Abstract
The origin and possible antiquity of the spectacularly diverse modern deep-sea fauna has been debated since the beginning of deep-sea research in the mid-nineteenth century. Recent hypotheses, based on biogeographic patterns and molecular clock estimates, support a latest Mesozoic or early Cenozoic date for the origin of key groups of the present deep-sea fauna (echinoids, octopods). This relatively young age is consistent with hypotheses that argue for extensive extinction during Jurassic and Cretaceous Oceanic Anoxic Events (OAEs) and the mid-Cenozoic cooling of deep-water masses, implying repeated re-colonization by immigration of taxa from shallow-water habitats. Here we report on a well-preserved echinoderm assemblage from deep-sea (1000–1500 m paleodepth) sediments of the NE-Atlantic of Early Cretaceous age (114 Ma). The assemblage is strikingly similar to that of extant bathyal echinoderm communities in composition, including families and genera found exclusively in modern deep-sea habitats. A number of taxa found in the assemblage have no fossil record at shelf depths postdating the assemblage, which precludes the possibility of deep-sea recolonization from shallow habitats following episodic extinction at least for those groups. Our discovery provides the first key fossil evidence that a significant part of the modern deep-sea fauna is considerably older than previously assumed. As a consequence, most major paleoceanographic events had far less impact on the diversity of deep-sea faunas than has been implied. It also suggests that deep-sea biota are more resilient to extinction events than shallow-water forms, and that the unusual deep-sea environment, indeed, provides evolutionary stability which is very rarely punctuated on macroevolutionary time scales.
Introduction
The deep sea is by far the largest environment on the planet, yet our knowledge of the diversity and evolutionary history of the deep-sea biota remains remarkably poor [1]. Early deep-sea research in the nineteenth century led to the discovery of peculiar organisms like stalked crinoids (sea lilies), until then known only as fossils from shallow-marine deposits. Such finds were the origin of the idea that the deep sea is a refuge for ancient lineages excluded from shelf habitats by superior competitors or increased predation pressure [2].
Although the antiquity of at least part of the deep-sea fauna is implicit in the notion of the apparent offshore migration of marine benthos over time [3], debates have increasingly focused on the impact of Mesozoic dysoxia (Oceanic Anoxic Events: OAEs) and the Cenozoic cooling of deep-water masses on the colonization of deep habitats [1], [4], [5]. Biogeographic patterns and molecular clock estimates calibrated against the fossil record of shallow-water sister groups of the modern deep-sea fauna have yielded dates from early Mesozoic to Pleistocene, with the majority converging to a latest Mesozoic or Cenozoic origin [5], [6], [7], [8].
The reason why this debate is unsettled is the lack of direct evidence of the geological history of deep-sea fauna due to the absence of outcrops of deep-sea sediments with preserved fossil remains [6]. While oceanic sediment cores provide an excellent fossil record of benthic foraminifers and ostracods, they have been assumed to almost never yield identifiable remains of larger deep-sea benthos [6].
Modern communities of deep-sea megabenthos are commonly dominated by echinoderms, both in terms of abundance and biomass [9]. In particular, ophiuroids (brittle stars) and holothuroids (sea cucumbers) can cover the deep-sea floor in dense concentrations of hundreds to thousands of individuals per square meter [10]. The echinoderm skeleton is made up of numerous plates composed of high Mg-calcite that have a high fossilization potential. Although the skeletal elements usually dissociate after death, many are morphologically diagnostic to genus or even species level, e.g. [11]–[12]. Therefore, echinoderms are likely to have left a fossil record in deep-sea deposits, and thus can provide insights into the composition and diversity of ancient deep-sea faunas.
Here, we describe an Early Cretaceous echinoderm assemblage providing the first fossil-based insights into Mesozoic deep-sea biodiversity. We compare the composition of the echinoderm assemblage with that of present-day equivalents and coeval shallow-water assemblages and discuss the implications for the origin of the modern deep-sea fauna.
Results
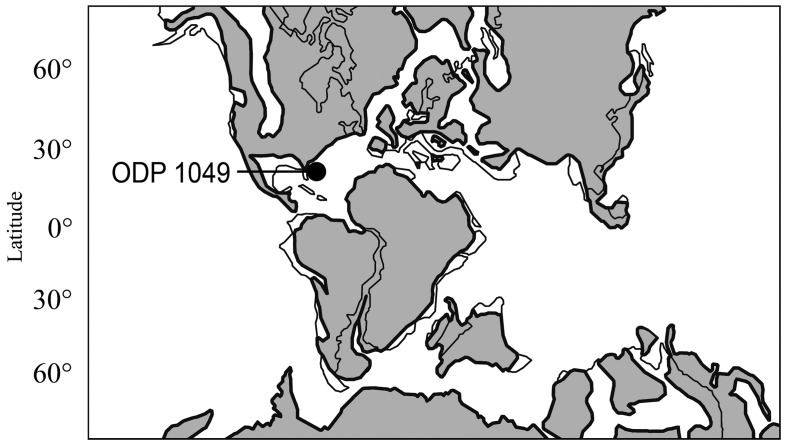
We here present an echinoderm assemblage based on microfossils retrieved from sediments drilled by the Ocean Drilling Program (ODP) Leg 171B on the Blake Nose escarpment in the western tropical Atlantic, off Florida [13] (Fig. 1). The echinoderm remains described herein originate from the chalk succession recovered at ODP site 1049 underlying the black shales of Oceanic Anoxic Event (OAE) 1b [13] and dated as middle/late Aptian to earliest Albian (Hedbergella trocoidea to Microhedbergella rischi planktonic zones, ca 114 Ma) [14]–[15]. The geologic history of site 1049 implies deposition of the Aptian–Albian chalk succession at lower bathyal depths (at least 1500 m), whereas benthic foraminiferal assemblages indicate middle bathyal (800–1000 m) or greater depths [13], [16]. There was no input of periplatform debris at the time the chalk was deposited, as indicated by the lack of shallow-water foraminifera [13], and the echinoderm plates are thus interpreted to represent an autochthonous bathyal assemblage.
Figure 1. Paleogeographic reconstruction for the late Aptian with the position of ODP site 1049 (Blake Nose).
Thick lines denote paleo-coastlines, grey areas represent emerged land (modified from ref. [51]).
