Abstract
Background
Reliable outcome measures are essential for preclinical modeling of spinal cord injury (SCI) in primates. Measures need to be sensitive to both increases and decreases in function in order to demonstrate potential positive or negative effects of therapeutics.
Objectives
To develop behavioral tests and analyses to assess recovery of function after SCI in the nonhuman primate.
Methods
In all, 24 male rhesus macaques were subjected to complete C7 lateral hemisection. The authors scored recovery of function in an open field and during hand tasks in a restraining chair. In addition, EMG analyses were performed in the open field, during hand tasks, and while animals walked on a treadmill. Both control and treated monkeys that received candidate therapeutics were included in this report to determine whether the behavioral assays were capable of detecting changes in function over a wide range of outcomes.
Results
The behavioral assays are shown to be sensitive to detecting a wide range of motor functional outcomes after cervical hemisection in the nonhuman primate. Population curves on recovery of function were similar across the different tasks; in general, the population recovers to about 50% of baseline performance on measures of forelimb function.
Conclusions
The behavioral outcome measures that the authors developed in this preclinical nonhuman primate model of SCI can detect a broad range of motor recovery. A set of behavioral assays is an essential component of a model that will be used to test efficacies of translational candidate therapies for SCI.
Keywords: nonhuman primate, spinal cord injury, recovery scale, hand function
Introduction
Behavioral recovery after spinal cord transection in primates has been the subject of many studies.1-4 Lesions of specific funiculi have been used to determine the role of ascending5-8 and descending tracts, especially the corticospinal tract (CST).9-15 Our objective was to develop a non-human primate model of spinal cord injury (SCI) that would permit assessment of candidate therapies for restoring both locomotor function and skilled hand use after SCI. Because the most common site of human SCI is the cervical spinal cord, and because sprouting or regeneration of axons over short distances beyond a cervical lesion could restore hand function, we chose to develop a model of C7 lateral hemisection in the rhesus monkey. This lesion model was used for initial studies of spontaneous plasticity of CST projections,16 growth factor administration,17 and locomotor function.16
Here, we report the development of a set of outcome measures for assessing motor function after C7 hemisection. Recovery after complete cervical hemisection was evaluated by assessing spontaneous behavior in an open field with opportunities for overground locomotion, climbing, foraging, and object manipulation. Additional chair-based tasks assessed forelimb reaching and grasping performance. We report that the measures we have developed capture a range of functional outcomes in monkeys subjected to C7 hemisections. Both increases and decreases in function are detected by these assays, providing a common “ruler” for evaluating the effects of experimental therapeutics. These data are added to a comprehensive database that also includes kinematic, physiological, and anatomic measures collected on the same animals to allow integrative statistical analysis of multiple facets of recovery.16
Methods
All procedures were approved by the Institutional Animal Care and Use Committee of the California National Primate Research Center (CNPRC) at the University of California, Davis. The experimental timeline is shown in Figure 1. Pooled data from 24 animals are included in this report: both treatment-control subjects with hemisections (n = 12) and those receiving experimental therapeutics (n = 12). The purpose of the present report is to communicate the development of outcome measures for this model of C7 hemisection and to determine whether the measures detect a broad range of possible outcomes. No attempt to communicate the efficacy of experimental therapeutics is made because this is the subject of continuing study. Here, we report the full range of outcomes possible without reference to treatment conditions, where experimenters were blinded to group inclusions for data acquisition and analysis.
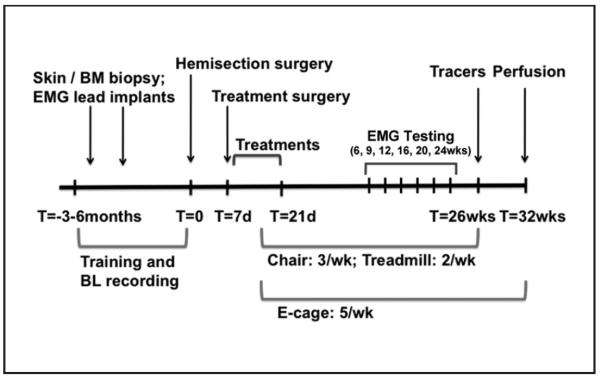
Figure 1.
Timeline: T = 0 indicates the time of spinal cord hemisection. In a period of 3 to 6 months before spinal cord hemisection, animals underwent 2 preparatory surgeries: a skin or bone marrow (BM) biopsy and implantation of EMG leads. In addition, animals were trained to perform chair, treadmill, and open-field tasks. Training and collection of baseline (BL) data on these tasks occurred up until the week of spinal cord hemisection. Then, 7 days after spinal cord hemisection, animals underwent a treatment surgery followed by 14 days of treatment delivery in the hospital and home cage. Behavioral testing in the chair (3 times weekly), treadmill (twice weekly), and open field (5 times weekly) resumed once animals were ready (during week 2 after spinal cord hemisection) and continued until week 25 (chair and treadmill) and week 32 (open field). EMG and 3D video recording sessions occurred before the lesion and at weeks 6, 9, 12, 16, 20, and 24. Animals underwent surgery 6 weeks prior to perfusion for delivery of tracers into the motor cortex, brainstem, and spinal cord.
Animal Subjects
Adult male rhesus monkeys (n = 24; mean age, 8 years; range, 6-10 years) were housed individually in cages (32 in. high, 34 in. wide, and 27 in. deep) with other animals in view. All animals were born at the CNPRC, and most (16/24) were reared outside in family groups. A mirror attached to the outside of the cage allowed animals to view activity in most of the room. A 12-hour day/night cycle was maintained, water was freely available, and food was provided according to CNPRC guidelines. Enrichment (mirrors, forage boards, chew toys, and radio) was provided daily. In addition, animals received puzzle toys and food reinforcements (produce items and treats) 5 times/wk. Testing occurred in the housing room.
Behavioral Testing Methods
Five months before hemisection, general adaptation and training to perform tasks in a restraint chair, to enter/exit a transfer box, to walk on a treadmill, and to enter/exit an open field—in this case a large exercise cage (size 5 × 7 × 10 ft3)—commenced. Animals were tested in the restraint chair 3 times/wk, on the treadmill 2 times/wk, and in the open field 5 times/wk for 30 min/session.
Open-field behavioral testing and scoring
A total of 20 animals were scored in the open field. The main entrance opened onto the lowest of 4 end-to-end perches (38.5 in. × 9 in. at 15, 23, 31, and 39 in. above ground level) that ascended along the back and side of the cage.
