Abstract
The pathogenesis of gastroesophageal reflux disease (GERD) remains elusive, but recent evidence suggests that early secretion of inflammatory cytokines and chemokines by the mucosa leads to influx of immune cells followed by tissue damage. We previously showed that exposure of esophageal mucosa to HCl causes ATP release, resulting in activation of acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine acetyltransferase (lyso-PAF AT), the enzyme responsible for the production of platelet-activating factor (PAF). In addition, HCl causes release of IL-8 from the esophageal mucosa. We demonstrate that esophageal epithelial cells secrete proinflammatory mediators in response to HCl and that this response is mediated by ATP. Monolayers of the human esophageal epithelial cell line HET-1A were exposed to acidified cell culture medium (pH 5) for 12 min, a total of seven times over 48 h, to simulate the recurrent acid exposure clinically occurring in GERD. HCl upregulated mRNA and protein expression for the acid-sensing transient receptor potential cation channel, subfamily vanilloid member 1 (TRPV1), lyso-PAF AT, IL-8, eotaxin-1, -2, and -3, macrophage inflammatory protein-1α, and monocyte chemoattractant protein-1. The chemokine profile secreted by HET-1A cells in response to repeated HCl exposure parallels similar findings in erosive esophagitis patients. In HET-1A cells, the TRPV1 agonist capsaicin reproduced these findings for mRNA of the inflammatory mediators lyso-PAF AT, IL-8, and eotaxin-1. These effects were blocked by the TRPV1 antagonists iodoresiniferatoxin and JNJ-17203212. These effects were imitated by direct application of ATP and blocked by the nonselective ATP antagonist suramin. We conclude that HCl/TRPV-induced ATP release upregulated secretion of various chemoattractants by esophageal epithelial cells. These chemoattractants are selective for leukocyte subsets involved in acute inflammatory responses and allergic inflammation. The data support the validity of HET-1A cells as a model of the response of the human esophageal mucosa in GERD.
Keywords: gastroesophageal reflux; chemokines; transient receptor potential cation channel, subfamily vanilloid member 1; esophageal epithelium
several studies using animal models and human tissue samples suggest that proinflammatory cytokine production may underlie the development of erosive esophagitis (EE) in gastroesophageal reflux disease (GERD) (8, 11, 15, 22, 56). The secretion of these mediators is believed to precede the infiltration of neutrophils and eosinophils into the mucosa and submucosa (4, 18, 21, 50), ultimately leading to tissue damage and ulcerations. This viewpoint is supported by endoscopically obtained esophageal biopsies from patients with EE showing infiltration of immune cells. Esophageal biopsies primarily contain epithelial cells, the major component of the esophageal mucosa, suggesting that chemoattractants may be present in and derived from epithelial cells. Enhanced expression of chemokines such as IL-8, a potent chemoattractant for neutrophils, has been detected in esophageal biopsy samples of EE patients, with IL-8 levels being associated with the endoscopic severity of EE (22). Thus production of chemokines by esophageal epithelial cells may be an important step in initiating the inflammatory process. However, the specific mediators responsible for infiltration of immune cells (4, 18) in EE have not been defined.
Given the nature of the inflammatory infiltrate in GERD, possible candidates for chemoattractants released by epithelial cells are IL-5, IL-8, regulated upon activation, normal T cell expressed and presumably secreted (RANTES, or CCL-5), eotaxin-1, -2, and -3, monocyte chemoattractant protein-1 (MCP-1, or CCL-2), and macrophage inflammatory protein 1α (MIP-1α, or CCL-3). These act primarily as chemotactic factors for eosinophils, monocytes, and other immune cells (29, 57). Platelet-activating factor (PAF) is a potent chemoattractant for eosinophils and selectively induces the migration of eosinophils over that of neutrophils (24, 49, 55).
We previously showed that the esophageal mucosa contains acid-sensing transient receptor potential cation channel, subfamily vanilloid member 1 (TRPV1) receptors and, when exposed to HCl, releases substance P, calcitonin gene-related peptide, PAF, and IL-8 (32). We further showed that HCl-induced activation of TRPV1 causes ATP release from esophageal epithelial cells, which, in turn, causes release of calcitonin gene-related peptide and substance P from esophageal submucosal neurons and activation of acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine acetyltransferase (lyso-PAF AT), the enzyme responsible for the production of PAF in epithelial cells (33). Repeated application of HCl or ATP causes upregulation of lyso-PAF AT in epithelial cells (33). These data point to ATP as a critical inducer for release of inflammatory mediators, such as PAF, and possibly for cytokines or chemoattractants, such as IL-8.
In the present investigation, we performed a systematic screening of possible chemoattractants or cytokines released by TRPV1 activation in the human esophageal epithelial cell line HET-1A and examined the role of ATP in mediating the release of these mediators, as well as their upregulation by repeated TRPV1 stimulation. After repeated acid exposure mimicking reflux episodes in GERD patients (2, 43, 47), HET-1A cells upregulate mRNA and protein for the TRPV1 receptor itself and increase expression for lyso-PAF AT, as well as selected cytokines and chemokines known to function as chemoattractants for neutrophils, eosinophils, and monocytes. The cytokine increase was inhibited by an ATP receptor antagonist. The HCl-induced cytokine increases observed in HET-1A cells were similar to those observed in human esophageal biopsies from patients with EE. Taken together, these data indicate that acid-induced changes in the esophageal mucosa may begin with epithelial cells responding to acid by releasing ATP. ATP, in turn, induces upregulation of TRPV1, as well as secretion of multiple cytokines, with preference for mediators that promote epithelial infiltration by eosinophils, neutrophils, and monocytes.
METHODS
HET-1A cell culture.
Human esophageal squamous (HET-1A) cells (American Type Culture Collection, Manassas, VA) were cultured at 37°C in a 5% CO2-humidified atmosphere in bronchial epithelial cell medium (BEGM BulletKit, Lonza, Walkersville, MD) containing basal medium (BEBM) plus additives (BEGM SingleQuots, Lonza) in wells precoated with a mixture of 0.01 mg/ml fibronectin and 0.03 mg/ml Vitrogen 100 (Cohesion, Palo Alto, CA). HET-1A cells were originally obtained from normal human esophageal autopsy tissue. They have been shown to retain epithelial morphology, stain positively for cytokeratins, and remain nontumorigenic (51).
Experimental procedure.
HET-1A cells were exposed to BEBM, pH 5, with 1.2 mM Ca2+ for 12-min on seven occasions over 48 h. HCl (2 N) was added to BEBM to bring the solution to pH 5.0. Cells were exposed for 12 min to acidified medium at 8:30 AM, 11:30 AM, and 2:30 PM each on days 1 and 2. On day 3, final exposure took place at 8:30 AM for 12 min. At 1 h after readdition of nonacidified culture medium, the cells were harvested. Gene (mRNA) expression was determined by real-time PCR and protein expression by multiplex ELISA cytokine assay (Eve Technologies, Calgary, AB, Canada). To confirm involvement of vanilloid receptors in acid-induced mRNA changes, cells were pretreated with the TRPV1 receptor antagonist iodoresiniferatoxin (IRTX; Sigma-Aldrich, St. Louis, MO) at 3 × 10−6 M or 10−6 M JNJ-17203212 (Tocris Bioscience, Minneapolis, MN) for 20 min before each exposure to acid.
To confirm involvement of the TRPV1 receptor in these acid-induced changes, we used the selective TRPV1 agonist capsaicin (10−5 M; Sigma-Aldrich) with the same repeated exposure protocol and measured mRNA for lyso-PAF AT, IL-8, and eotaxin-1 for comparison with acid-induced changes.
To confirm involvement of ATP in these acid-induced changes, we used the nonselective purinergic receptor antagonist suramin (10−6 M; Sigma-Aldrich) for 20 min before each exposure to acid or direct exposure to the ATP analog ATPγS (10−4 M; Sigma-Aldrich) with the same repeated exposure protocol and measured mRNA for lyso-PAF AT, IL-8, eotaxins, MCP-1, and MIP-1α for comparison with acid-induced changes.