Taxonomic Composition
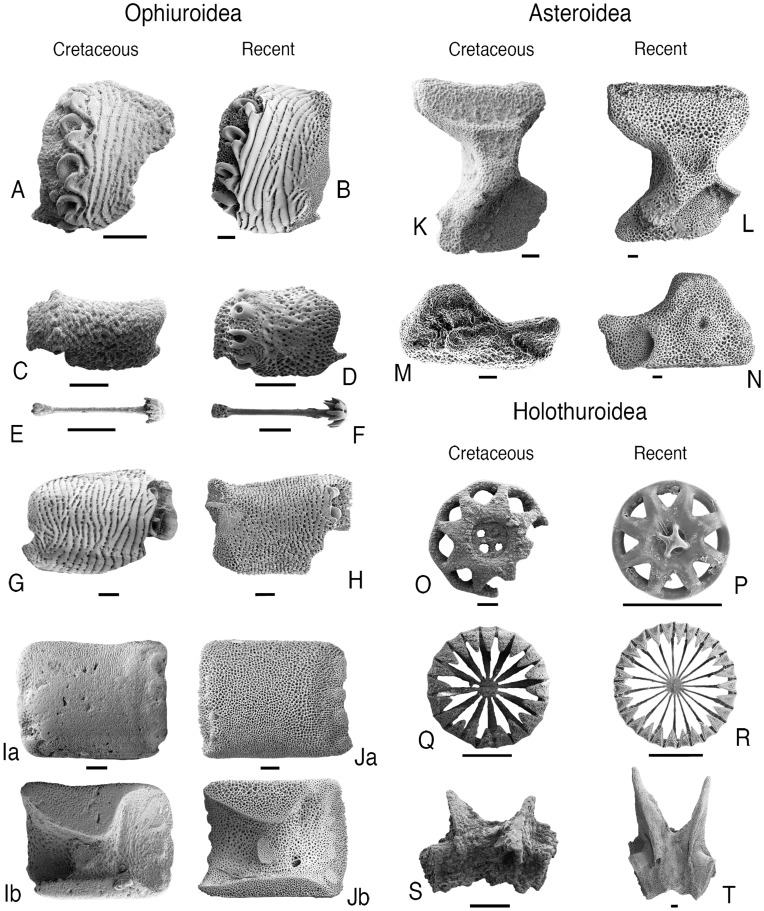
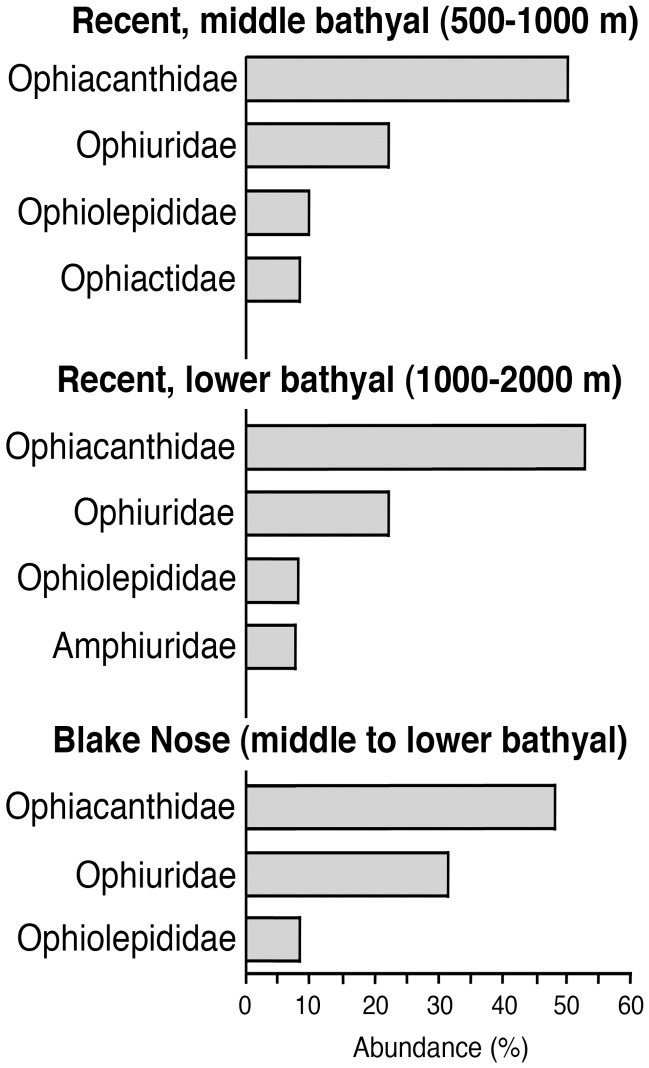
The ophiuroid (brittle star) remains from the Blake Nose samples are composed mostly of the highly diverse, spine-bearing lateral arm plates [17]. Nearly half of these plates (48%) are assignable to the extant, predominantly deep-sea family Ophiacanthidae [18] on account of the ear-shaped spine articulations with a sigmoidal fold, the relatively thin aspect of the plates and a vertical series of perforations on the inner side (Figs 2A–F, 3A–D). Within this group, lateral arm plates with a conspicuous vertical striation including a minutely denticulate distal edge of the stripes, spine articulations sunken into the distal edge of the lateral arm plates, and well-developed connecting ridges between the spine articulations and the vertical stripes are assignable to the extant genus Ophiolimna (Fig. 2A–B). Lateral arm plates with a moderately to weakly developed, fine vertical striation and freestanding spine articulations on an elevated vertical ridge are commonly found in the extant genus Ophiacantha (Fig. 3D). Parasol-shaped arm spines similar to those found in the Blake Nose material are an exclusive feature of the modern deep-sea genera Ophiohelus and Ophiotholia (Fig. 2E–F). The co-occurrence of lateral arm plates with vertical and single (Fig. 2C–D) rather than oblique and multiple rows of spine articulations precludes assignment to Ophiohelus [19]. Most of the remaining lateral arm plates of the assemblage are assignable to the subfamily Ophioleucinae within the Ophiuridae (31%) (Fig. 2G–H), on account of the fragile plate architecture, the large tentacle notches, an outer surface ornamentation consisting of minute, scale-like granules and small rhombic spine articulations sunken into the distal edge of the plate, and to the extant deep-sea ophiolepidid genus Ophiomusium (8%) [18] (Fig. 2I–J). Lateral arm plates of the latter are typically rectangular in outline, with tentacle openings developed as within-plate perforations, and with a conspicuous large dorsal contact surface with the opposite lateral arm plate on the inner side. The composition at family level of the Blake Nose ophiuroid assemblage is closest to that of modern lower bathyal communities, and clearly differs from modern and Cretaceous shallow-water assemblages (Figs 4–5).
Figure 2. Key echinoderm plates from the early Cretaceous of Blake Nose (ODP site 1049) with corresponding plates of Recent relatives.