A baited object requiring bimanual manipulation (eg, a hollow cone or ball loaded with raisins, nuts, or small carrots) was placed on the highest perch before each session, providing motivation to first run to perch 4 to retrieve the food when sessions began. Then, 5 food cups on the front of the cage (heights, 18.5, 32.5, 42.5, 51.5, and 56.2 in.; distance between cups, 27 in.) were baited. Animals retrieved food from these cups by standing on the ground for cups 1 and 2 and climbing to reach cups 3 to 5. During video recordings, animals were presented with an orange or apple. The duration of observation was 12 to 15 minutes. Baseline data were collected during 2 sessions that took place within 2 weeks before SCI. Weekly assessments of recovery were made live and from videos. Some sessions had simultaneous telemetric EMG recordings synchronized to the video.
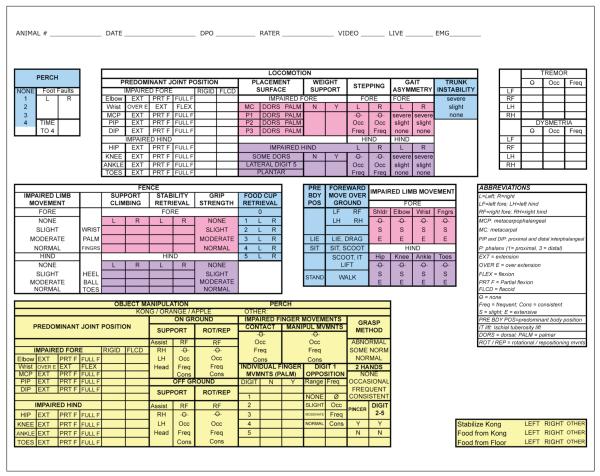
Behavioral observations recorded on a data sheet (Figure 2) included features of general movement, overground locomotion, climbing, perch use, and object/food manipulation. These data were used to develop a novel scale to describe recovery of function during spontaneous behavior in the open field. The initial analysis presented here (Table 1) assigned points for functional benchmarks; for example, right forelimb use for object support was rated as none, occasional (<50%), or frequent (>50%; 0, 1, and 2 points, respectively). A total of 88 points were assigned based on recovery of locomotion (67 points) and hand function during object manipulation (21 points). Locomotor scores consisted of general, hind limb, and forelimb subscores.
Figure 2.
Open field data collection form: This is the data form used to record function in the open field. Data were used to generate an overall total recovery score (Figures 3 and 4). This total score comprised a locomotion and object manipulation subscore (yellow). The locomotion score was generated from a general subscore (blue), a forelimb subscore (pink), and a hind limb subscore (purple).
Table 1.
Open-Field Scoring (Exercise Cage Scoring)
| Category | Subscore | Maximum Score | Score Composition |
|---|---|---|---|
| Locomotion | 67 | Overground locomotion and climbing | |
| General | (17) | Method of forward movement (dragging-walking), number of limbs used (2, 3, 4), number of perches reached (0-4), number of cups reached (0-5), presence of truncal instability (see also pale blue area in Figure 2) |
|
| Hind limb | (21) | Extent of movements made by hip, knee, ankle, and digits; presence of weight support; presence of stepping; ability and extent of use of the hind limb for climbing and support at the fence (see also pale purple area in Figure 2) |
|
| Forelimb | (28) | Extent of movements made by shoulder, elbow, wrist, and digits; presence of weight support, presence of stepping, ability and extent of use of the forelimb for climbing and support at the fence (see also pale pink area in Figure 2) |
|
| Object manipulation | 21 | Posture of the animal during object manipulation, use of the impaired hand for support and movement of the object, grasping method used, extent of wrist and digit movements for object manipulation (see also pale yellow area in Figure 2) |
|
| Total | 88 |
Chair tasks
A total of 20 monkeys were trained to perform 4 tasks with their right forelimb while seated in a standard primate-restraining chair. Each trial began with the limb in a trained starting position when a clear plexiglass partition was raised; the trial ended after 15 s if there was no response. The intertrial interval averaged 10 s. Detailed response data were recorded for each chairing session preinjury, and 2 to 25 weeks postoperatively. A baseline criterion of 80% was set for food transfer to the mouth for platform, stick, and drawer tasks and complete pull for the handle task. The best performance was recorded for each trial (10 trials/task/d). Response categories are shown in Table 2 for the chair tasks. Baseline values were binned across the 2 weeks prior to spinal cord hemisection and reported as a single value (±standard error of the mean [SEM]). The following tasks were assessed:
Platform task: As shown in Figure 5 and described in Rosenzweig et al,16 animals were required to retrieve consistently sized food objects from a platform (5 in. × 10 in.) with their impaired forelimb. A total of 20 trials per session were presented, 10 with small (raisins, half peanuts, etc) and 10 with medium-sized objects (grape, apple pieces, etc). Response approximations and latency to retrieve food were recorded. During EMG recordings, the platform (6 in. × 6 in. or 3.75 in. × 3.75 in.) was attached to a force transducer.
Stick: As shown in Figure 5 and described in Jindrich et al,18 animals were required to retrieve a food item (usually a grape) from a vertical post in the center of a platform. Unimpaired animals used a pincer grasp for this task. On half the trials, a funnel (base diameter, 3 in.; height, 3.25 in.) was placed over the bottom of the stick to promote pincer retrieval and to prevent alternate forms of grasping. A total of 10 trials per session were presented (5 with and 5 without the funnel). During EMG recordings, the post was attached to a force transducer.
Handle: As shown in Figure 5, animals were required to pull a round handlebar (red; 4.5 in. long × 2.5 in. wide × 0.25 in. diameter) attached to a plastic guide equipped with springs of different tension. The handle was presented vertically or horizontally (5 trials per position). Monkeys pulled the handle so the spring would be stretched approximately 7 cm to receive a reward. Successively greater spring tensions were used as recovery progressed (20 N, easy; 60 N, medium; 98 N, hard; and 107 N, extra hard). A force transducer measured pulling force during EMG recordings.
Drawer pull: Animals were required to grasp a small handle (ideally using the thumb and index digit) to open a drawer containing a sugar pellet located in a small trough. This task required the most dexterous use of the impaired hand.
Table 2.
Response Categories for the Hand Tasks in the Restraining Chair
| Platform | No attempt | Reach | Touch platform | Touch food | Grab food | Transfer to left hand | Transfer to mouth |
| Stick | No attempt | Reach | Touch platform | Touch food | Grab food | Transfer to left hand | Transfer to mouth |
| Handle | No attempt | Reach | Touch | Partial pull | Partial hold | Pull and hold |
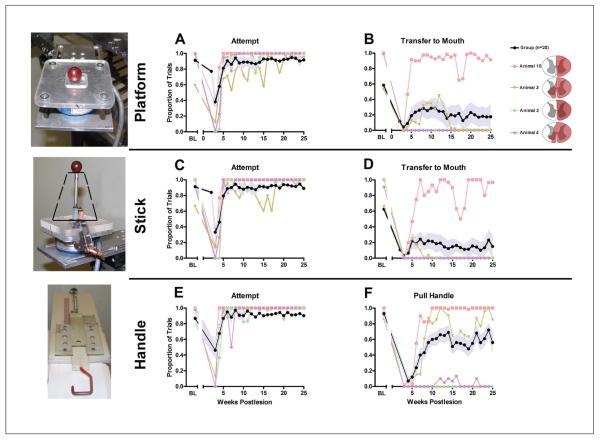
Figure 5.
