Abstract
Background
Pharmacogenetics involves complex interactions of gene products affecting pharmacodynamics and pharmacokinetics, but there is little information on the interaction of multiple genetic modifiers of drug response. Bucindolol is a β-blocker/sympatholytic agent whose efficacy is modulated by polymorphisms in the primary target (β1 adrenergic receptor [AR] Arg389 Gly on cardiac myocytes) and a secondary target modifier (α2C AR Ins [wild-type (Wt)] 322–325 deletion [Del] on cardiac adrenergic neurons). The major allele homozygotes and minor allele carriers of each polymorphism are respectively associated with efficacy enhancement and loss, creating the possibility for genotype combination interactions that can be measured by clinical trial methodology.
Methodology
In a 1,040 patient substudy of a bucindolol vs. placebo heart failure clinical trial, we tested the hypothesis that combinations of β1389 and α2C322–325 polymorphisms are additive for both efficacy enhancement and loss. Additionally, norepinephrine (NE) affinity for β1389 AR variants was measured in human explanted left ventricles.
Principal Findings
The combination of β1389 Arg+α2C322–325 Wt major allele homozygotes (47% of the trial population) was non-additive for efficacy enhancement across six clinical endpoints, with an average efficacy increase of 1.70-fold vs. 2.32-fold in β1389 Arg homozygotes+α2C322–325 Del minor allele carriers. In contrast, the minor allele carrier combination (13% subset) exhibited additive efficacy loss. These disparate effects are likely due to the higher proportion (42% vs. 8.7%, P = 0.009) of high-affinity NE binding sites in β1389 Arg vs. Gly ARs, which converts α2CDel minor allele-associated NE lowering from a therapeutic liability to a benefit.
Conclusions
On combination, the two sets of AR polymorphisms 1) influenced bucindolol efficacy seemingly unpredictably but consistent with their pharmacologic interactions, and 2) identified subpopulations with enhanced (β1389 Arg homozygotes), intermediate (β1389 Gly carriers+α2C322–325 Wt homozygotes), and no (β1389 Gly carriers+α2C322–325 Del carriers) efficacy.
Introduction
Genetic determination of drug action, or pharmacogenetics, involves complex interactions of gene products affecting pharmacodynamics and pharmacokinetics. These interactions are difficult to investigate and quantify, in part because of the uncertainty of the modifier mechanism and the imprecision of measuring drug clinical responses.
There is considerable evidence that genetic variation in bucindolol's primary target, the cardiac β1 adrenergic receptor (AR), as well as in a modifier of the signaling mechanism for the primary target, cardiac neuronal norepinephrine (NE) release, modifies the response to this β-blocker/sympatholytic agent and possibly to other anti-adrenergic compounds. Although randomized trials have demonstrated that β-blockers improve survival and clinical outcomes in patients with chronic heart failure (HF) and reduced left ventricular ejection fractions (LVEFs) [1]–[5], trial results vary, with all-cause mortality effect sizes ranging from about 35% [4] to 10% [5]. Differences in the patient populations investigated [6], including geographic origin of study populations [7], may contribute to this variability, but there is also marked response heterogeneity within trials [8]. Genetic variations in ARs [9], [10] could explain the observed high inter-individual variability of β-blocker response [8]. Studies with bucindolol, in development for the indications of chronic HF and atrial fibrillation, have demonstrated response variability dependent on two coding AR polymorphisms that affect signaling: an amino acid position 389 Arg→Gly of the cardiac myocyte located β1 AR resulting from a nucleotide position c.1165 C→G in the ADRB1 gene [11]; and a position 322–325 four amino acid deletion (Del) in the cardiac prejunctional sympathetic nerve terminal α2C AR resulting from a nucleotide position c.964–975 Del in the ADRA2C gene [12].
The 389 Arg vs. Gly β1 AR variants are pharmacologically distinct. Compared with the β1389 Gly AR, the more-common β1389 Arg AR has 3-to-4–fold greater signal transduction capacity [11], [13], a higher affinity for agonists [13], [14], and a larger proportion of constitutively active receptors [10], [11]. Bucindolol exerts a selective effect on β1389 Arg vs. Gly receptors in part through inverse agonist activity [11], a property that leads to a shift of constitutively active receptors to an inactive state. α2C ARs are localized to cardiac prejunctional nerve terminals, where they mediate inhibition of NE release in a negative feedback loop [15]. The α2C four amino acid Del imparts a loss-of-function phenotype [16] and is also associated with adrenergic dysregulation and an exaggerated sympatholytic response to bucindolol [12]. The presence of both β1389 Arg/Arg and α2C322–325 Del/Del genotypes appears to synergistically increase the risk of HF in African Americans [9], emphasizing the potential importance of therapeutic interactions between these two AR polymorphisms.
Prejunctional α2C wild-type (Wt) 322–325 Del and postjunctional β1Arg389 Gly genetic variants are positioned in-series in the cardiac adrenergic neuroeffector pathway, and their potential interaction provides an opportunity to investigate the genetic complexity [17], [18] of drug response. Most treatment effects are likely determined by the interplay of multiple genes [17], and the existence of two drug-response modifying polymorphisms in-series with a signaling pathway that is a major determinant of HF disease progression provides a unique model system for investigating complex pharmacogenetic interactions. Given the pharmacologic and clinical importance of these polymorphisms, in a 1,040 patient substudy of the Beta Blocker Evaluation of Survival Trial (BEST), we investigated their combined influence on major cardiovascular event responses to bucindolol by testing the primary hypothesis that the efficacy-modifying effects of each polymorphism would be additive and more pharmacogenetically informative when genotypes are combined. To provide adequate precision of detection of pharmacogenetic effects, we investigated effects on six BEST Endpoint Committee-adjudicated heart failure clinical outcomes, two of which (all-cause mortality and all-cause mortality or cardiac transplantation) were the primary endpoints of the substudy. In addition, when results were obtained that were inconsistent with the primary hypothesis, in experiments performed in left ventricular (LV) membrane preparations expressing β1 ARs, we tested the subhypothesis that marked differences in NE affinity accounted for the disparate results.