A: Ophiolimna sp. (Ophiacanthidae), lateral arm plate (LAP), Blake Nose (GZG.INV.78777). B: Ophiolimna bairdi (Lyman) (Ophiacanthidae), LAP, Recent, North Atlantic. C: Ophiohelinae gen. nov. (Ophiacanthidae), LAP (GZG.INV.78778), Blake Nose. D: Ophiotholia spathifer (Lyman) (Ophiacanthidae), LAP, Recent, Japan. E: Ophiohelinae gen. nov. (Ophiacanthidae), parasol spine (GZG.INV.78779), Blake Nose. F: Ophiotholia spathifer (Lyman) (Ophiacanthidae), parasol spine, Recent, Japan. G: Ophioleuce sp. (Ophioleucinae), LAP (GZG.INV.78780), Blake Nose. H: Ophioleuce seminudum Koehler (Ophioleucinae), LAP, Recent, Pacific. I: Ophiomusium sp. (Ophiolepididae), LAP (a: external, b: internal) (GZG.INV.78781), Blake Nose. J: Ophiomusium lymani (Wyville-Thomson) (Ophiolepididae), LAP (a: external, b: internal), Recent, North Atlantic. K: Benthopectinidae gen. nov., ambulacral (GZG.INV.78782), Blake Nose. L: Pectinaster filholi Perrier (Benthopectinidae), ambulacral, Recent, Atlantic. M: Benthopectinidae gen. nov., adambulacral (GZG.INV.78783), Blake Nose. N: Pectinaster filholi Perrier (Benthopectinidae), adambulacral, Recent, North Atlantic. O: Laetmogonidae gen. nov., body wall ossicle (lower side) (GZG.INV.45613), Blake Nose. P: Laetmogone olivacea Théel (Laetmogonidae), body wall ossicle (lower side), Recent, North Atlantic. Q: Hemisphaeranthos sp. (Myriotrochidae), body wall ossicle (upper side) (GZG.INV.45634), Blake Nose. R: Myriotrochus rinkii Steenstrup (Myriotrochidae), body wall ossicle, Recent, North Atlantic. S: (?)Myriotrochus sp. (Myriotrochidae), radial calcareous ring element (inner side) (GZG.INV.45623), Blake Nose. T: Myriotrochus rinkii Steenstrup (Myriotrochidae), radial calcareous ring element (inner side), Recent, North Atlantic. Scale bars equal 100 µm.
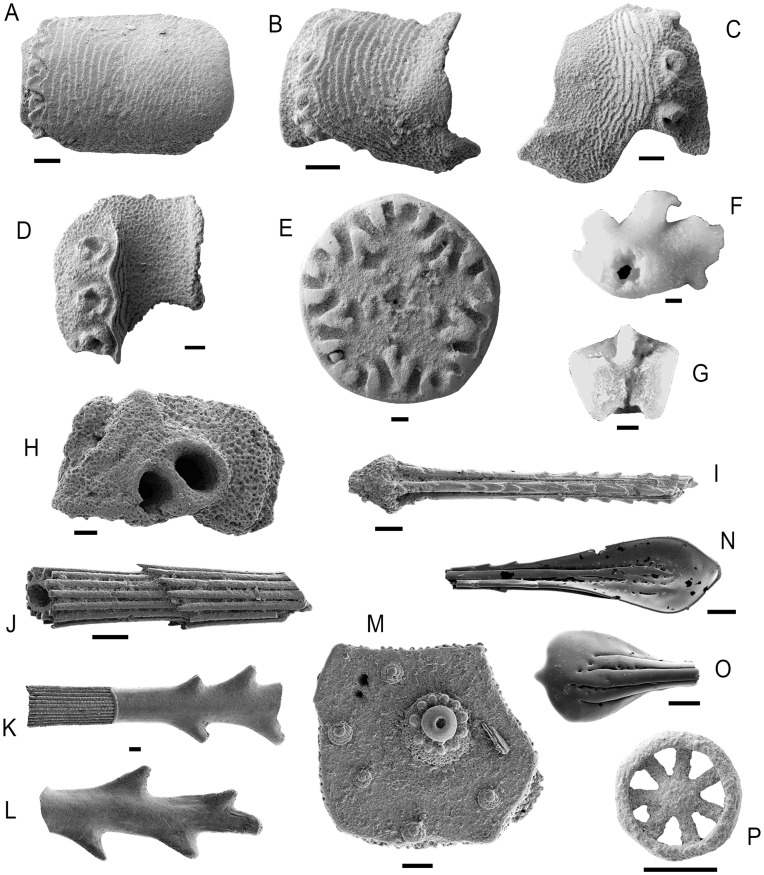
Figure 3. Additional diagnostic skeletal components of the echinoderm groups from the Aptian-earliest Albian (Early Cretaceous) of Blake Nose (ODP Site 1049).
A: Ophiacanthidae gen. et sp. nov., lateral arm plate (GZG.INV.78784). B: Ophiologimus sp. nov. (Ophiacanthidae), lateral arm plate (GZG.INV.78785). C: Ophiologimus sp. nov. (Ophiacanthidae), lateral arm plate (GZG.INV.78786). D: Ophiacantha sp. nov. (Ophiacanthidae), lateral arm plate (GZG.INV.78787). E: Balanocrinus sp. (Isocrinidae), columnal (GZG.INV.78788). F: Bathycrinus? sp. (Bathycrinidae), holdfast (GZG.INV.78789). G: Bathycrinus? sp. (Bathycrinidae), second primibrachial (GZG.INV.78790). H: echinothurioid ambulacral plate (GZG.INV.78791). I: echinothurioid? spine (GZG.INV.78792). J: diadematoid spine (GZG.INV.78793). K-L: Histocidaridae gen. et sp. indet., adoral spine fragments (GZG.INV.78794–78795). M: holasteroid ambulacral plate (GZG.INV.78796). N: holasteroid spine fragment (GZG.INV.78797). O: holasteroid spine fragment (GZG.INV.78798). P: Jumaraina sp. (Chiridotidae) body wall ossicle (upper side) (GZG.INV.78799). Scale bars equal 100 µm.