Recovery of function over time on skilled forelimb tasks (n = 20): performance on tasks of skilled forelimb use while seated in a restraining chair is shown on 3 tasks: object retrieval from a flat platform (upper panel), grape retrieval from a vertical stick (middle panel, funnel position indicated with black dashed line), and a handle-pull task (lower panel), with an example of each task apparatus shown at the left. A, C, E: The proportion of trials on which the animals attempted food retrieval (Attempt) on each chair-based task, whether successful or not. In the 4-week period after hemisection, few animals attempted to perform the task. Rapid recovery in ability to initiate movement is observed by week 5. B, D, F: Successful completions of platform task (food retrieval and placement in mouth). B. Stick task (food retrieval from vertical stick and placement in mouth). D. Handle task (pull handle). F. Group means ± standard error of the mean are shown in black and pale blue, respectively, and 4 individual recovery curves in color (individual lesion reconstructions are shown on the right). Three of the 4 selected individuals are the same for Figures 3, 4, and 5. A range of initial deficits are evident, with some followed by substantial recovery (eg, animals 3 and 10). Abbreviation: BL, baseline.
Spasticity assessment
At the beginning of each postoperative chairing session, the affected forelimb was gently massaged, joints were moved through the full range of motion, and spasticity was rated using the Modified Ashworth Scale19 (Table 3). This test was introduced at a later stage of the experiments, so 16 of the 24 animals were rated.
Table 3.
Modified Ashworth Scale19
| 0 | No increase in muscle tone |
| 1 | Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension |
| 2 | Slight increase in muscle tone, manifested by a catch followed by minimal resistance throughout the remainder (less than half) of the range of motion |
| 3 | More marked increase in tone throughout most of the range of motion but affected part(s) easily moved |
| 4 | Considerable increase in muscle tone, and passive movement is difficult |
| 5 | Affected part(s) rigid in flexion or extension |
Treadmill task
Animals were trained to walk quadrupedally on a motor-driven treadmill at speeds of 0.45, 0.89, 1.34, and 1.79 m/s as described previously.16,20 Postoperatively, treadmill speeds were appropriate to the level of recovery, not exceeding 1.79 m/s.
EMG and video recordings
Three-dimensional videos and EMG recordings were made during chair and treadmill testing (baseline, 6, 9, 12, 16, 20, and 24 weeks) after SCI as previously described.18,20,21 In some animals, EMG was also recorded and synchronized to video recordings in the open field. Most of these physiological data are the subject of ongoing studies and will be presented in a separate communication.
Other Procedures
Surgeries
Animals underwent skin and bone marrow biopsies, EMG lead implantation, spinal cord hemisection, delayed treatment surgery, and anatomic tracer surgery, all performed aseptically. Anesthesia was induced with ketamine (1 mg/kg) intramuscularly and maintained with 1.5% to 2.5% isofluorane (vol/vol). During surgery, body temperature, heart rate, respiration rate, blood gases, and indirect blood pressure were closely monitored and maintained within acceptable ranges. Analgesics (oxymorphone: 0.15 mg/kg, thrice a day for 3 days) and antibiotics (cephazolin: 25 mg/kg twice a day for 7 days) were administered. Pre-SCI, biopsies were collected as part of a cellular transplant treatment strategy, and bipolar intramuscular EMG electrodes (Konigsberg Instruments, Pasadena, California) were implanted into selected muscles for telemetric recording.18,20,21 A complete lateral hemisection at the C7 spinal cord level was created16,17 by intradural section by 1 surgeon (MHT) after C5 caudal and C6 total laminectomy. Then, 1 week postlesion, animals underwent treatment surgery.17 After reexposure, the lesion was debrided, and cellular transplants were placed in the lesion followed by intraparenchymal injections of treatments (n = 12) or vehicle (n = 12) aimed at enhancing axonal growth and connections below the lesion. In addition, animals received subcutaneous injections of rolipram or vehicle for 14 days beginning the day after the treatment surgery. Treatment effects are the subject of ongoing analysis and will be presented in a separate communication. Here, data for all animals are pooled to demonstrate the range of possible outcomes after hemisection and to show that the tests we use are capable of encompassing the full range of recovery, regardless of treatment condition. We used anatomic tracers to label the corticospinal, reticulospinal, and propriospinal systems 25 weeks posthemisection.16,17,22 After 6 weeks, animals were deeply anesthetized and transcardially perfused with 4% paraformaldehyde.
Postoperative care
After release from the hospital, animals were returned to their home cage, equipped with fleece pads and porous rubber mats to prevent pressure sores. Monkeys were attended every 2 to 4 hours during the day and encouraged, with preferred food items, to sit up, reach, and stand. Skin lesions were treated, and if necessary, limbs were bandaged. Some animals received gabapentin (15-60 mg/kg/d) and/or haloperidol (0.01-0.05 mg/kg/d) to prevent further skin damage. Medical records included observational information on home cage mobility. As soon as animals were able to enter the transfer box, they reentered the open field; movement was encouraged by baiting different parts of the enclosure. Chairing and treadmill training resumed at a similar time.
Anatomic methods
Rostrocaudal lesion location was determined by the dorsal root entry zones. An 8-mm block centered on the lesion was taken, cryoprotected in 20% glycerin, frozen, and sectioned horizontally at 30 μm. Every 12th section was stained for Nissl, and the lesion extent was reconstructed. Reorganization of cortical and brainstem projections to the spinal cord will be described in a separate communication; initial reports have been published.16
Statistical procedures
Recovery curves for open-field data (Figures 3 and 4) and the Modified Ashworth Scale (Figure 6) reflect weekly group means ± SEM at each time point. For chair data (Figure 5), group means reflect proportion of successful trials in a day, averaged across days to yield weekly means. Graphs were generated using GraphPad Prism v.5.04.