Methods
The 2,708-patient BEST trial [5], sponsored by the Department of Veterans Affairs and the National Heart, Lung, and Blood Institute, measured how bucindolol affected clinical endpoints in advanced chronic HF patients. The primary endpoint of BEST was all-cause mortality, which when analyzed according to the regulatory statistical analysis plan that included covariate adjustment for randomization stratifying variable and censoring for cardiac transplantation, yielded a hazard ratio (HR) and 95% confidence interval (CI) of 0.87 (0.76–1.00), P = 0.053. BEST contained a 1,040-patient DNA bank, which could be accessed by submission and successful peer review of a substudy protocol [11], [12]. Pharmacogenetic data presented here are from the DNA Oversight Committee-approved substudy “Pharmacogenomics of Beta-Adrenergic Receptor Polymorphisms and Response to Beta-Blockers in Heart Failure,” which was submitted prior to the trial's ending while patients were still being enrolled. The substudy tested the hypothesis that six previously identified AR variants [11], [12], of which five occur at a frequency adequate for hypothesis testing, could predict β-blocker response heterogeneity. This protocol included a provision for examining the effects of combinations of polymorphisms when positive findings were obtained for individual variants; an addendum to the protocol submitted prior to completion of the genetic analysis prospectively identified the potential importance of combinatorial interactions of β1389 and α2C322–325 polymorphisms.
The protocol-defined primary statistical method was Cox regression analysis [11], [12], yielding HRs and 95% CIs within genetic subgroups by treatment type or within treatment group by genotype. Because of racial differences in the distribution of AR polymorphisms, Cox models were covariate-adjusted for race and the three other BEST randomization stratification variables (+/− ischemic cardiomyopathy etiology of HF, LVEF, and sex). Tests for interaction were run on treatment group/genotype comparisons. HRs from Cox regression analyses or relative change ratios (RCRs) from HF hospitalization (HFH) days/patient data were transformed and normalized to the results in the entire DNA substudy cohort by the relative effect size (RES) method [12] of RES = Ln (HR or RCR genotype group)/Ln (HR or RCR DNA substudy cohort). Differential efficacy in percentage was calculated by adding the RES interval above unity to the amount below, and multiplying by 100. Results are presented as intention-to-treat analyses from the time of randomization and for the substudy's two primary clinical endpoints (times to all-cause mortality and all-cause mortality or cardiac transplantation), four secondary clinical endpoints (three of which were BEST parent protocol secondary endpoints, and the fourth, time to cardiovascular hospitalization, was requested post hoc by the U.S. Food and Drug Administration), and a negative control endpoint (time to non-cardiovascular hospitalization). All four of these efficacy secondary endpoints had a P<0.050 in the entire cohort. For efficacy analyses with genetic subgroups in this exploratory study, P values<0.050 in a two-tailed distribution were considered of interest and P values<0.010 were considered statistically significant based on a prespecified multiple comparison adjustment described for monotypes in the substudy grant application statistical section. A comparable multiple comparison adjustment for the four combination genotypes investigated in this study would yield a critical value of 0.0125.
High-affinity “agonist” binding of L-NE to β1 ARs was determined in non-failing human left ventricles obtained from unused organ donors as previously described [19].
The BEST trial was conducted according to Declaration of Helsinki principles. All participating patients gave informed written consent for both the parent protocol and the DNA substudy. Non-failing human hearts were provided by Donor Alliance, the Colorado-Wyoming organ procurement organization, which obtained written consent for research use of tissue from donor family members.
Results
Study population
The patient population was classified by the four possible β1389 Arg/Gly and α2CWt/Del combinations of major allele homozygote and minor allele carrier [11], [12] genotype combination groups 1–4, ordered by the number of major allele homozygous monotypes (Table 1).
Table 1. Baseline characteristics by genotype combination groups.
| Characteristic | Group 1A (n = 420) | Group 2B (n = 73) | Group 3C (n = 413) | Group 4D (n = 134) |
| β1389 genotype | Arg/Arg | Arg/Arg | Gly Carrier | Gly Carrier |
| α2C322–325 genotype | Wt/Wt | Del Carrier | Wt/Wt | Del Carrier |
| Age, yE | 60.7±11.3 | 56.9±13.7 | 61.0±12.4 | 58.9±12.7 |
| Male sex, n (%) | 339 (81) | 54 (74) | 332 (80) | 100 (75) |
| African American,E n (%) | 19 (5)F | 47 (64) | 50 (12)F | 91 (68) |
| CHF duration, mo | 46.6±49.6 | 55.9±56.8 | 41.4±41.9 | 48.1±48.8 |
| NYHA class, n (%) | ||||
| III | 397 (95) | 68 (93) | 373 (90) | 122 (91) |
| IV | 23 (5) | 5 (7) | 40 (10) | 12 (9) |
| Systolic BP, mmHgE | 117±17.8 | 123±19.8 | 118±17.2 | 120±18.7 |
| HF etiology, n (%) | ||||
| IschemicG | 242 (58) | 41 (56) | 258 (62)H | 65 (49) |
| Non-ischemicG | 178 (42) | 32 (44) | 155 (38)H | 69 (51) |
| LVEF, % | 23.3±7.1 | 23.6±7.1 | 24.0±7.0 | 23.3±7.1 |
| Diabetes, n (%) | 148 (35) | 30 (41) | 133 (32) | 51 (38) |
| History of hypertension,G n (%) | 202 (48) | 53 (73)I | 228 (55)H , J | 97 (72)I |
| Concomitant medications, n (%) | ||||
| ACEIs | 382 (91) | 69 (95) | 381 (92) | 128 (96) |
| Diuretics | 387 (92) | 67 (92) | 388 (94) | 125 (93) |
| Digoxin | 384 (91) | 63 (86) | 372 (90) | 117 (87) |
| NE, pg/mL | 472±279 (n = 352) | 517±349 (n = 59) | 488±250 (n = 363) | 523±354 (n = 101) |
Data presented as means ± standard deviations, unless otherwise noted.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; BP, blood pressure; CHF, chronic heart failure; HF, heart failure; LVEF, left ventricular ejection fraction; NE, norepinephrine; NYHA, New York Heart Association.