Real-time PCR.
Total RNA derived from HET-1A cells was isolated by RNeasy Mini Kit (Qiagen, Valencia, CA). A 2-μg sample of total RNA was treated with DNase I to eliminate DNA contamination. The sample was reverse-transcribed and subjected to real-time PCR using the GeneAmp Gold RNA PCR Reagent Kit (Applied Biosystems, Foster City, CA) and Power SYBR Green PCR Master Mix Kit (Applied Biosystems). Primers are shown in Table 1.
Table 1.
Primers
| Primer | Sequence | Amplicon, bp |
|---|---|---|
| Lyso-PAF AT | ||
| Sense | 5′-ATTGACTTCCGAGAGTATGTGA-3′ | 85 |
| Antisense | 5′-GCTTAAATGCCACCTGGATGA-3′ | |
| IL-8 | ||
| Sense | 5′-ACTGAGAGTGATTGAGAGTGGAC-3′ | 112 |
| Antisense | 5′-AACCCTCTGCACCCAGTTTTC-3′ | |
| hCCL-2 (MCP-1) | ||
| Sense | 5′-GATCTCAGTGCAGAGGCTCG-3′ | 153 |
| Antisense | 5′-TGCTTGTCCAGGTGGTCCAT-3′ | |
| hCCL-3 (MIP-1α) | ||
| Sense | 5′-TGCTTGTCCAGGTGGTCCAT-3′ | 212 |
| Antisense | 5′-CACTCAGCTCCAGGTCGCTGAC-3′ | |
| hCCL-5 (RANTES) | ||
| Sense | 5′-ACCACACCCTGCTGCTTTGC-3′ | 159 |
| Antisense | 5′-CCGAACCCATTTCTTCTCTGG-3′ | |
| hEot-1 | ||
| Sense | 5′-GAAACCACCACCTCTCACG-3′ | 190 |
| Antisense | 5′-GCTCTCTAGTCGCTGAAGGG-3′ | |
| hEot-2 | ||
| Sense | 5′-GCAGGAGCACATGCCTCAA-3′ | 113 |
| Antisense | 5′-GGCGTCCAGGTTCTTCATGTA-3′ | |
| hEot-3 | ||
| Sense | 5′-ATATCCAAGACCTGCTGCTTC-3′ | 152 |
| Antisense | 5′-TTTTTCCTTGGATGGGTACAG-3′ |
Lyso-PAF AT, acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine acetyltransferase; MCP-1, monocyte chemoattractant protein 1; MIP-1α, macrophage inflammatory protein 1α; RANTES, regulated upon activation, normal T cell expressed and secreted; hEot, human eotaxin.
Western blot analysis.
Cells, incubated in 1.2 mM Ca2+ BEBM, were acid-treated (see Experimental procedure) and then lysed in Triton X lysis buffer [50 mM Tris·HCl, pH 7.5, 100 mM NaCl, 50 mM NaF, 5 mM EDTA, 1% (vol/vol) Triton X-100, 40 mM β-glycerol phosphate, 40 mM p-nitrophenyl phosphate, 200 μM sodium orthovanadate, 100 μM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, and 1 μg/ml aprotinin]. The homogenate was centrifuged at 10,000 g for 5 min, and the protein concentration in the supernatant was determined using the Protein Assay Kit (Bio-Rad, Hercules, CA) based on the Bradford dye-binding method (7). The supernatant containing 80 μg of protein was used for Western blot assay. The primary antibody for lyso-PAF AT (Novus Biologicals, Littleton, CO) was diluted 1:1,500. The secondary antibody, horseradish peroxidase-conjugated anti-mouse antibody (Cell Signaling Technology, Danvers, MA), was diluted 1:2,000. Detection was achieved with Western Lightning ECL agent (Perkin Elmer, Waltham, MA). Molecular weight was estimated by comparison of sample bands with a prestained molecular weight marker (Bio-Rad, Melville, NY).
Multiplex ELISA.
The cell culture medium (from control and acid-treated HET-1A cells) was collected and sent to Eve Technologies (Calgary, AB, Canada) for multiplex ELISA 65 cytokine assay, including eotaxin-1, -2, and -3, IL-8, MCP-1, MIP-1α, and RANTES. The complete panel of cytokines is shown in Table 2.
Table 2.
Human cytokine/chemokine panel
| 6Ckine, BCA-1, CTACK, EGF, ENA-78, eotaxin-1, eotaxin-2, eotaxin-3, FGF-2, Fit-3L, fractalkine, G-CSF, GM-CSF, GRO, I-309, IFNα2, IFNγ, IL-1α, IL-1β, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-16, IL-17, IL-20, IL-21, IL-23, IL-28a, IL-33, IP-10, LIF, MCP-1, MCP-2, MCP-3, MCP-4, MDC, MIP-1α, MIP-1β, MIP-1d, PDGF-AA, PDGF-AB/BB, RANTES, SDF-1 α+β, sCD40K, SCF, sIL-2Rα, TARC, TGFα, TNFα, TNFβ, TPO, TRAIL, TSLP, VEGF |
BCA-1, B cell-attracting chemokine; CTACK, cutaneous T cell-attracting chemokine; ENA-78, epithelial neutrophil-activating peptide; G-CSF and GM-CSF, granulocyte and granulocyte-macrophage colony-stimulating factor; GRO, growth-regulated oncogene; IP-10, IFNγ-induced protein 10; LIF, leukemia-inhibitory factor; MDC, macrophage-derived chemokine; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; SDF-1, stromal cell-derived factor 1; sCD40K, soluble CD40; SCF, stem cell factor; sIL-2Rα, soluble IL-2 receptor-α; TARC, thymus and activation-regulated chemokine; TRAIL, TNF-related apoptosis-inducing ligand; TSLP, thymic stromal lymphopoietin.
Human data.
A total of 25 consecutive patients (mean age 63 yr, range 47–76 yr) attending the outpatient units of Campus Bio Medico University of Rome for recurrent typical GERD symptoms (heartburn and/or acid regurgitation) lasting >6 mo and with evidence of EE at endoscopy (grade A in 10 patients, grade B in 4 patients, and grade C in 1 patient, according to the Los Angeles classification) were invited to take part in the study. Exclusion criteria were as follows: presence of Barrett's esophagus, peptic ulcer disease, history of gastrointestinal (GI) cancer, and GI tract surgery (with the exception of appendectomy). Patients on proton pump inhibitors, H2 antagonists, or prokinetic drugs underwent a 3-wk pharmacological washout before upper endoscopy. Seventeen asymptomatic hospital staff volunteers with no history of GERD underwent the same protocol, thus representing a healthy control group.
After an overnight fast, patients and controls underwent upper endoscopy, performed by the same operator (A.A.). A combination of midazolam and propofol was used for sedation. The distal portion of the esophagus was carefully evaluated to determine the presence of mucosal injury. A total of four biopsies from each individual, two for routine histological evaluation and two for real-time PCR, were taken at 5 cm above the squamocolumnar junction in normal-appearing mucosa from EE patients and control subjects.
RESULTS
TRPV1 receptors in esophageal epithelium.
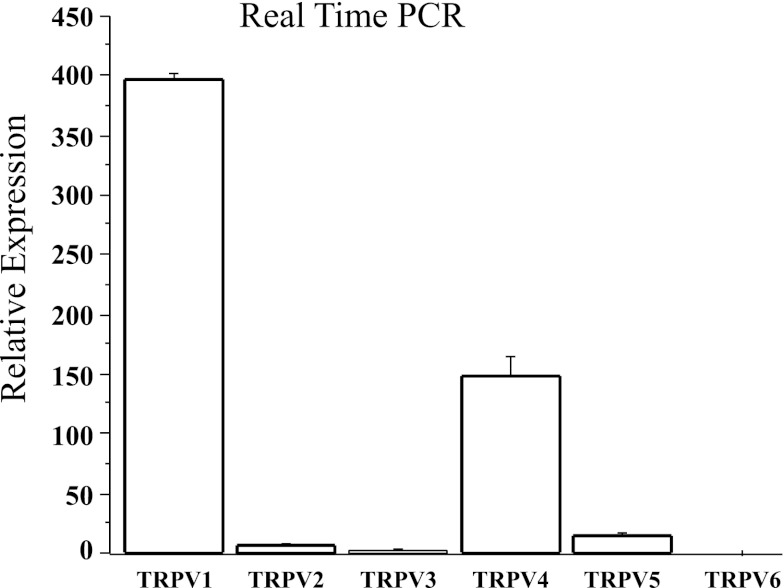
To verify the presence of acid-sensing receptors on HET-1A cells, we measured mRNA expression for TRPV1–TRPV6 (Fig. 1). Expression was higher for TRPV1 than TRPV2–TRPV6 mRNA (P < 0.05 by ANOVA), suggesting that TRPV1 is the dominant TRPV subfamily member transducing the effects of H+ in epithelial cells.