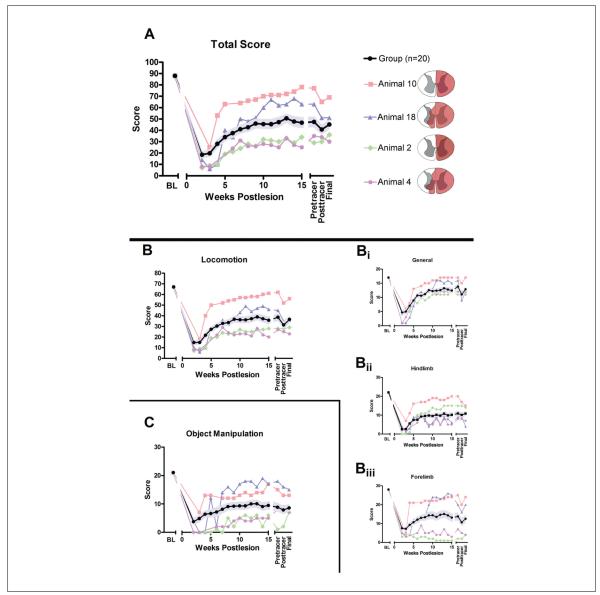
Figure 3.
Open-field analysis (n = 20): A. The overall total score for behavior assessed in the open field over time (maximum score = 88) for a group of 20 animals. The group mean ± standard error of the mean (SEM) is shown in black and light blue, respectively, and 4 representative individual subject recovery curves are also shown; schematic lesion reconstructions for these subjects are illustrated on the right. Immediately after hemisection, function is substantially reduced but shows recovery over time, although significant deficits remain. The total score comprises the locomotion score (B; maximum = 67) and the object manipulation score (C; maximum = 21). Similar to the total score, after hemisection, specific aspects of locomotor function are lost but show varying degrees of recovery (B). The subdivisions of the locomotion score (B), including a general subscore (Bi; maximum = 17), a hind limb subscore (Bii; maximum = 22), and a forelimb subscore (Biii; maximum = 28), all show loss of function followed by recovery similar to the overall locomotion and total scores. Group recovery on the general scale (Bi) was approximately 70% of baseline. This measured overall ability to independently move through the cage and retrieve food items. Specific methods, for example, ipsilateral hand participation in these maneuvers, are not taken into account in this subscore, and performance on this subcomponent showed that most animals recovered their ability to perform the tasks in the open field fairly well. Hind limb (Bii) and forelimb (Biii) functions recover to approximately 50% and 45% of baseline, respectively. On all graphs, the group mean ± SEM (n = 20) is shown in black and pale blue, respectively, and 4 individual recovery curves selected to illustrate the full range are also shown (as are their lesion reconstructions on the right). The 4 selected individuals are the same for Figures 3 and 4 and were selected to illustrate the range of recoveries. Abbreviation: BL, baseline.
Figure 4.
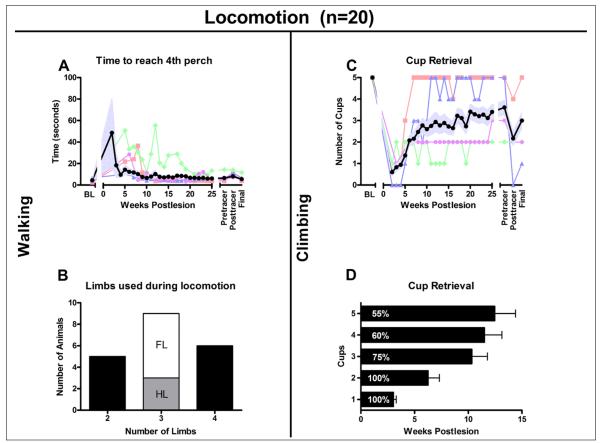
Recovery of walking (A, B) and climbing (C, D), n = 20. A. Time required to reach the fourth perch after entering the open field. Immediately after surgery, the time to fourth perch was prolonged, but animals recovered rapidly to achieve times that were just slightly longer than at baseline. Animals used 2, 3, or all 4 limbs for locomotion. B. The number of animals that use 2, 3 (white: using right forelimb; gray: using right hind limb), or all 4 limbs for walking. C and D, food retrieval from the cups. C. Mean number of cups emptied. D. Percentage of animals able to reach cups 1 to 5 and the average time ± standard error of the mean (SEM) they took to accomplish this. In A and C the group mean ± SEM (n = 20) is shown in black and pale blue, and 4 individual recovery curves are also shown. The 4 selected individuals are the same for Figures 3 and 4 and were selected to illustrate the range of recoveries. In D, the group mean ± SEM is shown. Abbreviation: BL, baseline.
Figure 6.
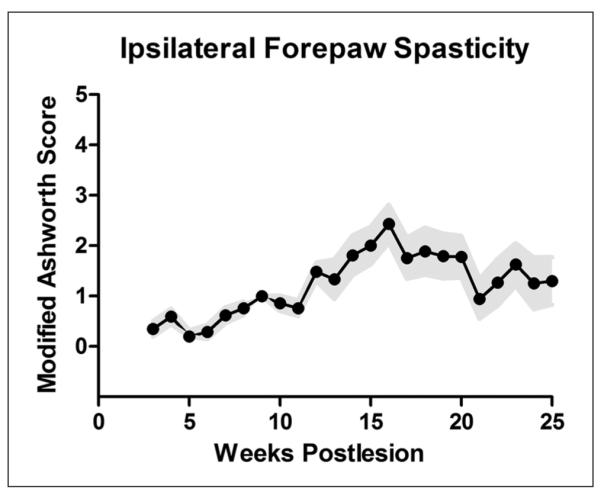
Spasticity assessment on the Ashworth Scale (n = 16): assessment of rigidity of the right forelimb using the Ashworth Scale. An increase in hand/arm spasticity was observed in most animals during the course of recovery. Wide variability was present, with some animals developing little increase in spasticity and occasional animals developing spastic distal forelimbs. Group means ± standard error of the mean are shown in black and pale blue, respectively.
Results
Animals performed the behavioral tasks to baseline criteria and survived spinal hemisection and treatment surgeries. By 4 days posthemisection, 90% were able to sit unassisted, and by 1 week, 65% were sitting more than 50% of the time. Most animals entered the open field, the chair, and treadmill by week 2 after treatment surgery. Also, 2 animals were prematurely killed in a humane manner (at 11 and 22 weeks) on the advice of veterinary staff as a result of non-healing skin lesions.
Spontaneous Behavior in the Open Field
Overall recovery scores and subscores showed gradual functional return starting from 2 weeks (Figure 3). The greatest rate of change was between 3 and 8 weeks. A range of individual initial deficits were evident, some followed by substantial recovery (eg, animals 10 and 18). The group (black line) recovered to approximately 50% of pre-SCI baseline performance.