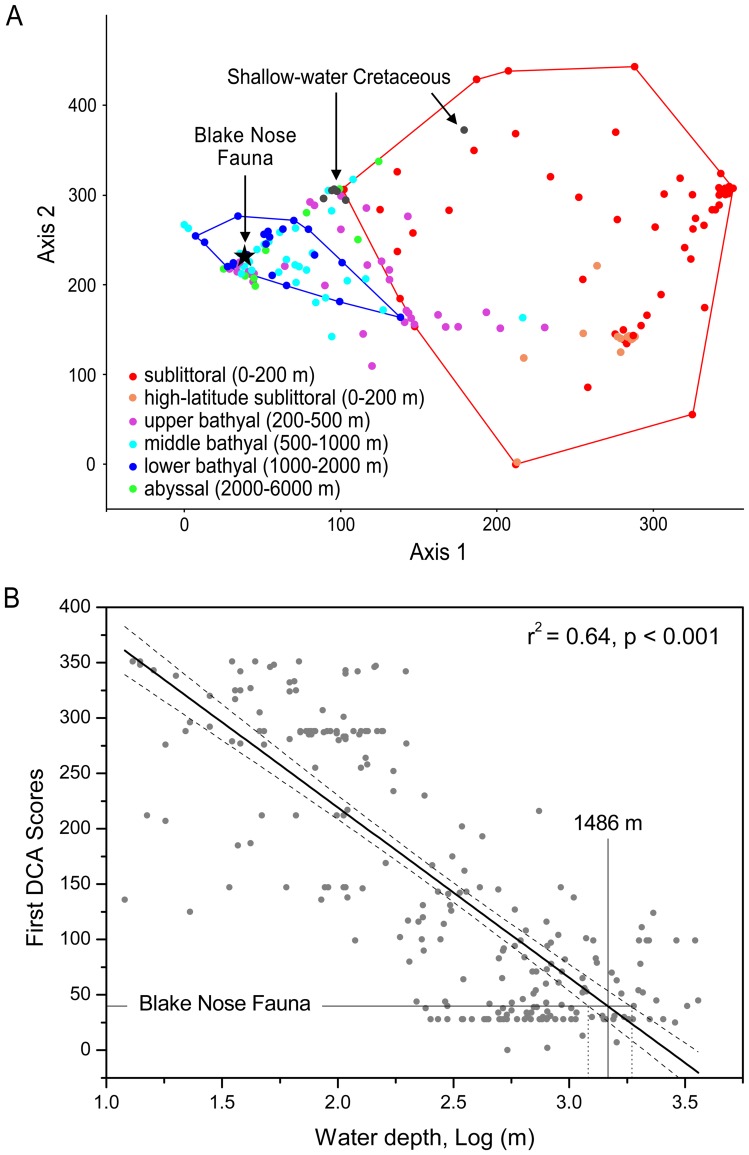
Figure 4. Relative abundances of the most common ophiuroid families in present-day middle and lower bathyal settings, in comparison with the middle to lower bathyal ophiuroid assemblages from the upper Aptian–lowermost Albian of Blake Nose (ODP site 1049).
Relative family-level abundances of the Blake Nose ophiuroid assemblage were inferred from lateral arm plate counts, assuming that the number of lateral arm plates serves as an approximation for the number of individuals.
Figure 5. Quantitative assessment of the Blake Nose ophiuroid assemblage.
A: Detrended Correspondence Analysis (DCA) of modern ophiuroid assemblages in comparison with the Blake Nose ophiuroid fauna and Cretaceous shelf assemblages. The Blake Nose assemblage plots within the modern lower bathyal communities, and strongly differs from modern shallow-water communities and Cretaceous shelf assemblages, challenging the possibility of repeated deep-sea recolonization from shelf depths in the Cretaceous. The analysis is based on the relative abundances of all 17 extant ophiuroid families minus the Ophiuridae, which are abundant at all depths (see Table 1 for abundance data). B: Linear correlation between DCA scores (axis 1) and LOG depth. The relationship is very strong (r = −0.80, adjusted r-square = 0.64). The probability of such strong correlation occurring by chance is virtually zero (4.8899E−29). When the score of the Blake Nose fauna is projected onto this relationship, it would be assigned a depth of 1,486 m (1,218–1,864 m) (uncertainty based on 95% confidence interval for regression line). Remarkably, this is exactly within the range of paleodepth reconstructions for this site [13], [16]. This means the fauna is similar to present-day lower bathyal assemblages to such degree that even the faunal composition versus depth relationship appears to have remained the same.
The asteroid (starfish) material from the Blake Nose samples (Fig. 2K–N) is dominated by the remains of a single taxon. The ambulacrals are hourglass shaped, with equally expanded triangular head and base, a benthopectinid synapomorphy. Furthermore, the ambulacral-adambulacral contact is highly modified [20] and unique to the Benthopectinidae, representatives of which are common elements of modern deep-sea asteroid assemblages [21].
The Blake Nose holothuroid (sea cucumber) assemblage consists of body wall wheels assignable to the Laetmogonidae on account of the presence of 6 to 15 spokes, a perforated hub with a central primary cross and a smooth rim (Fig. 2O–P). Body wall wheels presenting 10 to 20 spokes, a flat hub and a rim with small to medium-sized teeth are typical of the Myriotrochidae, a group which is furthermore represented in our material by calcareous ring elements the radial ones of which typically display a perforation for the passage of the radial nerve and long anterior prolongations (Fig. 2Q–T). A third type of wheels is characterized by the presence of 6 or rarely 7 to 8 spokes, a non-perforated hub which is complex at both sides, and a circular and finely denticulate rim, which unquestionably places them in the Chiridotidae (Fig. 3P). Modern myriotrochids and laetmogonids are typically bathyal groups, and, although not restricted to deep-sea settings, chiridotids are also commonly found in modern bathyal habitats [22].
The echinoid (sea urchin) assemblage studied herein includes spines with a smooth surface, a long collar, dorso-ventral flattening and ornamentation with coarse lateral thorns (Fig. 3K–L), a combination of characters known exclusively from the oral secondary spines of the extant family Histocidaridae. Strongly reduced ambulacral plates bearing a single large pore pair and lateral flanges bevelling under neighbor plates are assignable to the echinothurioids (Fig. 3H). Co-occurring secondary spines show morphologies consistent with those of extant echinothurioid secondary spines (Fig. 3I). Fragments of hollow, strongly verticillate spines were found in several samples, and clearly belong to a diadematoid echinoid (Fig. 3J), although attribution to any particular taxon within this large group is problematic. Almost all samples yield fragments and spines of thin-shelled atelostomate echinoids (Fig. 3M–O). Based on plate shape, ambulacral pore shape and position, as well as tuberculation, which consists of widely scattered primary tubercles with few distinctly smaller granules interspersed, an attribution to late stem-group holasteroids (tithoniids) [23] or early crown-group members seems likely. Although histocidarids, echinothurioids, diadematoids and holasteroids are typical components of modern deep-sea echinoid assemblages [6], the Blake Nose echinoid material fails to provide sufficient diagnostic elements for a detailed faunal analysis and comparison with modern equivalents. As predicted by phylogenetic analyses [24] our results indicate that echinothurioids and histocidarids evolved earlier than previously known, predating the oldest shallow-water occurrence of these groups. This may suggest that much of their evolution took place in deep-water settings.
The crinoid (sea lily) material from the Blake Nose assemblages includes columnals assignable to the isocrinid genus Balanocrinus on account of the short and uniform radiating crenulae along the margin, narrow radial ridges or ribbons of minute crenulae or granules, and large areolae (Fig. 3E). The assemblage furthermore comprises thin primibrachials 2 and a holdfast that are strongly reminiscent of the extant bourgueticrinid Bathycrinus (Fig. 3F–G). Modern isocrinids are found almost exclusively in bathyal settings, while Bathycrinus, and the modern bourgueticrinids in general, are among the deepest-dwelling of all extant crinoids, occurring from lower bathyal to hadal depths [25].