Fig. 1.
Human esophageal squamous epithelial (HET-1A) cells contain mRNA for several vanilloid receptors, with greater levels of transient receptor potential cation channel subfamily vanilloid (TRPV) member 1 (TRPV1) mRNA than TRPV2–TRPV6 mRNA (P < 0.05 ANOVA). Values are means ± SE of 3 different sets of data from 3 separate experiments.
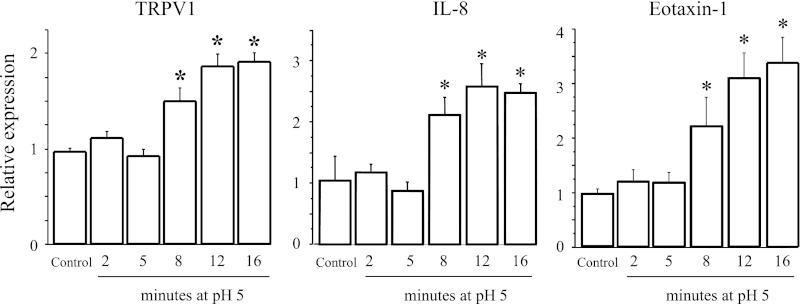
To simulate the recurrent epithelial exposure to acid in GERD (2, 43, 47), HET-1A cells were exposed seven times over 48 h to pH 5 for 2–16 min. TRPV1, IL-8, and eotaxin-1 mRNA increased on incubation with acidified medium, with a significantly higher mRNA expression with longer exposures (Fig. 2). For instance, when the cells were subjected to seven 2-min exposures to pH 5, their mRNA levels did not increase with respect to nonexposed cells. The maximum mRNA increase occurred for repeated 12-min exposures. Increasing the duration of the exposure to 16 min did not measurably increase mRNA for TRPV1, IL-8, or eotaxin-1. The maximally effective 12-min exposure was therefore used for subsequent experiments. This acid exposure protocol induced <2% cell death over the treatment period as measured by Trypan blue exclusion, with HET-1A monolayers remaining intact when examined microscopically. We previously demonstrated that in vitro exposure of esophageal mucosa to a more acidic pH, for example, pH 4, causes significant cell death (11), most likely because the preparation is not buffered by continuous blood perfusion, as occurs in vivo.
Fig. 2.
HET-1A cells were exposed 7 times to pH 5 for 0–16 min over a 48-h period. mRNA levels, obtained by real-time PCR, are shown as a function of duration of individual exposure to pH 5. mRNA increase was maximal for repeated 12-min exposures. Increasing exposure duration to 16 min did not measurably increase mRNA. For simplicity, data are shown only for TRPV1, IL-8, and eotaxin-1. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. *Significantly different from control (P < 0.05).
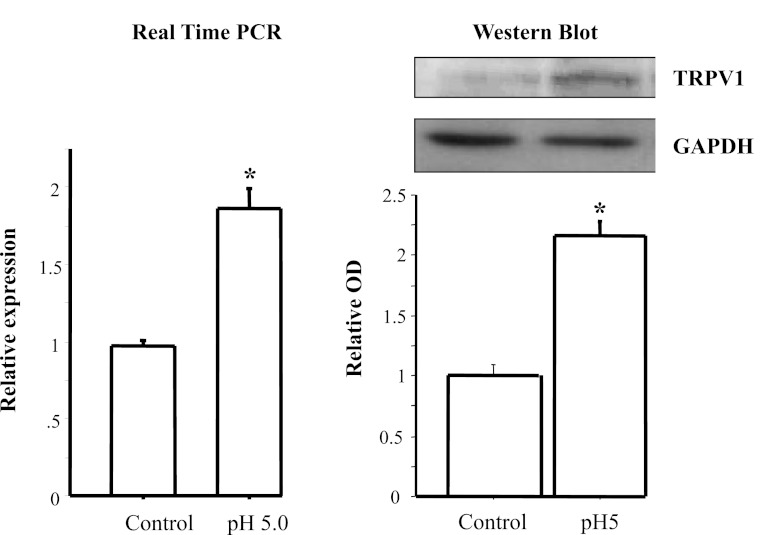
Figure 3 shows that the selected acid exposure protocol increases TRPV1 mRNA and protein, similar to the TRPV1 increase previously reported for EE patients (18).
Fig. 3.
Real-time PCR and Western blot for TRPV1. HET-1A cells were cultured in bronchial epithelial cell medium and treated with the selected HCl exposure protocol (12 min of exposure to pH 5 on 7 occasions over 48 h). Left: TRPV1 mRNA expression determined by real-time PCR. Right: TRPV1 protein expression determined by Western blotting. Relative mRNA expression was calculated with respect to mRNA from untreated cells. Western blot results are shown as relative optical density (OD) normalized to GAPDH. Exposure protocol caused a significant increase in mRNA and protein expression for TRPV1 (*P < 0.01). Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments.
HCl-induced inflammatory mediators and cytokines.
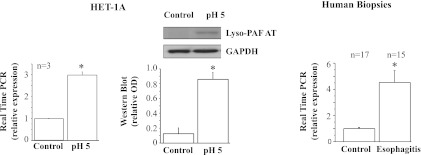
Consistent with previously reported data (33), the selected acid exposure protocol caused an increase in lyso-PAF AT mRNA and protein (Fig. 4), likely reflected by increased PAF production in response to HCl-induced stimulation (32). A similar increase in lyso-PAF AT mRNA was noted in esophageal biopsies from EE patients. Figure 5 shows a similar increase in IL-8 mRNA and protein in HET-1A cells and increased IL-8 mRNA in esophageal biopsies from EE patients, consistent with data previously demonstrated in rabbit mucosa (32).
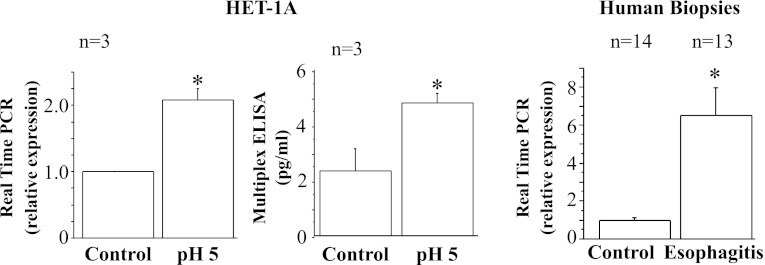
Fig. 4.
Real-time PCR and Western blot for acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine acetyltransferase (lyso-PAF AT), the enzyme responsible for production of platelet-activating factor. HET-1A cells were treated with the selected HCl exposure protocol and then used to determine lyso-PAF AT mRNA expression by real-time PCR (left) and protein expression (middle) by Western blotting. Relative mRNA expression was calculated with respect to mRNA from untreated cells. Repeated exposures to pH 5 over 48 h caused a significant increase in mRNA and protein expression for lyso-PAF AT (*P < 0.01). Western blot results are shown as relative optical density normalized to GAPDH. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. Right: real-time PCR for human biopsies from patients with erosive esophagitis (EE) and normal controls. Increased lyso-PAF AT mRNA in EE patients parallels increase in HET-1A cells after exposure to HCl.