During 2 to 8 weeks posthemisection, the animals rapidly recovered, sitting, standing, and ambulating on the ground and perches, and climbing. Figure 4A shows the time to reach the fourth perch on entering the open field. Before hemisection, animals immediately went to this highest perch to receive baited objects. After SCI, the time to fourth perch was longer but recovered quickly to near baseline values. However, limb recruitment patterns varied widely.
By 2 weeks, half the animals were able to sit and scoot overground using their unimpaired limbs plus an ipsilesional limb (7/20); some had weight support in an ipsilesional limb and could walk (3/20). Over the next 2 to 4 weeks, all were able to scoot or walk. Further recovery involved regaining trunk stability, increasing strength, and reestablishing weight-supported stepping ipsilesionally. Most animals ultimately used either 3 (45%) or 4 (30%) limbs for overground locomotion, but 25% continued to use only the 2 unaffected limbs (Figure 4B). Climbing recovered in 60% of the animals, with 55% able to retrieve from the highest cups (Figures 4C and 4D).
After SCI, the ipsilesional hind limb initially exhibited flaccid paralysis with the limb in extension, followed by gradual return of hip flexion (100%) and knee and ankle flexion in most animals. Some recovered hind limb weight support (Figure 4B) and showed limb use during locomotion (35%), plantar stepping, and rare dorsal stepping. Gait asymmetry decreased over time but deficits typically remained visible. Abnormal placement of digit 5 (D5) was consistently observed following SCI; D5 was placed adjacent to D4 with no spread visible (1-2 cm spread is normal) or slightly underneath D4, even in animals with good locomotor recovery. Two animals shown in Figure 3B (Bii) with good hind limb recovery walked on 4 (animal 10) and 3 (animal 2) limbs.
Post-SCI, some animals showed little use of the right forelimb (eg, animal 2, Figure 3B [Biii]). Most, however, rapidly recovered extensive shoulder and elbow use, and 60% developed limb use for weight support and stepping (Figure 3B). Dorsal digit placement, not uncommon early, disappeared in animals regaining palmar stepping; a few of these later developed excessive digit flexion, and dorsal placement returned. Animals not regaining weight support either (1) developed complete nonuse or (2) suspended the limb during locomotion but still used the limb (typically poorly) for object support during manipulation. All animals regaining good use of their impaired hands for object manipulation also used that hand for weight-supported stepping (n = 12/20).
Post-SCI, all animals recovered the ability to stand over 2 to 4 weeks, and most (60%) regained the ability to climb, with recovery continuing to 12 weeks (Figures 3C and 3D). Also, 5 animals used 4 limbs to climb, and 7 animals used 3 limbs; 6 animals did not use their right hind limb, and only 1 animal was able to climb without using the ipsilesional right forelimb. To retrieve food from cups 4 to 5, 1 hand needed to be freed from the fence. In most cases, the unimpaired hand was used for retrieval, and the ipsilesional forelimb was used for support by hooking it through the fence, either at the wrist or with the fingers (as at baseline). Only 2 subjects extracted food from the cups with the impaired hand; both had incomplete hemisections (Figure 7A: animals 19 and 20).
Figure 7.
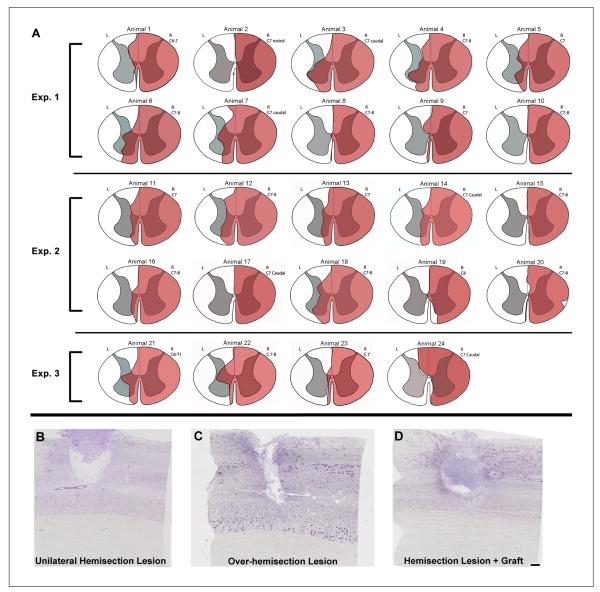
Lesions produced by hemisection (n = 24): A. Reconstructions of the lesion site for all animals described in this communication; red indicates lesioned area. In 4 animals, small areas of the right hemicord were spared. In 5 animals, there was a complete lesion of the right hemicord with no obvious damage to the left hemicord. The remaining 15 animals had complete lesions with varying degrees of left hemicord damage. B, C, and D. Nissl-stained horizontal sections through the lesion for 3 selected subjects. The lesions depicted include a complete hemisection that extended to the midline (B), an overhemisection that extended over the midline into the contralateral side (C), and a hemisection lesion with a treatment graft in the lesion site (D). In animals receiving a cellular transplant (D), the lesion site was generally completely filled with cells, likely a combination of transplanted and endogenous cells. Dashed line indicates midline in B, C, and D. Scale bar = 500 μm.
Object manipulation also showed a gradual recovery (Figure 3C). Prelesion, both hands manipulated large objects (eg, baited objects, apple, and orange), supporting, rotating, and repositioning using mostly wrist and digit movements. The space between individual digits was based partly on object size and shape. D1 moved independently and frequently opposed the other digits with a large spread between D1 and D2 to D5 (Figure 8A).
Figure 8.
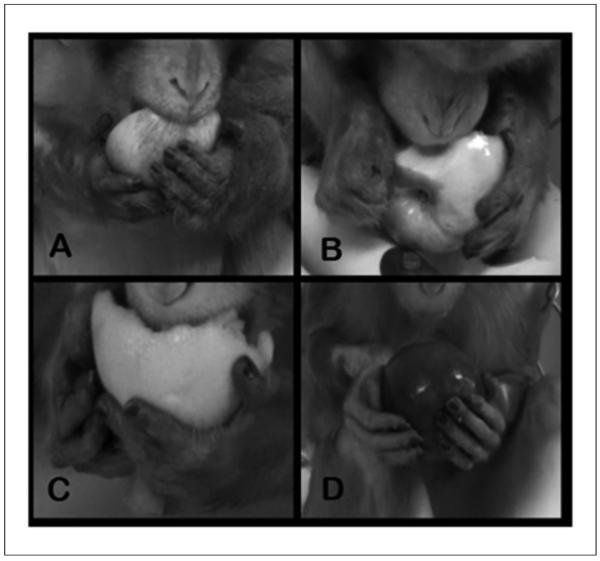
Range of recovery of hand function for object manipulation: Animals eating an orange or apple are shown. A. An animal prior to hemisection (baseline); both hands are used to manipulate the apple. Palmar hand and digit contact is present as is a normal distance between digits. Wrist and digital movements (not visible in this still image) are present. B. An example of poorly recovered hand function. The right hand is clubbed and the object is supported against the dorsal aspect of the digits. C. An animal using a slightly more normal grasping method to manipulate the orange; however, full palmar contact does not occur. D. An animal that regained a near-normal grasping method; note, however, that there is still no distance between digits 1 and 2. This lack of interdigital space and movement of digit 1 appear to be one of the last components of grasping to recover after the lesion. Also note the position of digit 1 on the normal left hand and digit 1 on the ipsilesional right hand in each picture.