Discussion
Implications for the Origin of the Modern Deep-sea Fauna
The discovery of a middle to lower bathyal echinoderm community of modern composition (Figs 4–5) of Early Cretaceous age thus implies that a significant part of the modern deep-sea fauna is much older than currently assumed. As ongoing invasion of deep habitats evidently precludes the determination of a single point of origin for the entire modern deep-sea fauna [26], it seems more appropriate to attempt to approximate the last point of major deep-sea faunal reorganization.
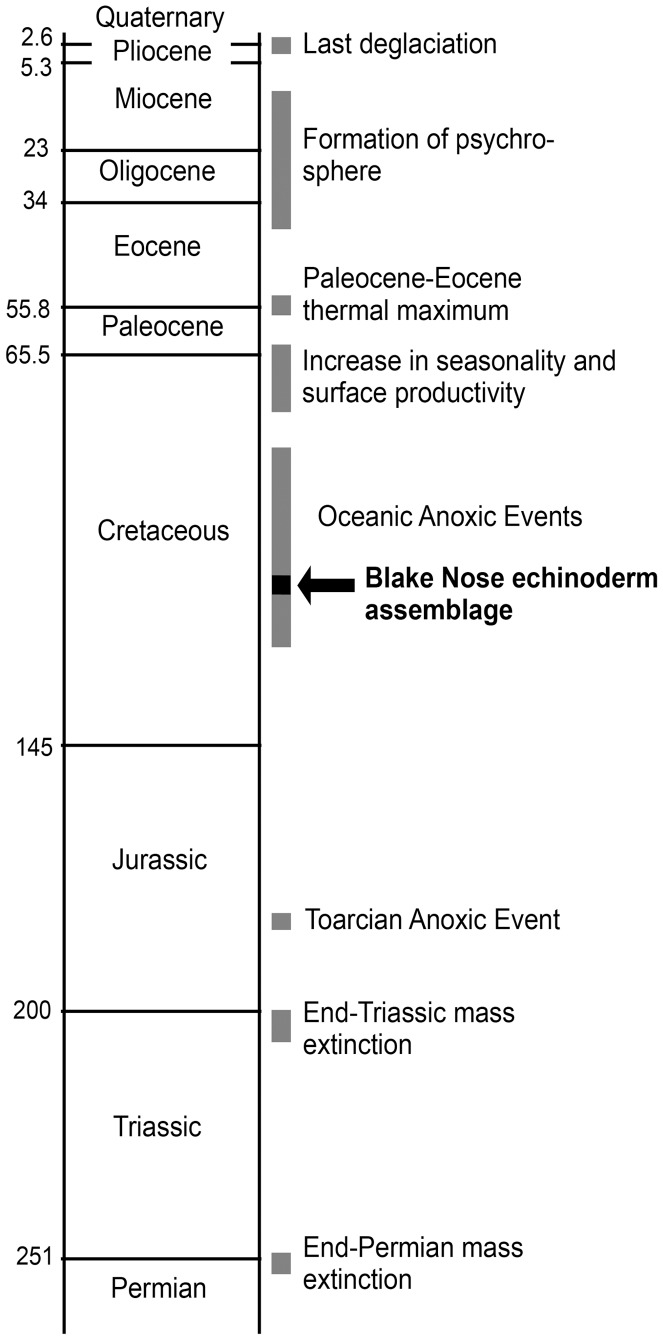
The Blake Nose assemblage postdates the major OAE 1a (Aptian, 124 Ma), but predates the minor dysoxic events of Albian age (OAE 1b–d; 112–100 Ma), and the extensive and widespread OAE 2 (late Cenomanian, 93 Ma) that was associated with photic zone anoxia in the Atlantic [27]. The Paleocene–Eocene thermal maximum (PETM; 60 Ma) [28] and the mid-Cenozoic deep-water cooling events (34 Ma) [29] are much later (Fig. 6). We therefore suggest that all these events were less important in controlling deep-sea biodiversity than previously assumed [4]–[5], although, strictly speaking, our observation can be spatially extrapolated for the North Atlantic at most. Then again, in spite of extensive sampling especially in Upper Cretaceous shelf deposits, at least four of the echinoderm groups recorded from the Blake Nose section have no known post-Aptian fossil record at shelf depths. These are parasol-spined and ophioleucinid ophiuroids, benthopectinid asteroids (the Maastrichtian specimens described in references [11] and [30] are of uncertain and/or non-benthopectinid affinities), and laetmogonid holothuroids. This is a significant piece of evidence since it effectively precludes the possibility that deep-sea recolonization from shallow habitats following episodic extinction occurred in echinoderms. Rather, the evidence points to an early Mesozoic or older colonization of deep-sea habitats by the modern fauna.
Figure 6. Position of the Blake Nose deep-sea echinoderm assemblage in the context of the events assumed to have triggered major reorganizations of the deep-sea fauna [4] –[6].
It has been suggested that the Middle Jurassic fossil Lagerstätte of La Voulte, France, was deposited in a bathyal setting [31]. This paleodepth estimate is entirely based on the presence of hexactinellid sponges and stalked crinoids, modern representatives of which are largely restricted to the deep sea. There are, however, well-documented shallow-water fossil occurrences of these groups, suggesting that water depth is not the main factor controlling their distribution [12], [32]. Therefore, in the absence of a robust paleodepth reconstruction, the La Voulte fauna does not contribute significantly to a discussion of the geologic history of the modern deep-sea fauna.
Deep-sea vent and seep communities have been considered relatively resistant to extinction events [33]. The Blake Nose assemblage corroborates previous assumptions that this resistance, rather than a result of the independent food source of vent communities, is a phenomenon of deep-sea communities in general, despite the bentho-pelagic coupling through food delivery that was previously assumed to control deep-sea biodiversity [34]. It can be speculated that wide biogeographic distribution and great dispersal potential, commonly observed in modern deep-sea organisms [1], [26], might have played an important role in buffering the deep-water fauna against major extinction events. Whatever the causes, our data clearly show that the factors controlling biodiversity and the resilience of ecosystems to oceanographic changes differ significantly between deep-sea and shallow-marine settings.
The origin of much of the modern deep-sea fauna must be sought in sediments older than late Aptian. The two major Mesozoic oceanic anoxic events preceding the Blake Nose assemblage (Toarcian OAE and early Aptian OAE 1a) were unlikely to have been more severe than the end-Cenomanian OAE 2 [27], post-dating our assemblage. It thus seems likely that they had a similarly negligible effect on deep-sea biodiversity. We thus speculate that the end-Permian mass extinction, accompanied by a long-term, worldwide deep-sea anoxia [35] and, probably to a lesser extent, the end-Triassic extinction and productivity collapse [36] were potential triggers for the last major reorganization of deep-sea communities and thus the origin of a significant part of the modern fauna.