Fig. 5.
Real-time PCR and ELISA for IL-8. HET-1A cells were treated with the selected HCl exposure protocol and then used to determine IL-8 RNA expression by real-time PCR (left) and protein expression by ELISA (middle). Relative mRNA expression was calculated with respect to mRNA from untreated cells. Repeated exposures to pH 5 over 48 h caused a significant increase in mRNA and protein expression for IL-8 (*P < 0.02). Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. Right: real-time PCR for human biopsies from EE patients and normal controls. Increased IL-8 mRNA expression in EE patients parallels increase in HET-1A cells after exposure to HCl.
A multiplex ELISA for 65 cytokines (Table 2) was used to explore the changed cytokines or chemokines on protein level in response to repeated HCl exposure.
HCl also caused an increase in mRNA for eotaxin-1, -2, and -3 (Fig. 6–8) that was associated with a corresponding increase in protein secretion. A similar increase in mRNA was observed in esophageal biopsies from EE patients.
Fig. 6.
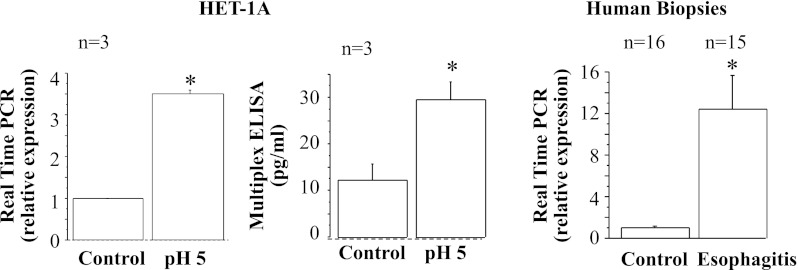
Real-time PCR and ELISA for eotaxin-1. HET-1A cells were treated with the selected HCl exposure protocol and then used to determine eotaxin-1 mRNA expression by real-time PCR (left) and protein expression by ELISA (middle). Repeated exposures to pH 5 over 48 h caused significant increase in mRNA and protein expression for eotaxin-1 (*P < 0.05). Relative mRNA expression was calculated with respect to mRNA from untreated cells. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. Right: real-time PCR for human biopsies from EE patients and normal controls. Increased eotaxin-1 mRNA in EE parallels increase in HET-1A cells after exposure to HCl.
Fig. 8.
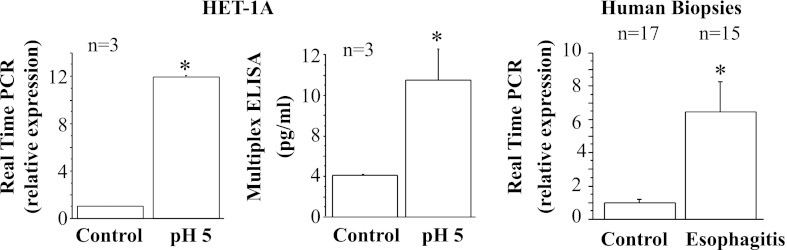
Real-time PCR and ELISA for eotaxin-3. HET-1A cells were treated with the selected HCl exposure protocol and then used to determine eotaxin-3 mRNA expression by real-time PCR (left) and protein expression by ELISA (middle). Repeated exposures to pH 5 over 48 h caused significant increase in mRNA and protein expression for eotaxin-3 (*P < 0.01). Relative mRNA expression was calculated with respect to mRNA from untreated cells. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. Right: real-time PCR for human biopsies from EE patients and normal controls. Increased eotaxin-3 mRNA in EE patients parallels increase in HET-1A cells after exposure to HCl.
Fig. 7.
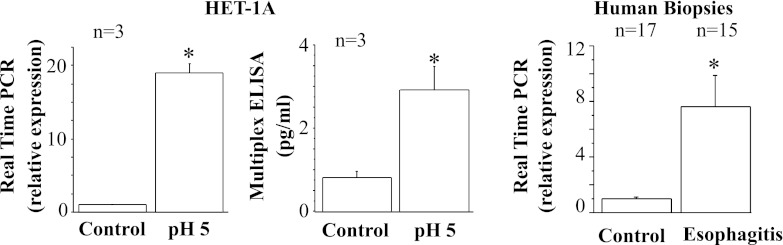
Real-time PCR and ELISA for eotaxin-2. HET-1A cells were treated with the selected HCl exposure protocol and then used to determine eotaxin-2 mRNA expression by real-time PCR (left) and protein expression by ELISA (middle). Repeated exposures to pH 5 over 48 h caused significant increase in mRNA and protein expression for eotaxin-2 (*P < 0.02). Relative mRNA expression was calculated with respect to mRNA from untreated cells. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. Right: real-time PCR for human biopsies from EE patients and normal controls. Increased eotaxin-2 mRNA in EE patients parallels increase in HET-1A cells after exposure to HCl.
A comparable HCl-induced increase in MCP-1 and MIP-1α mRNA and protein expression in HET-1A cells is shown in Figs. 9 and 10. A similar mRNA increase was observed in esophageal biopsies from EE patients.
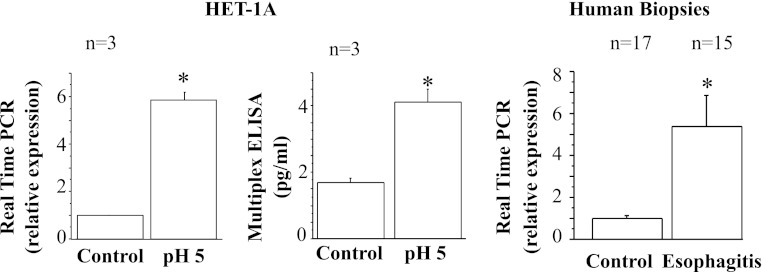
Fig. 9.
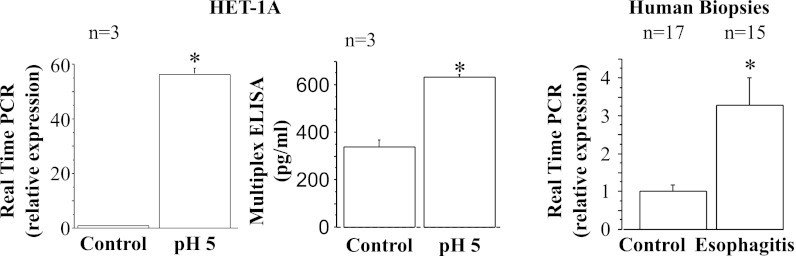
Real-time PCR and ELISA for monocyte chemoattractant protein 1 (MCP-1). HET-1A cells were treated with the selected HCl exposure protocol and then used to determine MCP-1 RNA expression by real-time PCR (left) and protein expression by ELISA (middle). Repeated exposures to pH 5 over 48 h caused a significant increase in mRNA and protein expression for MCP-1 (*P < 0.001). Relative mRNA expression was calculated with respect to mRNA from untreated cells. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. Right: real-time PCR for human biopsies from EE patients or from normal controls. Increased MCP-1 mRNA in EE patients parallels increase in HET-1A cells after exposure to HCl.
Fig. 10.
Real-time PCR and ELISA for macrophage inflammatory protein 1α (MIP-1α). HET-1A cells were treated with the selected HCl exposure protocol and then used to determine MIP-1α RNA expression by real-time PCR (left) and protein expression by ELISA (middle). Repeated exposures to pH 5 over 48 h caused a significant increase in mRNA and protein expression for MIP-1α (*P < 0.05). Relative mRNA expression was calculated with respect to mRNA from untreated cells. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. Right: real-time PCR for human biopsies from EE patients and normal controls. Increased MIP-1α mRNA in EE patients parallels increase in HET-1A cells after exposure to HCl.
Role of TRPV1 and ATP in upregulation of inflammatory mediators/cytokines.