β1389 Arg/Arg+α2C Wt/Wt.
β1389 Arg/Arg+α2C Del carrier.
β1389 Gly carrier+α2c Wt/Wt.
β1389 Gly carrier+α2C Del carrier.
P<0.05 by ANOVA (ANalysis Of Variance).
P<0.0083 by Bonferroni for all pair wise comparisons.
P<0.05 by Chi-square test.
P<0.0083 by Bonferroni vs. Group 4.
P<0.0083 by Bonferroni vs. Group 1.
P<0.0083 by Bonferroni vs. Group 2.
Baseline characteristics of the genotype combination groups are given in Table 1. The most striking difference between genotype combinations is race where, as expected [9], there was a greater percentage of African American patients in the two groups (2 and 4) containing α2C322–325 Del alleles. Other differences among genotype combinations, such as more non-ischemic cardiomyopathy etiology in Group 3 vs. Group 4 and a greater proportion of patients with a history of hypertension in groups 2 and 4, are likely due to the racial imbalance. The small, clinically insignificant ANOVA (ANalysis Of VAriance between groups) differences in age and systolic blood pressure are not statistically significant after a multiple comparison adjustment.
Outcomes by β1389 Arg/Gly and α2C322–325 Wt/Del AR genotype combinations
Table 2 gives the HRs or RCRs for the various clinical efficacy endpoints. For Groups 1 (β1389 Arg/Arg+α2C322–325 Wt/Wt) and 2 (β1389 Arg/Arg+α2C322–325 Del carrier), the six HRs and RCRs overlap with respective average effect sizes of 40% and 49%, with both groups exceeding the average effect size of 26% in the all-genotype 1,040-patient DNA substudy cohort. RES, which normalizes the effect size to that in the DNA substudy cohort, yielded average values of 1.70 and 2.32, respectively, in Groups 1 and 2. This means efficacy was increased by an average of 70% (Group 1) or 132% (Group 2) relative to the parent population containing all genotypes. However, the RES range for Groups 1 and 2 overlapped (Table 2; 1.48–2.14 for Group 1; 1.49–3.29 for Group 2). Because of the small numbers of patients and events in Group 2, only Group 1 HRs or RCRs achieved P values<0.010 (heart failure progression [HFP] and HFH days/patient) or <0.05 (all endpoints but all-cause mortality, P = 0.099).
Table 2. Hazard ratios or relative change ratios for bucindolol/placebo (95% confidence intervals), number of events, and log-rank P values for clinical endpoints and norepinephrine change by genotype combination groups.
| Endpoint (no. events in DNA substudy cohort)A | DNA substudy cohortB | Group 1C | Group 2D | Group 1/2E | Group 3F | Group 4G |
| n = 1040 (525P, 515B) | n = 420 (207P, 213B) | n = 73 (29P, 44B) | n = 493 (236P, 257B) | n = 413 (214P, 199B) | n = 134 (75P, 59B) | |
| ACM | ||||||
| HR/RCR (95% CI) | 0.77 (0.58–1.03) | 0.66 (0.39–1.09) | 0.50 (0.12–2.05) | 0.62H (0.39–0.99) | 0.75H (0.48–1.17) | 1.04H (0.43–2.54) |
| No. events | 189 | 67 | 13 | 80 | 85 | 24 |
| Log-rank P values | 0.077 | 0.099 | 0.33 | 0.042 | 0.21 | 0.93 |
| ACM/transplant | ||||||
| HR/RCR (95% CI) | 0.74 (0.56–0.98) | 0.59 (0.37–0.96) | 0.50 (0.12–2.05) | 0.57I (0.36–0.89) | 0.76I (0.50–1.16) | 1.04I (0.43–2.54) |
| No. events | 207 | 75 | 13 | 88 | 94 | 25 |
| Log-rank P values | 0.035 | 0.031 | 0.33 | 0.012 | 0.20 | 0.93 |
| CVM | ||||||
| HR/RCR (95% CI) | 0.66 (0.48–0.91) | 0.54 (0.31–0.97) | 0.40 (0.08–2.12) | 0.52I (0.31–0.88) | 0.60I (0.36–0.97) | 1.11I (0.45–2.78) |
| No. events | 159 | 54 | 10 | 64 | 73 | 22 |
| Log-rank P values | 0.011 | 0.035 | 0.27 | 0.014 | 0.036 | 0.82 |
| HFP | ||||||
| HR/RCR (95% CI) | 0.76 (0.62–0.92) | 0.65 (0.47–0.89) | 0.58 (0.27–1.25) | 0.66H (0.49–0.88) | 0.80H (0.60–1.08) | 0.99H (0.53–1.84) |
| No. events | 436 | 165 | 32 | 197 | 188 | 51 |
| Log-rank P values | 0.004 | 0.007 | 0.16 | 0.005 | 0.14 | 0.96 |
| HFH days/patientJ | ||||||
| HR/RCR (95% CI) | 0.71 (0.47–0.95) | 0.48 (0.18–0.78) | 0.60 (−0.11–1.30) | 0.52I (0.24–0.80) | 0.83I (0.47–1.20) | 1.19I (−0.17–2.55) |
| No. days | 5805 | 5805 | 343 | 2632 | 2281 | 892 |
| P values | 0.042 | 0.009 | 0.38 | 0.009 | 0.41 | 0.76 |
| CVH | ||||||
| HR/RCR (95% CI) | 0.79 (0.66–0.96) | 0.68 (0.50–0.93) | 0.46 (0.21–1.02) | 0.64H (0.48–0.86) | 0.91H (0.68–1.22) | 0.96H (0.53–1.76) |
| No. events | 447 | 171 | 33 | 204 | 190 | 53 |
| Log-rank P values | 0.016 | 0.016 | 0.051 | 0.002 | 0.53 | 0.90 |
| Average effect sizeK, % | 26 | 40 | 49 | 41 | 22 | −5.5 |
| Average RESL (range) | 1.00 | 1.70 (1.48–2.14) | 2.32 (1.49–3.29) | 1.76 (1.51–1.91) | 0.83 (0.40–1.22) | −0.14 (−0.51–0.17) |
| Differential efficacyM, % | – | 184 | 246 | 190 | 97 | – |
| Non-CVH | ||||||
| HR/RCR (95% CI) | 0.99 (0.80–1.22) | 0.87 (0.62–1.23) | 1.59 (0.66–3.87) | 0.94 (0.69–1.28) | 1.11 (0.80–1.55) | 0.99 (0.48–2.01) |
| No. events | 367 | 145 | 28 | 173 | 153 | 41 |
| Log-rank P values | 0.94 | 0.43 | 0.30 | 0.69 | 0.53 | 0.97 |
| ΔNE at 3 mo, pg/mL | ||||||
| Placebo | 17±15 | −4±19 | 38±110P | 1.0±21 | 28±21 | 31±60 |
| Bucindolol | −66±15 | −50±19 | −120±82 | −62±20 | −50±22 | −145±64 |
| Log-rank P values | <0.001 | 0.091 | 0.27 | 0.027 | 0.011 | 0.0496 |
| B group - P group as net change relative to placebo | −83 | −46 | −158 | −63 | −78 | −176 |
Abbreviations: ACM, all-cause mortality; B, bucindolol; CI, confidence interval; CVH, cardiovascular hospitalization; CVM, cardiovascular mortality; HFH, heart failure hospitalization; HFP, heart failure progression (composite of heart failure death, cardiac transplantation, heart failure hospitalization, or an emergency department visit for treatment of heart failure involving administration of intravenous heart failure medication); HR, hazard ratio; P, placebo; NE, norepinephrine; RCR, relative change ratio; RES, relative effect size.