Postlesion, distal forelimb position and use varied. Some animals regained little use of the wrist and digits, developing either wrist and finger joint extension or flexion (clubbed hand; Figure 8B). Few regained near-normal mobility and function (see individual recovery curves Figures 3B [Biii] and 3C). Poorly recovered animals had limited use of the ipsilesional hand (eg, Figure 8B), sometimes with shoulder and/or elbow rotational movements. Carpal and digit joints were immobile, and objects were contacted with the base of the palmar aspect of the hand plus the dorsal aspect of the digits. In animals with more normal use of the hand, there was more mobility in the wrist and digits, and they were able to partially extend the digits (Figure 8C), open the hand, and apply a more normal grasp (Figure 8D). Few animals (3/20) recovered the ability to use the ipsilesional hand for object manipulation with near-normal grasp and occasional normal interdigit distance. Only 1 animal (with an incomplete lesion) regained consistently normal D1 to D2 distance and the opposability of D1.
Recovery on Chair Tasks
Group mean recovery and selected individual recoveries are shown in Figures 5A and 6B for food retrieval on the platform task. A gradual recovery is evident with the sharpest rise in performance occurring at 4 to 5 weeks. On unsuccessful trials, responses were typically “touch-platform” or “touch-food” (see Table 1), indicating engagement in the task, but failure to reach the transfer-to-mouth criterion. The high proportion of “attempt” trials within the 15-s trial period is also consistent with engagement (Figure 5A). Prelesion, animals typically used a pincer grasp to pick up food, but after SCI, the hand was often used as a rake (trapping the food between either the fingers or the fingers and the palm).
Recovery on the stick task showed the same time course as the platform but a reduced success rate (Figures 5C and 5D) reflecting the greater dexterity required. Prelesion, D1 and D2 were used for pincer grip, but post-SCI, food was typically grasped between the fingers and palm, with the hand rotated vertically (thumb up), then lifting. Animals often included D3 and D4 to steady their grasp. This requires finger extension to accomplish. Criteria of “reach” and “touch-food” were common on unsuccessful trials (Table 3).
Recovery of handle pulling (Figures 5E and 5F) continued to improve over 15 weeks. Performance on this task was the best overall; data shown are collapsed over the 2 easiest spring tensions (20 N and 60 N). Almost no animals were able to pull the stiffer springs. This task required the least dexterous movement of the hand, yet some animals (eg, animal 2; Figure 5) still performed poorly. Individual curves also indicate that the most successful animal (animal 10) was able to perform all the hand tasks, had low spasticity, manipulated objects in the open field, and used his forelimb and hind limb for locomotion. Among the animals tested on the drawer task (n = 14), none were able to successfully perform the task post-SCI.
Spasticity, determined using the Modified Ashworth Scale (Table 2, Figure 6), was observed more frequently during months 3 to 6. Progressive development of contractures was seen in a few subjects even though the arm and hand were subjected to range-of-motion therapy prior to each chairing session.
Lesion Analysis
The lesion extended 2 to 3 mm rostrocaudally. Lesion reconstructions are shown in Figure 7A, and representative Nissl-stained sections through the lesion are shown in Figures 7B to 7D. Complete hemisections were accomplished in nearly all cases. Many lesions encroached slightly on the contralateral side of the spinal cord (Figures 7A and 7C), affecting the medial aspect of the dorsal and/or ventral funiculi. In 15 out of 24 animals, damage to the contralateral gray matter was observed as well (Figure 7A). The lesion level, indicated next to each reconstruction figure, was consistently in the C7 segment (n = 11) or at the C7/C8 junction (n = 10). One was at the C6/C7 junction, and 2 were at C8. One case with a lesion at C7/C8 (Figure 7A, animal 20) had sparing of a small region of the lateral funiculus and a small portion of the ventral aspect of the dorsal funiculus; this animal showed considerable recovery, and CST-tracing revealed sparing of CST axons distributing to the ipsilateral gray matter.
Discussion
Here, we summarize methods to test recovery in a primate model of SCI using complete C7 hemisections. Outcome measures assessed a variety of spontaneous behaviors in an open field and simple hand tasks in a primate chair. Our metrics were developed using a large sample (N = 24) spanning multiple experiments and treatment conditions to ensure that our outcome axes could detect both improvements and decrements in function after lesion. In both treated and untreated groups, animals spanned the range of outcomes measured here. Therefore, this variation in recovery of function after C7 hemisection is reported, regardless of treatment condition. Further rigorous statistical analyses are under way to determine whether treatments are significant contributors to these variations in behavioral recovery.
After C7 hemisection, motor impairments in the ipsilateral limbs were observed. Despite consistent lesions, a wide range of recovery was evident, from no or slight use to nearly normal use. Even animals showing poorer recovery were able to function effectively, ambulating, foraging, and consuming food. This overall functional recovery was reflected in the open-field behavioral scores, which provided a broad measure with improvement over time. Methods of task completion varied, but animals showed high motivation despite extensive SCIs. Recovery following less-extensive lesions, for example, subtotal lesions of the lateral funiculus, is more rapid and complete.3,10,11,14,23-25
Detailed aspects of recovery, such as limb use during overground locomotion, were particularly interesting. Some animals used only the contralateral unimpaired limbs for walking, others used the ipsilesional forelimb but not the hind limb, and vice versa, whereas some used all 4 limbs. This variation in overground locomotor patterns after cervical hemisection has not been previously reported for the primate.1,3 No clear relationship between lesion level and limb use during locomotion was evident. Similar to prior reports by others,1,2 we observed that recovery of hip flexion is the first component of hind limb function to be restored, followed by recovery of knee flexion; ankle flexion, however, was slow and incomplete.