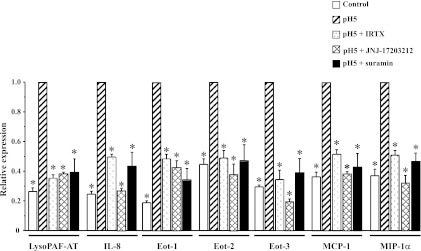
To investigate the mechanisms underlying the HCl-induced mediator secretion by HET-1A cells, we performed experiments exploring the role of TRPV1 and examining a possible role of ATP in this process. mRNA expression for lyso-PAF AT, IL-8, eotaxin-1, -2, and -3, MCP-1, and MIP-1α was significantly greater in HCl-exposed than control HET-1A cells, as previously shown (Fig. 11; P < 0.05). Preincubation with the TRPV1 receptor antagonists IRTX and JNJ-17203212 inhibited the increase in mRNA for lyso-PAF AT, IL-8, eotaxin 1, -2, and -3, MCP-1, and MIP-1α (P < 0.05). The values after TRPV1 blockade were not significantly different from control values, supporting a role of TRPV1 in mediating the HCl-induced mRNA increase. Similarly, preincubation with the nonselective ATP antagonist suramin inhibited the increase in mRNA for lyso-PAF AT, IL-8, eotaxin-1, -2, and -3, MCP-1, and MIP-1α (P < 0.05). The values after suramin exposure were not significantly different from control values, supporting a role of ATP in mediating the HCl-induced, TRPV1-mediated mRNA increase.
Fig. 11.
Role of TRPV1 and ATP in HCl-induced upregulation of mRNA for lyso-PAF AT, IL-8, eotaxin-1 (Eot-1), eotaxin-2 (Eot-2), eotaxin-3 (Eot-3), MCP-1, and MIP-1α. HET-1A cells were treated with the selected HCl exposure protocol. Some cells were pretreated with the TRPV1 antagonists iodoresiniferatoxin (IRTX, 3 × 10−6 M) and JNJ-17203212 (10−6 M) or the nonselective purinergic receptor antagonist suramin (10−4 M) for 20 min before each exposure to acid. Real-time PCR was used for mRNA determination. Relative mRNA expression was calculated with respect to mRNA values after acid exposure (pH 5, and reported as 1). Initial mRNA values, before exposure to HCl, are shown as control. For lyso-PAF AT and all the cytokines/chemokines, initial mRNA values were significantly lower (*P < 0.05) than values after acid exposure. There was no significant difference between initial mRNA values and values after TRPV1 antagonists or after suramin pretreatment, indicating that TRPV1 antagonists and suramin inhibit the HCl-induced increase in mRNA. None of the antagonists affected cell viability as assessed by Trypan blue exclusion (>98%). Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments. *Significantly different from pH 5 (P < 0.05).
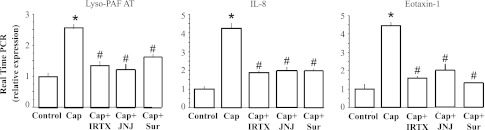
To confirm the involvement of the TRPV1 receptor and ATP in the HCl-induced changes, using the same repeated-exposure protocol used for HCl, we treated HET-1A cells with the selective TRPV1 agonist capsaicin and measured mRNA for lyso-PAF AT, IL-8, and eotaxin-1 (Fig. 12). Repeated capsaicin exposure increased lyso-PAF AT, IL-8, and eotaxin-1 mRNA to levels comparable to those induced by HCl exposure. As expected, the capsaicin-induced mRNA increase was significantly (P < 0.05) reduced by the TRPV1 antagonists IRTX, and JNJ-17203212, similar to antagonism for the HCl-mediated response. The capsaicin-induced mRNA increase was also blocked by the ATP antagonist suramin (P < 0.05), indicating a role of ATP in TRPV1-induced mRNA upregulation.
Fig. 12.
Selective TRPV1 agonist capsaicin (Cap) was used to confirm TRPV1-mediated upregulation of mRNA for lyso-PAF AT, IL-8, and eotaxin-1. HET-1A cells were exposed for 12 min to culture medium containing capsaicin (10−5 M) on 7 occasions over 48 h. mRNA upregulation produced by capsaicin-induced TRPV1 activation was similar to that produced by HCl exposure (see Figs. 4–6). mRNA values after repeated capsaicin exposure were not significantly different from those induced by HCl exposure and significantly greater (*P < 0.001) than control values. As expected, pretreatment with IRTX (3 × 10−6 M) or JNJ-17203212 (JNJ, 10−6 M) significantly reduced capsaicin-mediated mRNA increase (#P < 0.001), confirming that the antagonists properly inhibit TRPV1 activation. Inhibition by the nonselective ATP antagonist suramin (Sur, 10−4 M) supports a role of ATP in mediating mRNA upregulation.
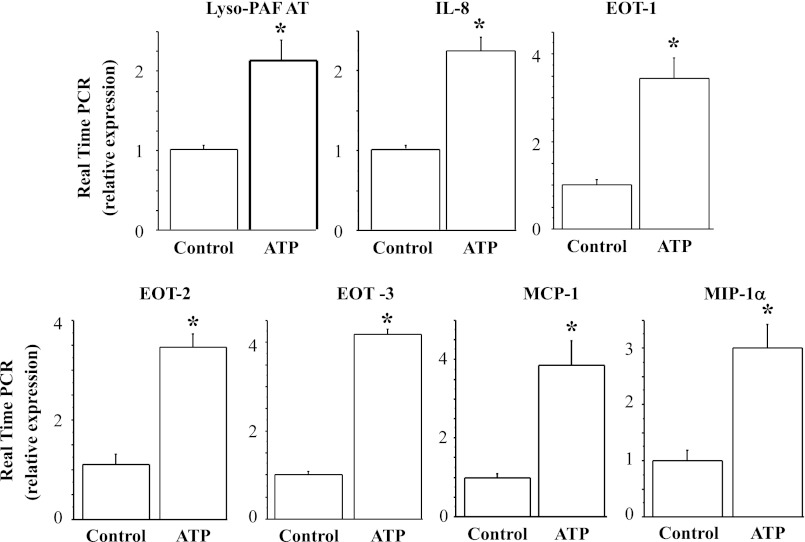
Direct and repeated exposure of the epithelial cells to the nonhydrolyzable ATP analog ATPγS using the above protocol reproduced the HCl- and TRPV1-induced upregulation of lyso-PAF AT, IL-8, eotaxin-1, -2, and -3, MCP-1, and MIP-1α, further supporting a role of ATP in the upregulation (Fig. 13).
Fig. 13.
ATP-induced upregulation of lyso-PAF AT, IL-8, eotaxin-1, -2, and -3, MCP-1, and MIP-1α mRNA. After seven 12-min exposures to the ATP analog ATPγS (10−4 M) over 48 h, HET-1A cells were used to determine mRNA by real-time PCR. Relative mRNA expression was calculated with respect to mRNA from untreated cells. Repeated exposure to ATP over 48 h caused a significant increase (*P < 0.01) in lyso-PAF AT, IL-8, eotaxin-1, -2, and -3, MCP-1, and MIP-1α mRNA expression. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments.
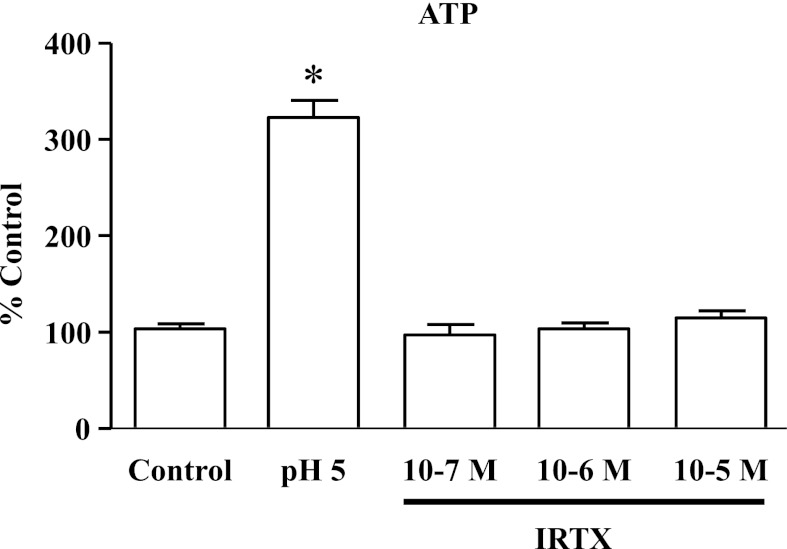
It has been suggested that IRTX may act as a TRPV1 agonist at 10−7–10−6 M, evoking a hypothermic response similar to that evoked by capsaicin. To exclude this possibility in our system, HET-1A cells were exposed for 5 min to 10−7–10−5 M IRTX. In addition, cells were exposed for 5 min to HCl (pH 5.0) as positive control. ATP released by HET-1A cells in response to these stimuli was measured using the ATP light luminescence ATP detection assay system (Perkin Elmer). The medium was used to measure ATP according to the manufacturer's instructions.