Methods
Sample Selection and Treatment
Only few pre-Cenomanian in situ deep-sea sediments meet the requirements of bathyal paleodepth and good preservation of calcareous microfossils necessary to investigate deep-sea communities preceding the majority of the Cretaceous OAEs, in particular OAE 2. The most promising among them is ODP site 1049. A total of 74 samples, each representing 1 cm3 of sediment, from intervals 171B–1049C−12×4, 141 cm to 13×2, 81 cm, and 171B–1049A−20×3, 0 cm to 20×5, 51 cm, were examined. Sample treatment included mechanical disintegration in de-ionized water using an orbital shaker, and washing over a 0.063 mm screen. Echinoderm remains were picked from the >125 µm fraction, and, when appropriate, the >63 µm fraction. The material described herein was deposited in the micropaleontological collection at the University of Tübingen for the residues, and the Geoscience Museum at the University of Göttingen (GZG) for the echinoderm remains. Extraction of skeletal plates from Recent specimens for comparison was performed using household bleach for maceration.
Quantitative Analysis
In order to compare the Blake Nose ophiuroid assemblage with modern brittle star communities in terms of abundance patterns, family-level specimen counts were compiled from various references covering all major ocean basins and depths ranging from shallow sublittoral to abyssal [37]–[44]. Abundance data for the Blake Nose assemblage were inferred from lateral arm plate counts (see Table 1), in which only unquestionably identifiable plates and fragments representing more than half a lateral arm plate were recorded. All other lateral arm plates and smaller fragments were counted as indeterminate and not included in the dataset. In addition, data from Cretaceous shallow-water ophiuroid communities were included in the analysis in order to test for possible similarities in family-level composition and abundance patterns with the bathyal Blake Nose assemblage. These were based on published Cretaceous assemblages [45]–[46], and on lateral arm plate counts of previously unpublished assemblages from the Aptian Mosqueruela Formation [47] of Mosqueruela (Spain), the middle Albian Gault Clay levels 2 and 6 [48] of Folkestone (United Kingdom), the upper Albian Duck Creek Formation of Saginaw, Texas (USA), and the Cenomanian Del Rio Formation [49] of Waco, Texas (USA) (See Table 2 for corresponding lateral arm plate counts). After removal of the Ophiuridae, ophiuroid assemblages with fewer than 10 individuals were deleted from the analysis to avoid overrating of rare components. In order to compare the above-mentioned assemblages, Detrended Correspondence Analysis (DCA) was performed using the PAST software [50].
Table 1. Ophiuroid lateral arm plate counts of the Blake Nose samples.
| Section | Depth below surface (m) | Ophiacanthidae | Ophiuridae | Ophiolepididae | indeterminate |
| 1049C 12×4 141–142 | 145,21 | 1 | 0 | 0 | 0 |
| 1049C 12×4 144–145 | 145,24 | 0 | 0 | 0 | 2 |
| 1049C 12×4 145–146 | 145,25 | 1 | 0 | 0 | 0 |
| 1049C 12×4 146–147 | 145,26 | 1 | 0 | 0 | 1 |
| 1049C 12×4 147–148 | 145,27 | 0 | 1 | 0 | 0 |
| 1049C 12×4 148–149 | 145,28 | 1 | 5 | 0 | 7 |
| 1049C 12×4 149–150 | 145,29 | 14 | 6 | 3 | 5 |
| 104912×5 0–1 | 145,30 | 23 | 17 | 1 | 6 |
| 104912×5 1–2 | 145,31 | 32 | 17 | 3 | 4 |
| 104912×5 3–4 | 145,33 | 5 | 4 | 0 | 1 |
| 104912×5 4–5 | 145,34 | 2 | 6 | 3 | 0 |
| 104912×5 5–6 | 145,35 | 5 | 2 | 3 | 1 |
| 104912×5 6–7 | 145,36 | 4 | 2 | 0 | 1 |
| 104912×5 7–8 | 145,37 | 4 | 7 | 0 | 2 |
| 104912×5 8–9 | 145,38 | 19 | 11 | 6 | 3 |
| 104912×5 9–10 | 145,39 | 30 | 12 | 1 | 4 |
| 104912×5 10–11 | 145,40 | 29 | 7 | 1 | 1 |
| 104912×5 11–12 | 145,41 | 15 | 6 | 0 | 7 |
| 104912×5 12–13 | 145,42 | 20 | 6 | 1 | 1 |
| 104912×5 13–14 | 145,43 | 8 | 16 | 1 | 2 |
| 104912×5 14–15 | 145,44 | 7 | 19 | 3 | 3 |
| 104912×5 15–16 | 145,45 | 9 | 14 | 0 | 1 |
| 104912×5 16–17 | 145,46 | 3 | 11 | 0 | 4 |
| 104912×5 17–18 | 145,47 | 5 | 7 | 0 | 0 |
| 104912×5 18–19 | 145,48 | 16 | 6 | 0 | 7 |
| 104912×5 19–20 | 145,49 | 27 | 24 | 5 | 2 |
| 104912×5 20–21 | 145,50 | 4 | 12 | 1 | 7 |
| 104912×5 21–22 | 145,51 | 14 | 15 | 1 | 10 |
| 104912×5 22–23 | 145,52 | 6 | 5 | 4 | 8 |
| 104912×5 23–24 | 145,53 | 8 | 13 | 4 | 12 |
| 104912×5 24–25 | 145,54 | 5 | 7 | 11 | 4 |
| 104912×5 25–26 | 145,55 | 3 | 13 | 4 | 6 |
| 104912×5 26–27 | 145,56 | 1 | 10 | 0 | 13 |
| 104912×5 27–28 | 145,57 | 3 | 12 | 0 | 5 |
| 104912×5 28–29 | 145,58 | 3 | 10 | 0 | 3 |
| 104912×5 29–30 | 145,59 | 3 | 2 | 0 | 1 |
| 104912×5 39–40 | 145,69 | 22 | 23 | 3 | 9 |
| 104912×5 59–60 | 145,89 | 33 | 40 | 7 | 2 |
| 104912×5 99–100 | 146,29 | 7 | 4 | 5 | 2 |
| 104912×5 139–140 | 146,69 | 18 | 6 | 1 | 1 |
| 1049C 12×6 0–1 | 146,80 | 4 | 1 | 0 | 3 |
| 1049C 12×6 40–41 | 147,20 | 11 | 5 | 0 | 7 |
| 1049C 12×6 60–61 | 147,40 | 29 | 0 | 0 | 4 |
| 1049C 12×6 70–71 | 147,50 | 8 | 0 | 6 | 1 |
| 1049C 12×6 100–101 | 147,80 | 24 | 0 | 2 | 5 |
| 1049C 12×6 120–121 | 148,00 | 6 | 0 | 3 | 3 |
| 1049C 13×1 0–1 | 148,90 | 10 | 0 | 0 | 2 |
| 1049C 13×1 30–31 | 149,20 | 9 | 3 | 0 | 5 |
| 1049C 13×1 60–61 | 149,50 | 1 | 0 | 0 | 3 |
| 1049C 13×1 130–131 | 150,20 | 107 | 8 | 13 | 4 |
| 1049C 13×1 140–141 | 150,30 | 29 | 2 | 0 | 6 |
| 1049C 13×1 40–41 | 150,80 | 45 | 37 | 3 | 5 |
| 1049C 13×1 50–51 | 150,90 | 12 | 5 | 12 | 2 |
| 1049C 13×1 60–61 | 151,00 | 9 | 5 | 1 | 6 |
| 1049C 13×1 80–81 | 151,20 | 41 | 11 | 1 | 1 |
| 1049C 13×1 100–101 | 151,40 | 14 | 6 | 3 | 1 |
| 1049C 13×1 130–131 | 151,70 | 3 | 0 | 0 | 0 |
| 1049C 13×1 149–150 | 151,89 | 9 | 12 | 7 | 7 |
| 1049A 20×3 80–81 | 157,30 | 0 | 1 | 0 | 0 |
| 1049A 20×4 60–61 | 158,60 | 7 | 2 | 1 | 4 |
| 1049A 20×4 70–71 | 158,70 | 5 | 5 | 2 | 2 |
| 1049A 20×4 100–101 | 159,00 | 63 | 54 | 22 | 1 |
| 1049A 20×4 110–111 | 159,10 | 13 | 9 | 3 | 11 |
| 1049A 20×4 140–141 | 159,40 | 2 | 3 | 1 | 3 |
| 1049A 20×4 150–151 | 159,50 | 33 | 19 | 8 | 3 |
| 1049A 20×4 10–11 | 159,70 | 68 | 57 | 8 | 8 |
| 1049A 20×4 30–31 | 159,90 | 3 | 6 | 2 | 2 |
| 1049A 20×4 50–51 | 160,10 | 3 | 5 | 0 | 0 |
| Total | 979 | 634 | 170 | 247 |
Samples barren of ophiuroid remains were omitted; each sample represented 1 cm3.