Number of events presented are using the unadjusted analysis, which differs slightly from covariate-adjusted because adjusted analyses are transplant-censored.
All genotypes.
β1389 Arg/Arg+α2C Wt/Wt.
β1389 Arg/Arg+α2C Del carrier.
β1389 Arg/Arg+any α2C.
β1389 Gly carrier+α2C Wt/Wt.
β1389 Gly carrier+α2C Del carrier.
Interaction P value≤0.20, >0.10.
Interaction P value≤0.10, >0.05.
Relative change ratio.
[1−HR or RCR]×100.
RES = Ln HR (genotype group)/Ln (DNA substudy cohort).
See Methods.
The HR/RCR and RES data for Group 2 therefore indicate that when combined with the β1389 major allele (Arg) homozygous monotype, the α2C322–325 Del variant does not exert any negative influence on bucindolol treatment effects despite Group 2 exhibiting a typical α2C322–325 Del variant-associated large decrease in NE (by 158 pg/mL compared with placebo), a degree of sympatholysis previously identified as compromising efficacy [12], [20]. In contrast, Group 1, which contains the Wt/Wt version of the α2C AR gene, exhibited only the expected mild NE lowering (average of 46 pg/mL compared with placebo) in bucindolol-treated patients (Table 2), a degree of sympatholysis that has been associated with increased efficacy [12], [20]. Therefore, there is no evidence that the presence of the α2C Del allele or degree of sympatholysis adversely influences outcomes when the β1389 polymorphism is Arg/Arg. Because the RES values for Groups 1 and 2 overlap, Group 1/2 was created as a combination genotype consisting of β1389 Arg/Arg+either α2C322–325 variant. Hazard ratios, RCRs, and P values in Group 1/2 are similar to those of Group 1 (Table 2), with respective average effect and relative effect sizes of 41% and 1.76 for Group 1/2, and 40% and 1.70 for Group 1. For Group 1/2, all P values for efficacy endpoints are <0.050; three are <0.010; and four, including the coprimary endpoint of ACM/transplant, are <0.0125 (the critical value for genotype combinations adjusted for multiple comparison).
As shown in Table 2, Group 3 (β1389 Gly carrier+α2C322–325 Wt/Wt) exhibited HRs/RCRs that were generally greater (less efficacy) than in Group 1 or Group 1/2 and similar to those in the 1,040-patient DNA substudy cohort. Despite a sample size similar to Group 1, only cardiovascular mortality had a P<0.05, and no endpoint had a P<0.010. The average effect size for Group 3 was 22%, compared with 26% in the entire DNA substudy, and the average RES was 0.83. The upper bound of the Group 3 RES range, 1.22, did not overlap with either the Group 1 (1.48), Group 2 (1.49) or Group 1/2 (1.51) lower bound of the RES range. Strikingly, Group 4 (β1389 Gly carrier+α2C322–325 Del carrier) exhibited HRs/RCRs of ∼1.0 with an average effect size of −5.5%, or no evidence of any efficacy. The average RES in Group 4 was −0.14 (range, −0.51–0.17), indicating higher event rates in the bucindolol group vs. the placebo group. This is comparable to respective average RES values of 0.40 (range, 0.22–0.60) and 0.22 (range −0.03–0.79) in the monotypes β1389 Gly carrier and α2C Del carrier, respectively (data not shown). The upper bound of the Group 4 RES range (0.17) does not overlap with the lower bound of the Group 3 RES range (0.40). Thus, when β1389 Gly carriers are combined with α2C322–325 Del carriers, an additive loss of efficacy occurs, compared with Groups 2 or 3, which contain only one monotype minor allele carrier. The reduction in NE at three months in Group 4 was expectedly large because of the presence of α2C322–325 Del alleles, and this degree of sympatholysis [12], [20] in patients with hypofunctional β1389 Gly receptors may have been the reason for efficacy loss.
Table 2 also gives the differential efficacy calculation [12], which is an important pharmacogenetic measure that expresses the maximal degree of efficacy separation of genetically defined groups. Compared with Group 4, Groups 1/2 and 3 had differential efficacies of 190% and 97%, respectively. These are comparable to differential efficacies of 136% for Group 1/2 vs. β1389 Gly carrier (average RES for the six clinical efficacy endpoints, 0.40) and 96% for α2C322–325 Wt/Wt (average RES,1.18) vs. Del carrier (average RES, 0.22). Thus, the differential efficacy gained by comparing Group 1/2 with Group 4 vs. the β1389 Gly carrier monotype is calculated as follows: 190%–136% = 54%. The advantage vs. the α2C322–325 Del carrier monotype is calculated as follows: 190%–96% = 94%.