Figure 14 indicates that whereas HCl induced significant release of ATP from epithelial cells, IRTX did not induce ATP release from these cells, excluding the possibility that the inhibitory role of IRTX in this system may be due to receptor desensitization.
Fig. 14.
HCl (pH 5, 5 min) induced significant release of ATP from epithelial cells (*P < 0.001) compared with untreated cells (control). IRTX (10−7–10−5 M) did not stimulate ATP release, demonstrating that IRTX concentrations used in the present study do not activate TRPV1 receptors in HET-1A cells. Values are means ± SE; n = 3 for 3 different sets of data from 3 separate experiments.
DISCUSSION
While in many chronic inflammatory processes the initiating event of inflammation remains elusive, in esophagitis a likely trigger is the effect of the gastroesophageal refluxate, containing HCl, enzymes such as pepsin, and sometimes bile acids, on the esophageal epithelium. We have focused our investigations on the mechanisms of HCl-induced inflammation in the esophageal mucosa. We previously reported that circular muscle contraction is not affected by exposure to low pH but is significantly reduced when the muscle is exposed to mucosa-derived mediators after preincubation of the mucosa with HCl (11). However, the relevance of HCl-induced esophageal mucosa-derived mediators might extend beyond influencing muscle contraction and may directly contribute to recruitment of the immune cell infiltrate seen in GERD and subsequent inflammatory damage. Epithelial cells constitute the first barrier encountered by acid reflux. Production of inflammatory mediators and cytokines by these cells may therefore be the first step in the inflammatory process, contributing to induction of esophagitis.
The human esophageal epithelial cell line HET-1A was originally obtained from normal human esophageal autopsy tissue. It retains epithelial morphology and cytokeratin expression and has remained nontumorigenic (51). HET-1A cells have been used to examine signaling pathways and transcriptional regulation of cytokine expression (44), to characterize the role of fibroblast growth factor in normal esophageal epithelium and in eosinophilic esophagitis (39), and to examine expression of mucin genes in the esophageal mucosa (54). It is a relevant and well-established tool to examine esophageal epithelial cell response to bile acids (30, 40, 41) and acid-induced activation of TRPV1 (34).
We previously showed that HCl-induced activation of TRPV1 causes release of ATP in HET-1A cells, which in turn activates lyso-PAF AT, inducing production of the inflammatory mediator PAF, and that repeated exposure to low pH or ATP enhances the expression of lyso-PAF AT mRNA and protein (33). Exposure of esophageal epithelium to HCl also causes production and release of the cytokine IL-8 at concentrations sufficient to promote directed migration of leukocytes (32).
To extend the above-described findings, we undertook a systematic investigation of cytokines and chemokines released by esophageal epithelial cells in response to HCl-induced activation of TRPV1 receptors. We used the selective TRPV1 agonist capsaicin to confirm the role of TRPV1 in mediator secretion and confirmed that the regulation is mediated by TRPV1-induced ATP release.
To simulate recurrent exposure to acid, as clinically observed in GERD patients, HET-1A cells were exposed to acidified medium multiple times over 48 h. The selected protocol (seven 12-min exposures to pH 5 over 48 h) caused the maximal increase in mRNA for TRPV1, IL-8, and eotaxin-1, which was not increased by extending the duration of low-pH exposure to 16 min. Thus 12-min HCl exposure may achieve maximal effects for release of inflammatory mediators/cytokines without damaging the cells and closely reproduces mRNA changes observed in human biopsies from EE patients (Figs. 4–10).
HCl-induced upregulation of TRPV1.
HET-1A cells contain mRNA for several vanilloid receptors, with substantially higher mRNA levels of TRPV1 than TRPV2–TRPV6. We show that the selected HCl exposure protocol caused mRNA and protein upregulation of TRPV1. This is in agreement with the previously found TRPV1 upregulation in esophageal biopsies from EE patients compared with controls (18).
HCl-induced upregulation of lyso-PAF AT.
In cat (9, 12), rabbit (32), and human (9, 10) esophageal mucosa, exposure to acid results in formation of PAF. PAF is an important chemoattractant and activator of immune cells (32), particularly eosinophils (55). In this study, we confirmed that HET-1A cells repeatedly exposed to acidified medium increase lyso-PAF AT mRNA and protein, presumably reflecting increased synthesis of PAF, as previously demonstrated (33). A similar increase in lyso-PAF AT mRNA was observed in esophageal biopsies from EE patients.
HCl-induced upregulation of other chemoattractants in epithelial cells mediated by TRPV1 receptors.
We recently showed that acid exposure of the esophageal mucosa causes release of IL-8 in concentrations sufficient to stimulate directed peripheral blood leukocyte migration in an experimental model of acid-induced inflammation (32), confirming the role of acid-induced release of this cytokine in immune cell recruitment. We now demonstrate that repeated acid exposure increases IL-8 mRNA and protein in HET-1A cells. The increase in IL-8 mRNA in HET-1A cells is similar to the increase in IL-8 previously demonstrated in rabbit mucosa (32) and similar to the increase in esophageal biopsies from EE patients. These results are consistent with the findings of Souza et al. (50) in several esophageal epithelial cell lines.
We next performed a screening approach using a multiplex ELISA for 65 cytokines demonstrating upregulation of selected mediators that induce infiltration of eosinophils, neutrophils, and monocytes. These include IL-8, a CXC chemokine with potent chemotactic activity for neutrophils; eotaxin-1, -2, and -3, CC chemokines that promote the recruitment of inflammatory cells, particularly eosinophils (45); and MCP-1 and MIP-1α, which are involved primarily in recruitment of monocytes and macrophages (3, 38). These findings were confirmed at the mRNA level using quantitative PCR.
There are three members of the eotaxin family, eotaxin-1 (CCL11), eotaxin-2 (CCL24), and eotaxin-3 (CCL26); their genes are located in different chromosomal positions, but all act on the same chemokine receptor, CCR3, which is highly expressed on eosinophils (1, 25, 26, 42). Thus eotaxins promote eosinophil recruitment and activation, enhanced adhesion, superoxide generation, and degranulation (17, 23, 57). An increase in eotaxin levels was also observed in esophageal biopsies from EE patients and is consistent with recruitment of eosinophils in esophageal inflammation during acid reflux injury (6, 31, 35, 36).
In addition, the data show an increase in MCP-1 and MIP-1α mRNA and protein expression after acid exposure. A similar mRNA increase was found in esophageal biopsies from EE patients. MCP-1 and MIP-1α are involved primarily in recruitment of monocytes and macrophages (3, 38). The presence of mononuclear cells in the esophageal lamina propria, but not in the epithelium, is indicative of EE (16, 20, 27). Production of these chemoattractants by esophageal epithelial cells after acid exposure may promote monocyte recruitment, even if the presence of monocytes is difficult to assess in esophageal biopsies of EE patients, because few biopsies are sufficiently deep to contain a significant amount of lamina propria (27).