Table 2. Ophiuroid lateral arm plate counts of Cretaceous shallow-water assemblages.
| Locality | Formation | Age | Ophiacanthidae | Ophiuridae | Ophiolepididae |
| Folkestone (UK) | Gault Clay, level 2 | Albian | 11 | 0 | 72 |
| Folkestone (UK) | Gault clay, level 6 | Albian | 0 | 0 | 206 |
| Mosqueruela (E) | Mosqueruela Formation | Aptian | 17 | 9 | 535 |
| Saginaw, Texas (USA) | upper Duck Creek Formation | Albian | 0 | 0 | 100 |
| Waco, Texas (USA) | Del Rio Formation | Cenomanian | 1 | 0 | 58 |
Acknowledgments
We thank H. Hess for assistance with the identification of the crinoid material, T. Fujita for providing Recent specimens for comparison, J.H. Nebelsick and F. Wiese for discussions, M. Kutscher for providing part of the data on Cretaceous shallow-water ophiuroid assemblages. We gratefully acknowledge the critical comments of the reviewers which helped to improve the present paper. This research used samples and data provided by the Integrated Ocean Drilling Program (IODP).
Funding Statement
The study was funded by the German Research Foundation (http://www.dfg.de/index.jsp), grant RE2599/6-1, and by the European Union funded Synthesys program (http://www.synthesys.info/), grants SE-TAF-2674 and SE-TAF-2969. Deposition of the described material in the collections of the Natural History Museum in London (UK), the micropaleontological collection at the University of Tübingen (D) and the Geoscientific Museum at the University of Göttingen (D) was done with the permission of the respective institutes. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Göttingen University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rex MA, Etter RJ (2010) Deep-Sea Biodiversity: Pattern and Scale. MA: Harvard University Press. 354 p.
- 2.Thomson CW (1873) The Depths of the Sea. London: Macmillan & Co. 527 p.
- 3. Jablonski D (2005) Evolutionary innovations in the fossil record: The intersection of ecology, development and macroeveolution. J Exp Zool (Mol Dev Evol) 304: 504–519. [DOI] [PubMed] [Google Scholar]
- 4. Jacobs DK, Lindberg DR (1998) Oxygen and evolutionary patterns in the sea: Onshore/offshore trends and recent recruitment of deep-sea faunas. Proc Natl Acad Sci USA 95: 9396–9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menzies RJ, George RY, Rowe GT (1973) Abyssal Environment and Ecology of the World Oceans. New York: Wiley-Interscience. 488 p.
- 6. Smith AB, Stockley B (2005) The geological history of deep-sea colonization by echinoids: roles of surface productivity and deep-water ventilation. Proc R Soc B 272: 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strugnell JM, Rogers AD, Prodöhl PA, Collins M, Allcock AL (2008) The thermohaline expressway: the Southern Ocean as a centre of origin for deep-sea octopuses. Cladistics 24: 853–860. [DOI] [PubMed] [Google Scholar]
- 8. Wilson GDF (1999) Some of the deep-sea fauna is ancient. Crustaceana 72: 1019–1030. [Google Scholar]
- 9.Gage JD, Tyler PA (1991) Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor. Cambridge: Cambridge University Press. 504 p.
- 10.Tyler PA, Rice AL, Young CM, Gebruk AA (1996) Walk on the deep side: animals in the deep sea. In: Summerhayes CP, Thorpe SA, editors. Oceanography – An Illustrated Guide. London: Manson. 195–211.
- 11. Jagt JWM (2000) Late Cretaceous-Early Palaeogene echinoderms and the K/T boundary in the southeast Netherlands and northeast Belgium. Part 5: Asteroids. Scripta Geologica 121: 377–503. [Google Scholar]
- 12. Hess H (1975) Die fossilen Echinodermen des Schweizer Juras. Veröff Naturhist Mus Basel 8: 1–130. [Google Scholar]
- 13.Norris RD, Kroon D, Klaus A, Alexander IT, Bardot LP, et al.. (1998) Proceedings of the Ocean Drilling Program, Initial Reports 171B. College Station, TX (Ocean Drilling Program): 1–749.
