamdy S, Mikulis DJ, Crawley A, Xue S, Lau H, Henry S, Diamant NE. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol Gastrointest Liver Physiol 277: G219–G225, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Hamdy S, Rothwell JC, Brooks DJ, Bailey D, Aziz Q, Thompson DG. Identification of the cerebral loci processing human swallowing with H215O PET activation. J Neurophysiol 81: 1917–1926, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab 22: 908–917, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Hu X, Le TH, Ugurbil K. Evaluation of the early response in fMRI in individual subjects using short stimulus duration. Magn Reson Med 37: 877–884, 1997 [DOI] [PubMed] [Google Scholar]
- 26. Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Kern M, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, Shaker R. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 280: G531–G538, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol 280: G354–G360, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412: 150–157, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Loubinoux I, Carel C, Alary F, Boulanouar K, Viallard G, Manelfe C, Rascol O, Celsis P, Chollet F. Within-session and between-session reproducibility of cerebral sensorimotor activation: a test–retest effect evidenced with functional magnetic resonance imaging. J Cereb Blood Flow Metab 21: 592–607, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Margulies DS, Vincent JL, Kelly C, Lohmann G, Uddin LQ, Biswal BB, Villringer A, Castellanos FX, Milham MP, Petrides M. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA 106: 20069–20074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin RE, Goodyear BG, Gati JS, Menon RS. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 85: 938–950, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Martin RE, MacIntosh BJ, Smith RC, Barr AM, Stevens TK, Gati JS, Menon RS. Cerebral areas processing swallowing and tongue movement are overlapping but distinct: a functional magnetic resonance imaging study. J Neurophysiol 92: 2428–2443, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia 8: 195–202, 1993 [DOI] [PubMed] [Google Scholar]
- 35. McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP. Variability in fMRI: an examination of intersession differences. Neuroimage 11: 708–734, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Miki A, Raz J, van Erp TG, Liu CS, Haselgrove JC, Liu GT. Reproducibility of visual activation in functional MR imaging and effects of postprocessing. AJNR Am J Neuroradiol 21: 910–915, 2000 [PMC free article] [PubMed] [Google Scholar]
- 37. Mosier K, Patel R, Liu WC, Kalnin A, Maldjian J, Baredes S. Cortical representation of swallowing in normal adults: functional implications. Laryngoscope 109: 1417–1423, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Mosier KM, Liu WC, Maldjian JA, Shah R, Modi B. Lateralization of cortical function in swallowing: a functional MR imaging study. AJNR Am J Neuroradiol 20: 1520–1526, 1999 [PMC free article] [PubMed] [Google Scholar]
- 39. Naidich TP, Kang E, Fatterpekar GM, Delman BN, Gultekin SH, Wolfe D, Ortiz O, Yousry I, Weismann M, Yousry TA. The insula: anatomic study and MR imaging display at 1.5 T. AJNR Am J Neuroradiol 25: 222–232, 2004 [PMC free article] [PubMed] [Google Scholar]
- 40. Narita N, Yamamura K, Yao D, Martin RE, Sessle BJ. Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res 824: 140–145, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Noll DC, Genovese CR, Nystrom LE, Vazquez AL, Forman SD, Eddy WF, Cohen JD. Estimating test-retest reliability in functional MR imaging. II: Application to motor and cognitive activation studies. Magn Reson Med 38: 508–517, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Paine TL, Conway CA, Malandraki GA, Sutton BP. Simultaneous dynamic and functional MRI scanning (SimulScan) of natural swallows. Magn Reson Med 65: 1247–1252, 2011 [DOI] [PubMed] [Google Scholar]
- 43. Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci 360: 781–795, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Rev Neurosci 5: 184–194, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Ramsey NF, Tallent K, van Gelderen P, Frank JA, Moonen CT, Weinberger DR. Reproducibility of human 3D fMRI brain maps acquired during a motor task. Hum Brain Mapp 4: 113–121, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Rasmussen T, Penfield W. Further studies of the sensory and motor cerebral cortex of man. Fed Proc 6: 452–460, 1947 [PubMed] [Google Scholar]
- 48. Rasmussen T, Penfield W. The human sensorimotor cortex as studied by electrical stimulation. Fed Proc 6: 184, 1947 [PubMed] [Google Scholar]
- 49. Rombouts SA, Barkhof F, Hoogenraad FG, Sprenger M, Scheltens P. Within-subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. Magn Reson Imaging 16: 105–113, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Rose JE, Woolsey CN. The orbitofrontal cortex and its connections with the mediodorsal nucleus in rabbit, sheep and cat. Res Publ Assoc Res Nerv Ment Dis 27: 210–232, 1948 [PubMed] [Google Scholar]
- 51. Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage 44: 839–848, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nature Neurosci 9: 569–577, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36: 1195–1210, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Smith AT, Williams AL, Singh KD. Negative BOLD in the visual cortex: evidence against blood stealing. Hum Brain Mapp 21: 213–220, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soltysik DA, Hyde JS. Strategies for block-design fMRI experiments during task-related motion of structures of the oral cavity. Neuroimage 29: 1260–1271, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Soros P, Inamoto Y, Martin RE. Functional brain imaging of swallowing: an activation likelihood estimation meta-analysis. Hum Brain Mapp 30: 2426–2439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sumi T. Modification of cortically evoked rhythmic chewing and swallowing from midbrain and pons. Jpn J Physiol 21: 489–506, 1971 [DOI] [PubMed] [Google Scholar]
- 59. Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia 18: 71–77, 2003 [DOI] [PubMed] [Google Scholar]
- 60. Talaraich J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, 1988 [Google Scholar]
- 61. Teismann IK, Dziewas R, Steinstraeter O, Pantev C. Time-dependent hemispheric shift of the cortical control of volitional swallowing. Hum Brain Mapp 30: 92–100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Veltman DJ, Friston KJ, Sanders G, Price CJ. Regionally specific sensitivity differences in fMRI and PET: where do they come from? Neuroimage 11: 575–588, 2000 [DOI] [PubMed] [Google Scholar]
- 63. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Rev Neurosci 6: 533–544, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wade AR. The negative BOLD signal unmasked. Neuron 36: 993–995, 2002 [DOI] [PubMed] [Google Scholar]
- 65. White LE, Andrews TJ, Hulette C, Richards A, Groelle M, Paydarfar J, Purves D. Structure of the human sensorimotor system. I: Morphology and cytoarchitecture of the central sulcus. Cerebral Cortex 7: 18–30, 1997 [DOI] [PubMed] [Google Scholar]
- 66. Yetkin FZ, Haughton VM, Cox RW, Hyde J, Birn RM, Wong EC, Prost R. Effect of motion outside the field of view on functional MR. AJNR Am J Neuroradiol 17: 1005–1009, 1996 [PMC free article] [PubMed] [Google Scholar]
- 67. Yetkin FZ, McAuliffe TL, Cox R, Haughton VM. Test-retest precision of functional MR in sensory and motor task activation. AJNR Am J Neuroradiol 17: 95–98, 1996 [PMC free article] [PubMed] [Google Scholar]
- 68. Zald DH, Pardo JV. The functional neuroanatomy of voluntary swallowing. Ann Neurol 46: 281–286, 1999 [PubMed] [Google Scholar]
- 69. Zilles K, Schlaug G, Matelli M, Luppino G, Schleicher A, Qu M, Dabringhaus A, Seitz R, Roland PE. Mapping of human and macaque sensorimotor areas by integrating architectonic, transmitter receptor, MRI and PET data. J Anat 187: 515–537, 1995 [PMC free article] [PubMed] [Google Scholar]