Interaction tests with Groups 1/2, 3, and 4 as a continuous ordinal variable were P = 0.13 and 0.093, respectively, for all-cause mortality and all-cause mortality or transplant, 0.073 for HFH days/patient, and <0.20 for two of the three other efficacy endpoints. In contrast to the efficacy endpoints, there was no evidence of an effect of bucindolol or genotype combination on time to non-cardiovascular hospitalization, with an average HR/RCR of 0.99 in the entire cohort and a range of 0.87–1.59 in genotype groups.
Time-to-event curves for β1389 Arg/Gly and α2C322–325 Wt/Del combination genotypes
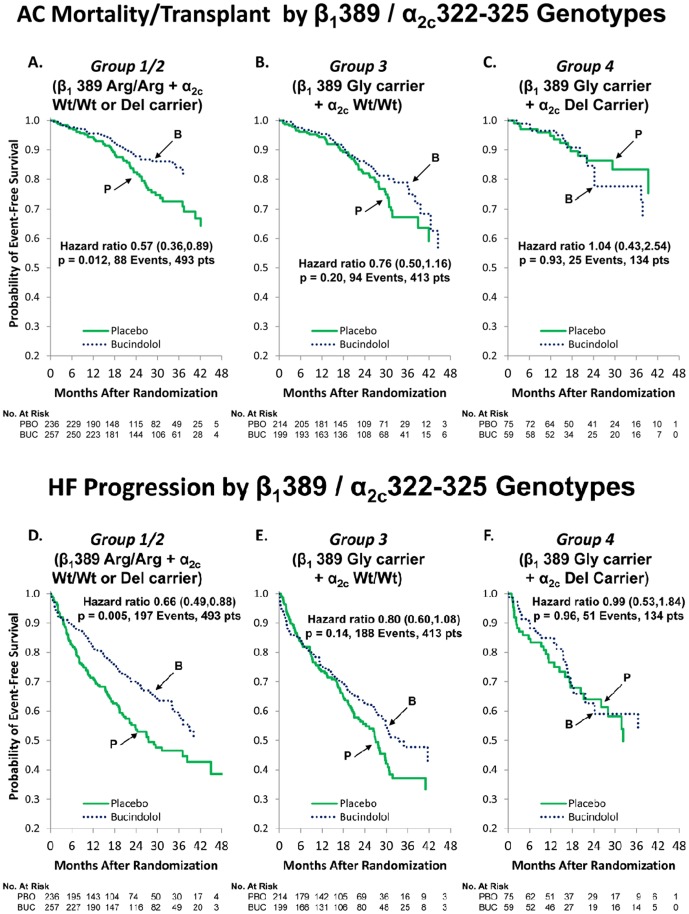
Figures 1A, 1B, and 1C give Kaplan-Meier curves for the primary endpoint of all-cause mortality or cardiac transplantation (ACM/Tx) for the efficacy-enhanced Group 1/2 (1A), the intermediate-efficacy Group 3 (1B), and the loss-of-efficacy Group 4 (1C) genotype combinations. Figures 1D, 1E, and 1F give the Kaplan-Meier curves for the combined endpoint of HFP in these same genotype groups. For both clinical endpoints, the pattern is substantial, statistically significant separation of the bucindolol and placebo curves in Group 1/2, moderate separation but non-significant P values in Group 3, and no curve separation (Figure 1F) or even curve crossover (Figure 1C) in Group 4. The HRs (95% CIs) for the pharmacogenetic groups are given in Table 2 and can be compared with those for the 2,708-patient entire cohort (ACM/Tx HR, 0.86; 95% CI, 0.75–0.98; P = 0.021) (HFP HR, 0.80; 95% CI, 0.72–0.89; P<0.0001) or the similar values for the 1,040-patient DNA substudy (Table 2). The Group 1/2 HRs of 0.57 (95% CI, 0.36–0.89; P = 0.012) for ACM/Tx and 0.66 (95% CI, 0.49–0.88; P = 0.005) for HFP (Table 2) are substantially less than the respective HRs for the entire or DNA substudy cohorts, with P values that are at or below the prespecified statistical analysis plan critical values.
Figure 1. Time to all-cause mortality or cardiac transplantation for Group 1/2 (A), Group 3 (B), and Group 4 (C), and time to heart failure progression (combination endpoint of heart failure mortality, cardiac transplantation, heart failure hospitalization, or emergency department care that includes intravenous therapy not requiring hospitalization) for Group 1/2 (D), Group 3 (E), and Group 4 (F).
Abbreviations: AC, all-cause; BUC, bucindolol; Del, deletion; HF, heart failure; PBO, placebo.