Both forelimb and hind limb recovery ranged from poor to very good as measured by different subscores in the open field. Animals that manipulated objects well also had forelimb use during locomotion. Although early articles on recovery after cervical spinal hemisection describe animals with persistent deficits,1-3 most recent studies describe consistent forelimb recovery.10,11,23-29 The lesions in the older studies are extensive (either complete or overhemisections); those in recent studies primarily damage the lateral funiculus. In the present series, lesions were complete or overhemisections, and we attribute the persistent deficits in recovery to cutting all descending input from the forebrain, brainstem, and rostral cervical spinal cord on the side of the lesion. However, we did not observe a clear relationship between lesion size and function; animals with large lesions that damaged parts of the contralateral side had poor and good recovery, and animals with smaller lesions (or even some ipsilateral sparing) had poor and good recovery. Rostrocaudal lesion location was consistent; only 3 of 24 lesions were outside the C7 segment. One lesion was at the C6/C7 border, and that animal regained use of its hind limb but not the forelimb. Two others were in the C8 region; 1 of these animals recovered well (but also had some ipsilateral spared tissue), and 1 showed moderate recovery. This suggests that there are additional factors besides lesion size and rostrocaudal location that affect behavioral outcome. Continued investigations into the roles of plasticity and sprouting and other changes in the connectivity of forebrain- and brainstem-spinal and propriospinal networks are necessary and may further explain these findings.
Our scale for describing open-field recovery is similar to the numeric scales that are used to study activities of daily living and assess rehabilitation in humans: for example, the Functional Independence Measure (FIM) or the Spinal Cord Independence Measure (SCIM).30,31 The FIM, like our open-field scale, is an ordinal scale containing many subscores. The SCIM is a 100-point disability scale developed specifically for SCI with emphasis on activities associated with self-care, respiration, sphincter management, and mobility.31 These scales are recommended for clinical trial use in SCI.32 It seems appropriate to have similar outcome measures in the nonhuman primate28,29 to facilitate bench-to-bedside translation. Complementarily, we developed kinematic analyses of gait and fine-motor control performance16,18 and detailed multifactorial statistical analysis16 that provide a more refined evaluation of function. These time-consuming examinations define a practical framework to address mechanistic hypotheses regarding the relationships between functional recovery and anatomic changes.16
Detailed forelimb recovery was also tested using constrained tasks in the chair. The pattern of overall recovery was similar to that observed in the open field; mean recovery reached approximately 50% of baseline. Similar to the open-field behavior, some animals showed poor recovery post-SCI and were unable to perform the tasks to baseline criteria. Others showed remarkable recovery and were able to grasp small food items and transfer them to their mouth. Three of the animals that recovered exceptionally well were also able to use the impaired forelimb well for locomotion and object manipulation in the open field. These animals also used a pincer grasp to obtain the reward and transfer it to their mouth in the chair tasks. Animals showing intermediate recovery in the open-field and chair tasks did not recover digital dexterity or the ability to oppose their index finger and thumb. Studies using subtotal hemisections from C3/C4 to C7/C8 report good recovery of precision grip and thumb opposition for food retrieval using a variety of testing situations.10,11,14,23-26,33 Recovery of such dexterity was rarely observed in the present study, and the 3 animals that did recover exhibited high functioning early in the recovery period. The motor columns for the distal forelimb in the monkey are located mostly below C7.34 Thus, loss of rostral input to these regions would seem to be responsible for the lack of recovery of dexterity. However, 2 of 3 subjects that recovered well had either complete or overhemisections (Figure 7A, animals 12 and 18), whereas the third had slight sparing (animal 20). Thus, the role of inputs from the contralateral side of the cord must be taken into consideration, and robust sprouting to the denervated side could also play a role.16 An additional consideration for the recovery of hand function is damage to sensory inputs to the cord and to ascending sensory systems to the brainstem and thalamus. The hand and forearm sensory inputs distribute to C4 to C8, with the digits especially represented in the dorsal horn at the C5 to C7 cord levels.35 The lesions in the present study most frequently damaged the cord in the C7 region, mostly above the hand muscle motor pools but at the cord level where sensory information is processed. Indeed, monkeys with lesions isolated to the fasciculus cuneatus at C4/C5 or C5/C6 show disrupted finger control.6 Currently, we do not have clear measures of sensory and autonomic function, 2 important aspects of recovery for individuals with SCI.36 We will be adding these to further delimit the course of recovery in ongoing studies. Although sensory tests are difficult to establish in animals, monkeys have been successfully trained to report detailed sensory experience, including pain.37,38
Our approach in these preclinical studies is to measure as much as we can, behaviorally, electrophysiologically, and anatomically in the same animals so that we can test overall recovery (which is a combination of all these dimensions). We expect that simultaneously considering all these distinct parameters using a multivariate statistical approach should provide a better reflection of therapeutic efficacy. As the present article illustrates, our outcome measures are sensitive to the effects of injury and recovery over time, and our scales indicate that we have sufficient room for evaluating both improvements and losses of function. These aggregate data should contribute to our understanding of the range of functional outcomes possible after spinal lesions in higher primates and humans. Understanding the substrates of this recovery will potentially help us exploit residual connectivity and function to enhance recovery after SCI in humans.
Acknowledgments
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support by US National Institutes of Health (NS42291, NS049881, NS053059, NS067092, and NS069537); Veterans Administration; California Roman Reed Fund; Bernard and Anne Spitzer Charitable Trust; Dr Miriam and Sheldon G. Adelson Medical Research Foundation; and NYS CoRE CO19772.