- 14. Huber BT, Leckie RM (2011) Planktic foraminiferal species turnover across deep-sea Aptian-Albian boundary sections. J Foram Res 41: 53–95. [Google Scholar]
- 15. Huber BT, MacLeod KG, Gröcke DR, Kucera M (2011) Paleotemperature and paleosalinity inferences and chemostratigraphy across the Aptian/Albian boundary in the subtropical North Atlantic. Paleoceanography 26: PA4221. [Google Scholar]
- 16. Holbourn A, Kuhnt W (2001) No extinctions during Oceanic Anoxic Event 1b: the Aptian–Albian benthic foraminiferal record of ODP Leg 171. In: Geol Soc London, Spec Publ Kroon D, Norris RD, Klaus A, editors. Western North Atlantic Palaeogene and Cretaceous Palaeoceanography. 183: 73–92. [Google Scholar]
- 17. Thuy B, Stöhr S (2011) Lateral arm plate morphology in brittle stars (Echinodermata: Ophiuroidea): new perspectives for ophiuroid micropalaeontology and classification. Zootaxa 3013: 1–47. [Google Scholar]
- 18. Tyler PA (1980) Deep-sea ophiuroids. Oceanogr Mar Biol Ann Rev 18: 125–153. [Google Scholar]
- 19. Litvinova NM (1992) Revision of the genus Ophiotholia (Echinodermata, Ophiuroidea). Zool Zh 72: 47–57. [Google Scholar]
- 20. Gale AS (2011) The phylogeny of post-Palaeozoic Asteroidea (Neoasteroidea, Echinodermata). Spec Pap Palaeont 85: 5–112. [Google Scholar]
- 21.Clark AM, Downey ME (1992) Starfishes of the Atlantic. London, New York, Tokyo, Melbourne, Madras: Chapman & Hall. 794 p.
- 22. Hansen B (1975) Systematics and biology of the deep-sea holothurians. Part 1. Elasipoda. Galathea Rep Sc Res Danish Deep-Sea Exp (1950–1952) 13: 1–262. [Google Scholar]
- 23. Barras CG (2007) Phylogeny of the Jurassic to Early Cretaceous ‘disasteroid’ echinoids (Echinoidea; Echinodermata) and the origins of spatangoids and holasteroids. J Syst Palaeontol 5: 134–161. [Google Scholar]
- 24. Kroh A, Smith AB (2010) The phylogeny and classification of post-Palaeozoic echinoids. J Syst Palaeontol 8: 147–212. [Google Scholar]
- 25.Hess H (2011) Treatise on Invertebrate Paleontology, Part T, Revised, Echinodermata 2, volume 3, Crinoidea Articulata. Lawrence: KU Paleontological Institute, Univ. of Kansas. xxix +261 p.
- 26. McClain CR, Mincks Hardy S (2010) The dynamics of biogeographic ranges in the deep sea. Proc R Soc B 277: 3533–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jenkyns HC (2010) Geochemistry of Oceanic Anoxic Events. Geochem Geophys Geosyst 11: 1–30. [Google Scholar]
- 28. Kennett JP, Stott LD (1991) Abrupt deep-sea warming, palaeoceanographic changes and benthic extinctions at the ed of the Palaeocene. Nature 353: 225–229. [Google Scholar]
- 29. Benson RH (1975) The origin of the psychrosphere as recorded in changes of deep-sea ostracode assemblages. Lethaia 8: 69–83. [Google Scholar]
- 30. Blake DB, Jagt JWM (2005) New latest Cretaceous and earliest Paleogene asteroids (Echinodermata) from the Netherlands and Denmark and their palaeobiological significance. Bull Inst Royal Sc Nat Belg, Sci Terre 75: 183–200. [Google Scholar]
- 31. Charbonnier S, Vannier J, Gaillard C, Bourseau JP, Hantzpergue P (2007) The La Voulte Lagerstätte (Callovian): Evidence for a deep water setting from sponge and crinoid communities. Palaeogeo Palaeoclim Palaeoecol 250: 216–236. [Google Scholar]
- 32. Gammon PR, James NP, Pisera A (2000) Eocene spiculites and spongiolites in southwestern Australia: Not deep, not polar, but shallow and warm. Geology 28: 855–858. [Google Scholar]
- 33. Kiel S, Little CTS (2006) Cold-seep molluscs are older than the general marine mollusc fauna. Science 313: 1429–1431. [DOI] [PubMed] [Google Scholar]
- 34. Cronin TM, Raymo ME (1997) Orbital forcing of deep-sea benthic species diversity. Nature 385: 624–627. [Google Scholar]
- 35. Isozaki Y (1997) Permo-Triassic boundary superanoxia and stratified superocean: records from lost deep sea. Science 276: 235–238. [DOI] [PubMed] [Google Scholar]
- 36. Ward PD, Haggart JW, Carter ES, Wilbur D, Tipper HW, et al. (2001) Sudden productivity collapse associated with the Triassic-Jurassic boundary mass extinction. Science 292: 1148–1151. [DOI] [PubMed] [Google Scholar]
- 37. Boos K, Franke H-D (2006) Brittle stars (Echinodermata: Ophiuroidea) in the German Bight (North Sea) – species diversity during the past 130 years. J Mar Biol Ass UK 86: 1187–1197. [Google Scholar]
- 38. Clark HL (1911) North Pacific ophiurans in the collection of the United States National Museum. Bull US Nat Mus 75: 1–302. [Google Scholar]
- 39. Clark HL (1939) Ophiuroidea. Sci Rep John Murray Exped 6: 29–136. [Google Scholar]
- 40. Koehler R (1922) Ophiurans of the Philippine seas and adjacent waters. Bull US Nat Mus 100: 1–486. [Google Scholar]
- 41.Koehler R (1901) Echinides et ophiures. Résultats du voyage du S.Y. Belgica en 1897–1898–1899 sous le commandement de A. de Gerlache de Gomery. Anvers: Rapports Scientifiques (1901–1913) Buschmann. 42 p.
- 42. Koehler R (1914) Ophiures des mers profondes. Siboga-Exped 45: 1–176. [Google Scholar]
- 43. Koehler R (1914) A contribution to the study of ophiurans of the United States National Museum. Bull US Nat Mus 84: 1–173. [Google Scholar]
- 44. Manjón-Cabeza ME, Ramos A (2009) Ophiuroid community structure of the South Shetland Islands and Antarctic Peninsula region. Polar Biol 26: 691–699. [Google Scholar]
- 45. Hess H (1970) Schlangensterne und Seesterne aus dem oberen Hauterivien “Pierre jaune" von St-Blaise bei Neuchâtel. Eclogae geol Helv 63: 1069–1091. [Google Scholar]
- 46. Storc R, Zitt J (2008) Late Turonian ophiuroids (Echinodermata) from the Bohemian Cretaceous Basin, Czech Republic. Bull Geosci 83: 123–140. [Google Scholar]
- 47.Vera JA (2004). editor. Geología de España. Madrid: SGE-IGME. 890 p.
- 48. Young JR, Gale AS, Knight RI, Smith AB (2010) Fossils of the Gault Clay. Palaeontological Association Field Guide to Fossils 12: 1–342. [Google Scholar]
- 49. Blake DB, Reid R III (1998) Some Albian (Cretaceous) asteroids (Echinodermata) from Texas and their paleobiological implications. J Paleont 72: 512–532. [Google Scholar]
- 50. Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electron 4: 1–9. [Google Scholar]
- 51. Erbacher J, Huber BT, Norris RD, Markey M (2001) Increased thermohaline stratification as a possible cause for an ocean anoxic event in the Cretaceous period. Nature 409: 325–327. [DOI] [PubMed] [Google Scholar]