The TRPV1 antagonists IRTX and JNJ-17203212 significantly inhibited HCl-induced increases in mRNA levels for the investigated mediators, supporting a role of TRPV1. IRTX is a classic TRPV1 antagonist, but it may also have other effects (14): it may act as an agonist at high doses (48). In the current study, however, IRTX completely inhibited the effect of the selective TRPV1 agonist capsaicin, and there was no evidence that it acted as an agonist (Fig. 14). In any case, the more recently developed antagonist JNJ-17203212 (52), a second-generation TRPV1 antagonist (5), was used for comparison and had exactly the same effect as IRTX. The critical involvement of TRPV1 in HCl-induced mRNA upregulation of lyso-PAF AT and selected cytokines is confirmed by the finding of a comparable mRNA upregulation by the selective TRPV1 agonist capsaicin.
Similarly, the ATP antagonist suramin significantly inhibited HCl- or capsaicin-induced increases in mRNA levels for the cytokines examined, supporting a role of ATP in the upregulation. For all cytokines, there were no significant differences in mRNA levels between control values and values after exposure to HCl + TRPV1 antagonists or HCl + suramin. Repeated direct exposure to ATP over 48 h results in an increase in mRNA for lyso-PAF AT and all relevant cytokines. Interaction between TRPV1 and ATP has been demonstrated in several experimental preparations, with TRPV1 inducing ATP release (33, 46) and ATP, in turn, potentiating TRPV1-mediated signaling (28, 53).
These results using HET-1A cells are consistent with data obtained in esophageal biopsies from EE patients, in which mRNA for lyso-PAF AT, IL-8, eotaxin-1 and -2, MIP-1α, and MCP-1 was significantly elevated compared with biopsies from healthy controls.
The current study shows that a highly selective spectrum of mediators is induced by exposure of human esophageal epithelial cells to low pH, as occurs in patients with GERD. Among the significantly elevated cytokines, there was a remarkable dominance for mediators that induce infiltration of eosinophils, neutrophils, and monocytes. This suggests that acid exposure is not an indiscriminate inducer of mediator release that leads to nonspecific recruitment of inflammatory cells. HCl can be considered a highly selective inducer of epithelial cell-released mediators recruiting restricted leukocyte subsets involved in acute inflammatory responses (neutrophils and monocytes) and allergic inflammation (eotaxins). This observation may help clarify, at least in part, the poorly understood clinical overlap between GERD and eosinophilic esophagitis: in fact, esophageal eosinophils at levels consistent with eosinophilic esophagitis can be found in up to 30% of patients with reflux esophagitis (13). In addition, our findings may also help explain why suppression of acid production can be beneficial in eosinophilic esophagitis and GERD patients (19, 37). Both diseases likely begin at the epithelial level, and the acidic refluxate may be seen as a regulator of a highly selective set of mediators by esophageal epithelial cells that determine the fate of the inflammatory process.
In summary, this study suggests that exposure to acid activates TRPV1 receptors in esophageal epithelial cells, causing release of ATP. ATP then causes upregulation of the TRPV1 receptors, as well as PAF, IL-8, eotaxins, MCP-1, and MIP-1α, which may contribute to inflammation and injury of the esophageal mucosa. Parallel findings in HET-1A cells treated with this HCl-exposure protocol and esophageal biopsies from EE patients provide a simple and powerful experimental model to examine inflammation-related changes in the esophageal epithelium.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-57030.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M., A.A., M.P.G., and D.L. performed the experiments; J.M. and P.B. analyzed the data; J.M., A.A., M.C., F.R., C.F., W.C., J.B., P.B., and K.M.H. interpreted the results of the experiments; J.M., A.A., M.P.G., M.C., F.R., C.F., W.C., J.B., P.B., and K.M.H. edited and revised the manuscript; J.M., A.A., M.P.G., M.C., F.R., C.F., W.C., J.B., P.B., and K.M.H. approved the final version of the manuscript; M.C., F.R., C.F., J.B., P.B., and K.M.H. are responsible for conception and design of the research; P.B. and K.M.H. prepared the figures; P.B. and K.M.H. drafted the manuscript.
REFERENCES
- 1. Abouheif E, Akam M, Dickinson WJ, Holland PW, Meyer A, Patel NH, Raff RA, Roth VL, Wray GA. Homology and developmental genes. Trends Genet 13: 432–433, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Azpiroz F, Baudet JS, Benages A, Canga F, Carrasco J, Ciriza C, Cucala M, Dominguez E, Faro V, Garrigues V, Giganto F, Herrerias JM, Iglesias J, Lacima G, Lopez P, Llabres M, Mearin F, Minguez M, Mones J, Mora F, Munoz C, Perez de la Serna J, Ponce J, Rodriguez-Tellez M, Romero MJ, Ruiz de Leon A, Ruiz-Cabello M, Sanchez-Gey S, Sanchiz V, Serra J, Sevilla MC, Sopena F, Soria MJ. Normal values in ambulatory oesophageal pH monitoring at two levels in Spain. Rev Esp Enferm Dig 102: 406–412, 2010 [PubMed] [Google Scholar]
- 3. Baggiolini M, Loetscher P, Moser B. Interleukin-8 and the chemokine family. Int J Immunopharmacol 17: 103–108, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Basseri B, Levy M, Wang HL, Shaye OA, Pimentel M, Soffer EE, Conklin JL. Redefining the role of lymphocytes in gastroesophageal reflux disease and eosinophilic esophagitis. Dis Esophagus 23: 368–376, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Bhattacharya A, Scott BP, Nasser N, Ao H, Maher MP, Dubin AE, Swanson DM, Shankley NP, Wickenden AD, Chaplan SR. Pharmacology and antitussive efficacy of 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethyl-pyridin-2-yl)-amide (JNJ17203212), a transient receptor potential vanilloid 1 antagonist in guinea pigs. J Pharmacol Exp Ther 323: 665–674, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bhattacharya B, Carlsten J, Sabo E, Kethu S, Meitner P, Tavares R, Jakate S, Mangray S, Aswad B, Resnick MB. Increased expression of eotaxin-3 distinguishes between eosinophilic esophagitis and gastroesophageal reflux disease. Hum Pathol 38: 1744–1753, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 8. Cao W, Cheng L, Behar J, Fiocchi C, Biancani P, Harnett KM. Proinflammatory cytokines alter/reduce esophageal circular muscle contraction in experimental cat esophagitis. Am J Physiol Gastrointest Liver Physiol 287: G1131–G1139, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Cheng L, Cao W, Behar J, Fiocchi C, Biancani P, Harnett KM. Acid-induced release of platelet-activating factor by human esophageal mucosa induces inflammatory mediators in circular smooth muscle. J Pharmacol Exp Ther 319: 117–126, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis. Am J Physiol Gastrointest Liver Physiol 290: G1307–G1317, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. In vitro model of acute esophagitis in the cat. Am J Physiol Gastrointest Liver Physiol 289: G860–G869, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Cheng L, de la Monte S, Ma J, Hong J, Tong M, Cao W, Behar J, Biancani P, Harnett KM. HCl-activated neural and epithelial vanilloid receptors (TRPV1) in cat esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 297: G135–G143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeBrosse CW, Collins MH, Buckmeier Butz BK, Allen CL, King EC, Assa'ad AH, Abonia JP, Putnam PE, Rothenberg ME, Franciosi JP. Identification, epidemiology, and chronicity of pediatric esophageal eosinophilia, 1982–1999. J Allergy Clin Immunol 126: 112–119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dogan MD, Patel S, Rudaya AY, Steiner AA, Szekely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol 143: 1023–1032, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fitzgerald RC, Onwuegbusi BA, Bajaj-Elliott M, Saeed IT, Burnham WR, Farthing MJ. Diversity in the oesophageal phenotypic response to gastro-oesophageal reflux: immunological determinants. Gut 50: 451–459, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frierson HF., Jr Histology in the diagnosis of reflux esophagitis. Gastroenterol Clin North Am 19: 631–644, 1990 [PubMed] [Google Scholar]