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
References
- 1.Turner WA. On hemisection of the spinal cord. Brain. 1891;14:496–522. [Google Scholar]
- 2.Denny-Brown D. The Cerebral Control of Movement. Liverpool University Press; Liverpool, UK: 1966. [Google Scholar]
- 3.Mettler FA. Observations on the consequences of large, subtotal lesions of the simian spinal cord. J Comp Neurol. 1944;81:339–360. [Google Scholar]
- 4.Mettler FA, Liss H. Functional recovery in primates after large subtotal spinal cord lesions. J Neuropathol Exp Neurol. 1959;18:509–516. [Google Scholar]
- 5.Brinkman J, Bush BM, Porter R. Deficient influence of peripheral stimuli on precentral neurones in monkeys with dorsal column lesions. J Physiol. 1978;276:27–48. doi: 10.1113/jphysiol.1978.sp012218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glendinning DS, Vierck CJ, Jr, Cooper BY. The effect of fasciculus cuneatus lesions on finger positioning and long-latency reflexes in monkeys. Exp Brain Res. 1993;93:104–116. doi: 10.1007/BF00227785. [DOI] [PubMed] [Google Scholar]
- 7.Vierck CJ, Jr, Hamilton DM, Thornby JI. Pain reactivity of monkeys after lesions to the dorsal and lateral columns of the spinal cord. Exp Brain Res. 1971;13:140–158. doi: 10.1007/BF00234083. [DOI] [PubMed] [Google Scholar]
- 8.Vierck CJ, Jr, Luck MM. Loss and recovery of reactivity to noxious stimuli in monkeys with primary spinothalamic cordotomies, followed by secondary and tertiary lesions of other cord sectors. Brain. 1979;102:233–248. doi: 10.1093/brain/102.2.233. [DOI] [PubMed] [Google Scholar]
- 9.Tower S. Pyramidal lesions in the monkey. Brain. 1940;63:36–90. [Google Scholar]
- 10.Galea MP, Darian-Smith I. Manual dexterity and corticospinal connectivity following unilateral section of the cervical spinal cord in the macaque monkey. J Comp Neurol. 1997;381:307–319. [PubMed] [Google Scholar]
- 11.Galea MP, Darian-Smith I. Corticospinal projection patterns following unilateral section of the cervical spinal cord in the newborn and juvenile macaque monkey. J Comp Neurol. 1997;381:282–306. [PubMed] [Google Scholar]
- 12.Hepp R, Trouche E, Wiesendanger M. Effects of unilateral and bilateral pyramidotomy on a conditioned rapid precision grip in monkeys (Macaca fascicularis) Exp Brain Res. 1974;21:519–527. doi: 10.1007/BF00237170. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey: I. The effects of bilateral pyramidal lesions. Brain. 1968;91:1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki S, Isa T, Pettersson LG, et al. Dexterous finger movements in primate without monosynaptic corticomotoneuronal excitation. J Neurophysiol. 2004;92:3142–3147. doi: 10.1152/jn.00342.2004. [DOI] [PubMed] [Google Scholar]
- 15.Rouiller EM, Yu XH, Moret V, Tempini A, Wiesendanger M, Liang F. Dexterity in adult monkeys following early lesion of the motor cortical hand area: the role of cortex adjacent to the lesion. Eur J Neurosci. 1998;10:729–740. doi: 10.1046/j.1460-9568.1998.00075.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig ES, Courtine G, Jindrich DL, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brock JH, Rosenzweig ES, Blesch A, et al. Local and remote growth factor effects after primate spinal cord injury. J Neurosci. 2010;30:9728–9737. doi: 10.1523/JNEUROSCI.1924-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jindrich DL, Courtine G, Liu JJ, et al. Unconstrained three-dimensional reaching in rhesus monkeys. Exp Brain Res. 2011;209:35–50. doi: 10.1007/s00221-010-2514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 20.Courtine G, Roy RR, Hodgson J, et al. Kinematic EMG determinants in quadrupedal locomotion of a non-human primate (rhesus) J Neurophysiol. 2005;93:3127–3145. doi: 10.1152/jn.01073.2004. [DOI] [PubMed] [Google Scholar]
- 21.Courtine G, Roy RR, Raven J, et al. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta. Brain. 2005;128(pt 10):2338–2358. doi: 10.1093/brain/awh604. [DOI] [PubMed] [Google Scholar]
- 22.Rosenzweig ES, Brock JH, Culbertson MD, et al. Extensive spinal decussation and bilateral termination of cervical corticospinal projections in rhesus monkeys. J Comp Neurol. 2009;513:151–163. doi: 10.1002/cne.21940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura Y, Onoe H, Morichika Y, Perfiliev S, Tsukada H, Isa T. Time-dependent central compensatory mechanisms of finger dexterity after spinal cord injury. Science. 2007;318:1150–1155. doi: 10.1126/science.1147243. [DOI] [PubMed] [Google Scholar]
- 24.Schmidlin E, Wannier T, Bloch J, Rouiller EM. Progressive plastic changes in the hand representation of the primary motor cortex parallel incomplete recovery from a unilateral section of the corticospinal tract at cervical level in monkeys. Brain Res. 2004;1017:172–183. doi: 10.1016/j.brainres.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 25.Schmidlin E, Wannier T, Bloch J, Belhaj-Saif A, Wyss AF, Rouiller EM. Reduction of the hand representation in the ipsilateral primary motor cortex following unilateral section of the corticospinal tract at cervical level in monkeys. BMC Neurosci. 2005;6:56. doi: 10.1186/1471-2202-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishimura Y, Morichika Y, Isa T. A subcortical oscillatory network contributes to recovery of hand dexterity after spinal cord injury. Brain. 2009;132(pt 3):709–721. doi: 10.1093/brain/awn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babu RS, Namasivayam A. Recovery of bipedal locomotion in bonnet macaques after spinal cord injury: footprint analysis. Synapse. 2008;62:432–447. doi: 10.1002/syn.20513. [DOI] [PubMed] [Google Scholar]
- 28.Suresh Babu R, Muthusamy R, Namasivayam A. Behavioural assessment of functional recovery after spinal cord hemisection in the bonnet monkey (Macaca radiata) J Neurol Sci. 2000;178:136–152. doi: 10.1016/s0022-510x(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard CD, Slotkin JR, Yu D, et al. Establishing a model spinal cord injury in the African green monkey for the preclinical evaluation of biodegradable polymer scaffolds seeded with human neural stem cells. J Neurosci Methods. 2010;188:258–269. doi: 10.1016/j.jneumeth.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6–18. [PubMed] [Google Scholar]
- 31.Catz A, Itzkovich M, Tesio L, et al. A multicenter international study on the Spinal Cord Independence Measure, version III: Rasch psychometric validation. Spinal Cord. 2007;45:75–291. doi: 10.1038/sj.sc.3101960. [DOI] [PubMed] [Google Scholar]
- 32.Steeves JD, Lammertse D, Curt A, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 2007;45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 33.Freund P, Schmidlin E, Wannier T, et al. Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med. 2006;12:790–792. doi: 10.1038/nm1436. [DOI] [PubMed] [Google Scholar]
- 34.Jenny AB, Inukai J. Principles of motor organization of the monkey cervical spinal cord. J Neurosci. 1983;3:567–575. doi: 10.1523/JNEUROSCI.03-03-00567.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florence SL, Wall JT, Kaas JH. Somatotopic organization of inputs from the hand to the spinal gray and cuneate nucleus of monkeys with observations on the cuneate nucleus of humans. J Comp Neurol. 1989;286:48–70. doi: 10.1002/cne.902860104. [DOI] [PubMed] [Google Scholar]
- 36.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 37.Goldberger ME, Bregman BS, Vierck CJ, Jr, Brown M. Criteria for assessing recovery of function after spinal cord injury: behavioral methods. Exp Neurol. 1990;107:113–117. doi: 10.1016/0014-4886(90)90149-m. [DOI] [PubMed] [Google Scholar]
- 38.Vierck CJ, Jr, Greenspan JD, Ritz LA. Long-term changes in purposive and reflexive responses to nociceptive stimulation following anterolateral chordotomy. J Neurosci. 1990;10:2077–2095. doi: 10.1523/JNEUROSCI.10-07-02077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]