High-affinity agonist binding by L-NE to Arg or Gly β1389 ARs
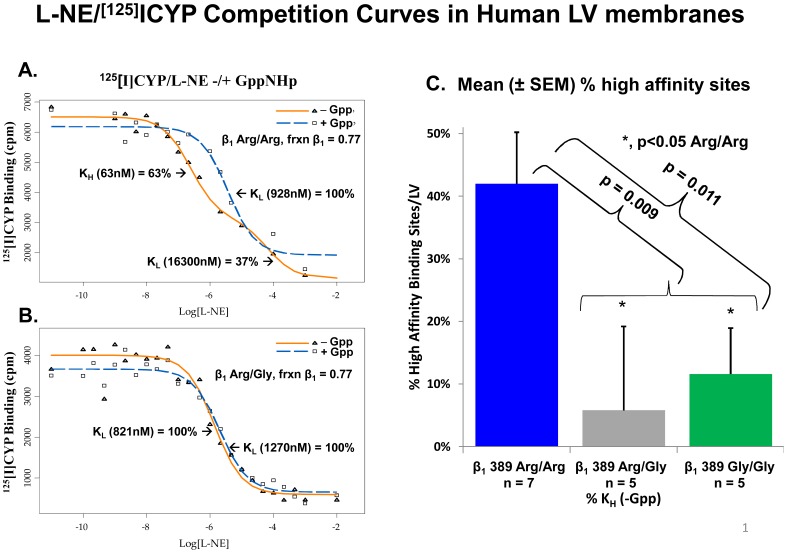
To investigate the affinity of NE for β1 ARs of various genotypes, we measured high-affinity agonist binding in membranes prepared from 17 human non-failing LV membranes that contained at least 75% β1 ARs (mean, 80.0±5.8%). Figure 2 gives representative competition curves for L-NE and 125[I]CYP (cyanopindolol) in a β1389 Arg/Arg (Figure 2A) or a β1389 heterozygote preparation (Figure 2B). In the β1389 Arg/Arg preparation, there is high-affinity displacement of 125[I]CYP by L-NE, with a dissociation constant (KH) of 63 nM. Computer modeling yielded a two-site fit, with the high-/low-affinity percentages of β1 ARs estimated to be 63/37. When incubated with the non-hydrolyzable guanine nucleotide Gpp(NH)p, which uncouples high-affinity agonist binding, only low-affinity (KL 928 nM) receptors are identified. In contrast, in the competition curve from a left ventricle genotyped as β1389 Arg/Gly, there is no evidence of any high-affinity L-NE binding. In all, 6/7 Arg/Arg; 1/5 Arg/Gly; and 2/5 Gly/Gly left ventricles exhibited two-site fits in the absence of Gpp(NH)p, with an average KH of 74.9±87.0 nM. The low-affinity binding constant (KL) in all 17 left ventricles averaged 7,254±12,250 nM. Figure 2C also gives the mean percentages of high-affinity L-NE β1 ARs in the three possible genotypes. Both heterozygotes (5.8%) and β1389 Gly homozygotes (11.6%) have much lower percentages of high-affinity L-NE binding sites than Arg homozygotes (42.0%, ANOVA P = 0.011; P = 0.009 for Arg/Arg vs. Gly carriers).
Figure 2. Representative competition curves between 50 pM 125[I]CYP and L-NE at increasing concentrations, in the absence and presence of 30 µM Gpp(NH)p in membranes from a non-failing human left ventricle with 77% β1 AR that was β1389 Arg/Arg genotype (A) and in membranes from a non-failing human heart with 77% β1 AR that was β1389 Arg/Gly genotype (B); mean±SEM (%) of high-affinity L-NE binding sites identified in seven β1389 Arg/Arg, five β1389 Arg/Gly, and five β1389 Gly/Gly left ventricles (C).
Abbreviations: AR, adrenergic receptor; CYP, cyanopindolol; Gpp(NH)p, non-hydrolyzable guanine nucleotide; KH, dissociation constant; KL, low-affinity binding constant; L-NE, L-norepinephrine; SEM, standard error measurement.
Discussion
Based on an analysis of three clinical endpoints, we have previously reported [11] that the the β-blocker/sympatholytic agent bucindolol exhibits enhanced clinical efficacy in ∼50% of the population that is homozygous for an Arg allele at position 389 of the β1 AR, compared with patients with a Gly allele at this position. Two of these endpoints (time to all-cause mortality and all-cause mortality/HFH) were also measured in the current study, and the other (time to first HFH) was the major component of time to HF progression in the current report. In another study [12] based on five clinical endpoints, all of which are included in the current report, we found that the ∼80% of patients with an Ins (Wt) at amino acids 322–325 of the α2C AR had therapeutic responses that were consistently better than in patients with a deletion of amino acids 322–325. This α2C AR 322–325 polymorphism-associated differential clinical response was found to be related to dysregulation of NE release in patients with Del genotypes, who had exaggerated sympatholytic responses to bucindolol that obviated efficacy. The major allele homozygotes of these two AR polymorphisms are therefore associated with enhanced response. In the case of β1389, this response is enhanced to a degree that tends to be greater than the response to standard β-blockers in all genotypes. In the case of α2C322–325, this response is enhanced to a degree comparable to standard β-blockers in all genotypes. Thus, the question arises as to whether the combination of two major allele genotypes would yield an even greater therapeutic response than in either monotype alone. This provides an opportunity to assess the interactive effects of two genetic variants that are intimately related to disease pathophysiology as well as drug response, a rare opportunity in pharmacogenetics. To test the hypothesis that the effects of each of these AR polymorphisms would be additive for HF clinical response, we investigated the effects of bucindolol vs. placebo on six clinical endpoints that included the three previously measured for β1389 Arg/Gly effects [11] and the five for α2C322–325 Wt/Del effects [12]. We added an additional adjudicated efficacy endpoint, cardiovascular hospitalization, and also included a negative control, non-efficacy endpoint of non-cardiovascular hospitalization.
Surprisingly, no additional therapeutic benefit of bucindolol was observed in the major allele homozygote combination of β1389 Arg/Arg+α2C322–325 Wt/Wt. That is, efficacy (as measured by the RES method) [12] across the six efficacy endpoints was enhanced by an average of 1.70-fold in the major allele homozygotes combination genotype, compared with 2.32-fold in the combination genotype of β1389 Arg/Arg+α2C322–325 Del carrier. The non-efficacy, non-cardiovascular hospitalization endpoint did not exhibit pharmacogenetic enhancement or decrement. In contrast, for carriers of β1389 and α2C322–325 minor alleles (Group 4) as a combination genotype, there was additive diminished efficacy to the point of complete efficacy loss (RES = −0.14). This indicates an average RES for which the bucindolol treatment effect is 14% worse than placebo and worse than in patients with the β1389 Gly carrier or α2C Del carrier monotype, for which the respective RES values indicated efficacies 40% or 22% better than placebo. In Group 4, the further decrement in efficacy compared with the β1389 Gly carrier monotype was due to the removal of the β1389 Gly carrier+α2C Wt/Wt combined genotype as Group 3. Compared with the α2C Del carrier monotype, this was due to the removal of the β1389 Arg/Arg+α2C Del carrier combined genotype to constitute Group 2. Thus the hypothesis that combining predictive individual monotypes into combined genotypes improves pharmacogenetic targeting was supported only for the minor alleles.