- 17. Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med 2: 449–456, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Guarino MP, Cheng L, Ma J, Harnett K, Biancani P, Altomare A, Panzera F, Behar J, Cicala M. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol Motil 22: 746–751, e219, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Hirano I. Eosinophilic esophagitis and gastroesophageal reflux disease: there and back again. Clin Gastroenterol Hepatol 9: 99–101, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Ismail-Beigi F, Horton PF, Pope CE., 2nd Histological consequences of gastroesophageal reflux in man. Gastroenterology 58: 163–174, 1970 [PubMed] [Google Scholar]
- 21. Isomoto H, Saenko VA, Kanazawa Y, Nishi Y, Ohtsuru A, Inoue K, Akazawa Y, Takeshima F, Omagari K, Miyazaki M, Mizuta Y, Murata I, Yamashita S, Kohno S. Enhanced expression of interleukin-8 and activation of nuclear factor-κB in endoscopy-negative gastroesophageal reflux disease. Am J Gastroenterol 99: 589–597, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K, Murata I, Kohno S. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol 98: 551–556, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Jose PJ, Griffiths-Johnson DA, Collins PD, Walsh DT, Moqbel R, Totty NF, Truong O, Hsuan JJ, Williams TJ. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med 179: 881–887, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kato M, Kita H, Tachibana A, Hayashi Y, Tsuchida Y, Kimura H. Dual signaling and effector pathways mediate human eosinophil activation by platelet-activating factor. Int Arch Allergy Immunol 134 Suppl 1: 37–43, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, Murphy PM, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem 271: 7725–7730, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Kitaura M, Suzuki N, Imai T, Takagi S, Suzuki R, Nakajima T, Hirai K, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine (eotaxin-3) that is a functional ligand of CC chemokine receptor 3. J Biol Chem 274: 27975–27980, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi S, Kasugai T. Endoscopic and biopsy criteria for the diagnosis of esophagitis with a fiberoptic esophagoscope. Am J Dig Dis 19: 345–352, 1974 [DOI] [PubMed] [Google Scholar]
- 28. Lakshmi S, Joshi PG. Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol 25: 819–832, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy 59: 793–805, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Reuter NP, Li X, Liu Q, Zhang J, Martin RC. Colocalization of MnSOD expression in response to oxidative stress. Mol Carcinog 49: 44–53, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Lucendo AJ, De Rezende L, Comas C, Caballero T, Bellon T. Treatment with topical steroids downregulates IL-5, eotaxin-1/CCL11, and eotaxin-3/CCL26 gene expression in eosinophilic esophagitis. Am J Gastroenterol 103: 2184–2193, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Ma J, Altomare A, de la Monte S, Tong M, Rieder F, Fiocchi C, Behar J, Shindou H, Biancani P, Harnett KM. HCl-induced inflammatory mediators in esophageal mucosa increase migration and production of H2O2 by peripheral blood leukocytes. Am J Physiol Gastrointest Liver Physiol 299: G791–G798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma J, Altomare A, Rieder F, Behar J, Biancani P, Harnett K. ATP: a mediator for HCl-induced TRPV1 activation in esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 301: G1075–G1082, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma J, Harnett KM, Behar J, Biancani P, Cao W. Signaling in TRPV1-induced platelet-activating factor (PAF) in human esophageal epithelial cells. Am J Physiol Gastrointest Liver Physiol 298: G233–G240, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mastracci L, Spaggiari P, Grillo F, Zentilin P, Dulbecco P, Ceppa P, Baccini P, Mansi C, Savarino V, Fiocca R. Microscopic esophagitis in gastro-esophageal reflux disease: individual lesions, biopsy sampling, and clinical correlations. Virchows Arch 454: 31–39, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Mishra A. Mechanism of eosinophilic esophagitis. Immunol Allergy Clin North Am 29: 29–40, viii, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, Duenas C, Fernandez-Gonzalez N, Quintana EM, Gonzalez-Nunez MA. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol 9: 110–117, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Mukaida N, Harada A, Yasumoto K, Matsushima K. Properties of pro-inflammatory cell type-specific leukocyte chemotactic cytokines, interleukin 8 (IL-8) and monocyte chemotactic and activating factor (MCAF). Microbiol Immunol 36: 773–789, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Mulder DJ, Pacheco I, Hurlbut DJ, Mak N, Furuta GT, MacLeod RJ, Justinich CJ. FGF9-induced proliferative response to eosinophilic inflammation in oesophagitis. Gut 58: 166–173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Naran S, Abrams P, de Oliveira PQ, Hughes SJ. Bile salts differentially sensitize esophageal squamous cells to CD95 (Fas/Apo-1 receptor) mediated apoptosis. J Surg Res 171: 504–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen KD, Blain SW, Gress F, Treem WR. Inflammatory mediators of esophagitis alter p27 Kip1 expression in esophageal epithelial cells. J Pediatr Gastroenterol Nutr 51: 556–562, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, Gutierrez-Ramos JC, Mackay CR. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest 97: 604–612, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Portale G, Peters J, Hsieh CC, Tamhankar A, Arain M, Hagen J, DeMeester S, DeMeester T. When are reflux episodes symptomatic? Dis Esophagus 20: 47–52, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Rafiee P, Nelson VM, Manley S, Wellner M, Floer M, Binion DG, Shaker R. Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): role of PKC, MAPKs, and NF-κB. Am J Physiol Gastrointest Liver Physiol 296: G388–G398, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today 6: 20–27, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Sadananda P, Shang F, Liu L, Mansfield KJ, Burcher E. Release of ATP from rat urinary bladder mucosa: role of acid, vanilloids and stretch. Br J Pharmacol 158: 1655–1662, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scheffer RC, Wassenaar EB, Herwaarden MA, Holloway RH, Samsom M, Smout AJ, Akkermans LM. Relationship between the mechanism of gastro-oesophageal reflux and oesophageal acid exposure in patients with reflux disease. Neurogastroenterol Motil 17: 654–662, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Shimizu I, Iida T, Horiuchi N, Caterina MJ. 5-Iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro. J Pharmacol Exp Ther 314: 1378–1385, 2005 [DOI] [PubMed] [Google Scholar]
- 49. Sigal CE, Valone FH, Holtzman MJ, Goetzl EJ. Preferential human eosinophil chemotactic activity of the platelet-activating factor (PAF) 1-O-hexadecyl-2-acetyl-sn-glyceryl-3-phosphocholine (AGEPC). J Clin Immunol 7: 179–184, 1987 [DOI] [PubMed] [Google Scholar]
- 50. Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137: 1776–1784, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Stoner GD, Kaighn ME, Reddel RR, Resau JH, Bowman D, Naito Z, Matsukura N, You M, Galati AJ, Harris CC. Establishment and characterization of SV-40 T-antigen immortalized human esophageal epithelial cells. Cancer Res 51: 365–371, 1991 [PubMed] [Google Scholar]
- 52. Swanson DM, Dubin AE, Shah C, Nasser N, Chang L, Dax SL, Jetter M, Breitenbucher JG, Liu C, Mazur C, Lord B, Gonzales L, Hoey K, Rizzolio M, Bogenstaetter M, Codd EE, Lee DH, Zhang SP, Chaplan SR, Carruthers NI. Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J Med Chem 48: 1857–1872, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA 98: 6951–6956, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Roon AH, Mayne GC, Wijnhoven BP, Watson DI, Leong MP, Neijman GE, Michael MZ, McKay AR, Astill D, Hussey DJ. Impact of gastro-esophageal reflux on mucin mRNA expression in the esophageal mucosa. J Gastrointest Surg 12: 1331–1340, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Wardlaw AJ, Moqbel R, Cromwell O, Kay AB. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest 78: 1701–1706, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yoshida N, Uchiyama K, Kuroda M, Sakuma K, Kokura S, Ichikawa H, Naito Y, Takemura T, Yoshikawa T, Okanoue T. Interleukin-8 expression in the esophageal mucosa of patients with gastroesophageal reflux disease. Scand J Gastroenterol 39: 816–822, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Zimmermann N, Daugherty BL, Stark JM, Rothenberg ME. Molecular analysis of CCR-3 events in eosinophilic cells. J Immunol 164: 1055–1064, 2000 [DOI] [PubMed] [Google Scholar]