The differences between minor and major alleles in pharmacogenetic combination are likely due to the pharmacologic differences in β1389 Arg vs. Gly receptors. Compared with its Gly counterpart, the β1389 Arg receptor has higher signal transduction capacity [11], [13], more receptors in a constitutively active state [10], [11], and higher-agonist affinity [13], [14]. We demonstrated that high-affinity agonist binding to NE does not extend to heterozygotes, as Gly β1389 ARs appear to exert a dominant negative effect on NE high-affinity binding as well as on bucindolol's clinical efficacy. Therefore, in advanced HF patients, the 389 Arg version of the β1 AR is much better-equipped to support cardiac function in the face of marked NE lowering, where its higher-agonist binding affinity allows it to better utilize low levels of adrenergic activity. In contrast, the hypofunctioning, lower-NE affinity β1389 Gly version of the β1 AR needs higher NE levels to support the failing heart, and in the presence of the α2C322–325 Del-associated marked sympatholysis, likely cannot adequately support cardiac function, leading to an increase in mortality and hospitalizations that cancels bucindolol efficacy. These relationships likely explain the marked differences in bucindolol's clinical efficacy between Group 2 (has only β1389 Arg receptors) and Group 4 (contains ≥50% β1389 Gly receptors). Moreover, because of its much higher affinity for NE, the β1389 Arg receptor is an NE receptor, whereas the β1389 Gly variant is not, having an NE affinity that is similar to the β2 AR. The sympatholytic effects of bucindolol therefore preferentially inhibit β1389 Arg signalling, providing a basis for the selective clinical effects of bucindolol in β1389 Arg/Arg genotypes vs. any Gly-containing genotype.
The β1389 and α2C322–325 combinations of genetic biomarkers conferred a nearly two-fold difference in averaged efficacy (a differential efficacy of 190%) between the efficacy-enhanced (β1389 Arg/Arg) genotype Group 1/2 and the loss-of-efficacy (β1389 Gly carrier, α2C322–325 Del carrier) genotype Group 4. The goal of pharmacogenetic targeting is to identify subgroups with large differences in treatment efficacy, or “outliers” [21], so that the more responsive group can be offered the likelihood of benefit that is better than that in the general population, and the less-responsive group can avoid treatment exposure. In this regard, the use of β1389 and α2C combination genotypes yielded numerically greater degrees of high-low response differential efficacy compared with β1389 or α2C monotypes, by respective absolute amounts of 54% and 94%. Based on the above and non-overlap of RES ranges, combinations of β1389 Arg/Gly and α2C322–325 genotypes therefore identified a 47% subpopulation (Group 1/2 [β1389 Arg/Arg+any α2C]) with a bucindolol-enhanced clinical response profile compared with the parent population that generally exceeds effect sizes associated with other β-blockers in a variety of HF populations [1]–[8], a 40% subpopulation (Group 3 [β1389 Gly carriers+α2C322–325 Wt/Wt]) with clinical responses similar to that in the parent population of all genotypes, and a small but non-trivial 13% subpopulation with complete loss of efficacy that should not be treated with bucindolol.
Although tests for interaction between β1389 and α2C genotype combinations and treatment effects did not achieve statistical significance, two P values were <0.10 and most were <0.20. However, interaction tests, commonly used to assess heterogeneity of subgroups within a clinical trial population without regard to any particular mechanistic interaction with the tested treatment, have limited statistical power to detect pharmacogenetic efficacy differences that can be expected to be small, unidirectional, and present in limited sample sizes. The demonstration of statistically significant, robust treatment effects across multiple relevant clinical endpoints in one pharmacogenetic subset but not its allelic counterpart, especially when supported by biologic plausibility, achieves the goal of identifying a subset of patients highly likely to respond favorably to a treatment. The β1389 Arg/Arg genotype meets these criteria for a favorable response pharmacogenetic subgroup.
Limitations
There are some limitations to this study. First, although the substudy was prospectively designed and hypothesis-driven, the pharmacogenetic data were generated and analyzed after the trial's main results were analyzed and published [5]. However, the investigators generating the pharmacogenetic data remained blinded to the treatment code and to clinical outcomes throughout. Second, approximately two-thirds of the patients were enrolled into the DNA substudy after being randomized into the parent trial. This “late entry” phenomenon has been extensively analyzed, by both L-truncation [12] and, most recently, propensity score statistical methods (unpublished observations). The effect of late entry into the DNA substudy is only to lower event rates for all clinical endpoints, without affecting genotype-specific treatment effects.
Conclusions
The combinatorial interaction of two sets of AR polymorphisms that influence bucindolol's drug action resulted in unanticipated effects on HF clinical responses, non-additivity in efficacy enhancement for the major allele homozygotes, and additive effects for minor allele carrier-associated efficacy loss. An explanation for these disparate results was provided by the effects of the α2C322–325 minor (Del) allele on facilitating bucindolol's NE-lowering properties, where excessive NE lowering abolished efficacy when the β1389 Gly minor allele and low NE affinity AR were present but did not alter or even enhance efficacy in the presence of the major allele homozygous β1389 Arg genotype, which encodes ARs with a NE affinity of ∼100-fold more than 389 Gly ARs.
Combinatorial genotyping led to improvement in pharmacogenetic differentiation of drug response compared with monotype genotyping. The use of β1389 Arg/Gly and α2C322–325 Wt/Del genotype combinations accomplishes the goal of pharmacogenetics to identify response outliers from both ends of the therapeutic spectrum. Compared with the use of β1389 Arg/Gly or α2C322–325 Wt/Del monotypes, the differential efficacy gained by the use of genotype combinations was increased by respective amounts of 54% and 94%. The new identification of a completely unresponsive genotype, supported by biologic plausibility and bolstered by data consistency across multiple clinical endpoints, is especially important inasmuch as a major goal of pharmacogenetics is to identify patients with no likelihood of benefit who can then be spared drug side effects [21]. Other β-blockers that have been used to treat HF do not have these pharmacogenetic interactions [22], [23], but rather exhibit response heterogeneity through other, unknown mechanisms [8]. Thus, the ability to predict drug response through pre-treatment pharmacogenetic testing should improve therapeutic response to this drug class but will need to be confirmed by prospective studies.
Finally, the unexpected results of this study, (i.e., the additive loss of efficacy by minor allele combinations in the absence of additive gain of efficacy by major allele homozygotes) emphasizes that combinations of response-altering polymorphisms may behave in unpredictable ways and in-silico predictions of combinatorial genetic effects will need to be supported by empirical data.
Acknowledgments
Additional Contributions
The authors thank Rachel Rosenberg and Morgan deBlecourt for manuscript processing and Richard Bittman for statistical input.
Clinical Trial Registration
ClinicalTrials.gov unique identifier: NCT00000560
Funding Statement
This study was supported by United States National Institutes of Health (NIH) grants HL077101, awarded to SB Liggett, and 2R01 HL48013, awarded to MR Bristow; the Department of Veterans Affairs Cooperative Studies Program; the National Heart, Lung, and Blood Institute Division of Cardiovascular Sciences; and ARCA biopharma. The BEST Trial and DNA substudy were funded by the NIH/National Heart, Lung, and Blood Institute. No other funding was used to support this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. [No authors listed] (1999) The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 353: 9–13. [PubMed] [Google Scholar]
- 2. Flather MD, Shibata MC, Coats AJ, Van Veldhuisen DJ, Parkhomenko A, et al. (2005) Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 26: 215–225. [DOI] [PubMed] [Google Scholar]
- 3. [No authors listed] (1999) Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353: 2001–2007. [PubMed] [Google Scholar]
- 4. Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, et al. (2001) Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 344: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 5. The Beta Blocker Evaluation of Survival Trial Investigators (2001) A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 344: 1659–1667. [DOI] [PubMed] [Google Scholar]
- 6. Domanski MJ, Krause-Steinrauf H, Massie BM, Deedwania P, Follmann D, et al. (2003) A comparative analysis of the results from 4 trials of beta-blocker therapy for heart failure: BEST, CIBIS-II, MERIT-HF, and COPERNICUS. J Card Fail 9: 354–363. [DOI] [PubMed] [Google Scholar]
- 7. O'Connor CM, Fiuzat M, Caron MF, Deedwania P, Follmann D, et al. (2011) Influence of global region on outcomes in large heart failure β-blocker trials. J Am Coll Cardiol 58: 915–922. [DOI] [PubMed] [Google Scholar]
- 8.Metra M, Bristow MR (2010) Beta-blocker therapy in chronic heart failure. In: Mann DL, ed. Heart Failure: A companion to Braunwald's Heart Disease.
- 9. Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB (2002) Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 347: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 10. Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, et al. (2003) Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med 9: 1300–1305. [DOI] [PubMed] [Google Scholar]
- 11. Liggett SB, Mialet-Perez J, Thaneemit-Chen S, Weber SA, Greene SM, et al. (2006) A polymorphism within a conserved beta(1)-adrenergic receptor motif alters cardiac function and beta-blocker response in human heart failure. Proc Natl Acad Sci U S A 103: 11288–11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bristow MR, Murphy GA, Krause-Steinrauf H, Anderson JL, Carlquist JF, et al. (2010) An α2C-adrenergic receptor polymorphism alters the norepinephrine lowering effects and therapeutic response of the beta blocker bucindolol in chronic heart failure. Circ Heart Fail 3: 21–28. [DOI] [PubMed] [Google Scholar]
- 13. Mason DA, Moore JD, Green SA, Liggett SB (1999) A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J Biol Chem 274: 12670–12674. [DOI] [PubMed] [Google Scholar]
- 14. Sandilands AJ, O'Shaughnessy KM, Brown MJ (2003) Greater inotropic and cyclic AMP responses evoked by noradrenaline through Arg389 β1-adrenoceptors versus Gly389 β1-adrenoceptors in isolated human atrial myocardium. Br J Pharmacol 138: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hein L, Altman JD, Kobilka BK (1999) Two functionally distinct alpha2-adrenergic receptors regulate sympathetic neurotransmission. Nature 402: 181–184. [DOI] [PubMed] [Google Scholar]
- 16. Small KM, Forbes SL, Rahman FF, Bridges KM, Liggett SB (2000) A four amino acid deletion polymorphism in the third intracellular loop of the human alpha 2C-adrenergic receptor confers impaired coupling to multiple effectors. J Biol Chem 275: 23059–23064. [DOI] [PubMed] [Google Scholar]
- 17. Evans WE, Relling MV (2004) Moving towards individualized medicine with pharmacogenomics. Nature 429: 464–468. [DOI] [PubMed] [Google Scholar]
- 18. Sadee W, Dai Z (2005) Pharmacogenetics/genomics and personalized medicine. Hum Mol Genet 14 (Spec No.2) R207–R214. [DOI] [PubMed] [Google Scholar]
- 19. Hershberger RE, Wynn JR, Sundberg L, Bristow MR (1990) Mechanism of action of bucindolol in human ventricular myocardium. J Cardiovasc Pharm 15: 959–967. [DOI] [PubMed] [Google Scholar]
- 20. Bristow MR, Krause-Steinrauf H, Nuzzo R, Liang CS, Lindenfeld J, et al. (2004) Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial (BEST). Circulation 110: 1437–1442. [DOI] [PubMed] [Google Scholar]
- 21. Woodcock J, Lesko LJ (2009) Pharmacogenetics–tailoring treatment for the outliers. N Engl J Med 360: 811–813. [DOI] [PubMed] [Google Scholar]
- 22. White HL, de Boer RA, Maqbool A, Greenwood D, van Veldhuisen DJ, et al. (2003) An evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism in individuals with heart failure: a MERIT-HF sub-study. Eur J Heart Fail 5: 463–468. [DOI] [PubMed] [Google Scholar]
- 23. Sehnert AJ, Daniels SE, Elashoff M, Wingrove JA, Burrow CR, et al. (2008) Lack of association between beta adrenergic receptor genotype and survival in heart failure patients treated with carvedilol or metoprolol. J Am Coll Cardiol 52: 644–651. [DOI] [PubMed] [Google Scholar]