Abstract
Whether persons with dementia benefit from fall prevention exercise is unclear. Applying the Positive Emotion-Motivated Tai Chi protocol, preliminary findings concerning adherence and effects of a dyadic Tai Chi exercise program on persons with Alzheimer’s disease (AD) are reported. Using pre/ posttest design, 22 community-dwelling AD-caregiver dyads participated in the program. Fall-risk-relevant functional mobility was measured using Unipedal Stance Time (UST) and Timed Up and Go (TUG) tests. Results showed that 19/22 (86.4%) AD patients completed the 16-week program and final assessment; 16/19 dyads (84.2%) completed the prescribed home program as reported by caregivers. UST adjusted mean improved from 4.0 to 5.1 (Week 4, p < .05) and 5.6 (Week 16, p < .05); TUG improved from 13.2 to 11.6 (Week 4, p < .05) and 11.6 (Week 16, p > .05) post intervention. Retaining dementia patients in an exercise intervention remains challenging. The dyadic Tai Chi approach appears to succeed in keeping AD-caregiver dyads exercising and safe.
Keywords: dementia, Tai Chi, exercise, positive emotional motivators, fall risks
According to the Alzheimer’s Association, an estimated 5.4 million Americans suffer from Alzheimer’s disease (AD) or another dementia. The number of patients with AD in the United States is expected to triple by 2050. Falls are frequent among elderly and cause significant morbidity. Falls are particularly problematic for patients with AD. AD patients have a higher incidence rate of falls as well as more serious consequences of fractures and hospitalization (Buchner & Larson, 1987; Morris, Rubin, Morris, & Mandel, 1987; Oleske, Wilson, Bernard, Evans, & Terman, 1995).
Tai Chi is an ancient Chinese exercise grounded in the martial arts, which involves slow movements of the limbs and trunk, and gradually improves range of motion and standing balance. Due to the balance-training properties of Tai Chi, considerable evidence exists to suggest that Tai Chi is effective in preventing falls. Participants in a 15-week Tai Chi group training achieved a 47.5% (relative risk [RR] = .525, p = .01) reduction in risk of multiple falls and decreased fear of falling, as compared with an education-only control group (Wolf et al., 1996). Clinical practice guidelines have subsequently been adopted to suggest that Tai Chi is an effective form of fall prevention exercise (The American Geriatrics Society and the British Geriatrics Society Guideline for the Prevention of Falls in Older Persons, 2010), giving an “A” rating in the recommendation of Tai Chi intervention.
However, no recommendation was made for older persons with dementia living in the community or in long-term care facilities, due to insufficient evidence of available exercise programs for fall prevention in this population. Whether patients with dementia are able to benefit from a proven-effective exercise, such as Tai Chi, is a gap in research. Although a growing body of research suggests that program adaptations are critical when providing exercise interventions to patients with cognitive impairment (Logsdon, McCurry, Pike, & Teri, 2009; Teri et al., 1998; Yao, Giordani, & Alexander, 2008), appropriate techniques for program adaptations have been neither well studied nor accepted by researchers.
Purpose
The purpose of this study is to assess the acceptability of an adapted dyadic Tai Chi intervention, and its preliminary effect on fall risk-relevant functional mobility performance in patients with AD, estimating preliminary effect size for determining adequate sample size for a later randomized controlled trial (RCT). We hypothesize as follows:
Hypothesis 1: With program adaptation, Tai Chi is acceptable to participants with AD.
Hypothesis 2: AD participants will demonstrate improved fall-risk-relevant functional mobility performance after 4 weeks of group intervention and 12 weeks of home-based practice, compared with performance at baseline.
We also explored the relationship between baseline cognitive impairment and mobility outcomes in the AD patients.
Method
The dyadic Tai Chi intervention is an application of the Positive Emotion-Motivated Tai Chi (PEM-TC) protocol (Yao et al., 2008). It emphasizes enhanced communication techniques using positive emotional motivators (PEMs) and sensory assistance through a caregiver partner to enable a person with AD to participate in a Tai Chi exercise intervention. Pleasant Events Schedule (Logsdon & Teri, 1997) was used as a proxy for the PEMs. Caregivers were guided to document individually identified PEMs, and incorporate them into the Tai Chi practice, during the exercise itself (such as practice with a partner, being with family) and/or served as an incentive/reward before and afterward (such as having a snack, watching TV together). Verbal encouragement and cuing, relaxation music accompaniment, and sensory assistance from caregiver’s body movements through the Sticky Hands technique were also applied. “Sticky Hands” is a Tai Chi training technique practiced with a partner by maintaining physical contact (Patience T’ai Chi Association, 2011; Yao et al., 2008). AD patients and caregivers were taught to maintain hand contact with each other while practicing weight shifts (including one-legged stance) and reciprocal arm motions so that the AD patients could mirror the caregivers’ movements, with enhanced postural control (Yao et al., 2008). All exercise moves were practiced slowly and in a relaxed fashion to promote attention, engagement, and awareness of body alignment.
Design
The study uses a pretest/posttest design. Measurements were taken at baseline, at the end of the group training (end of Week 4) and the end of home practice (end of Week 16).
Sample
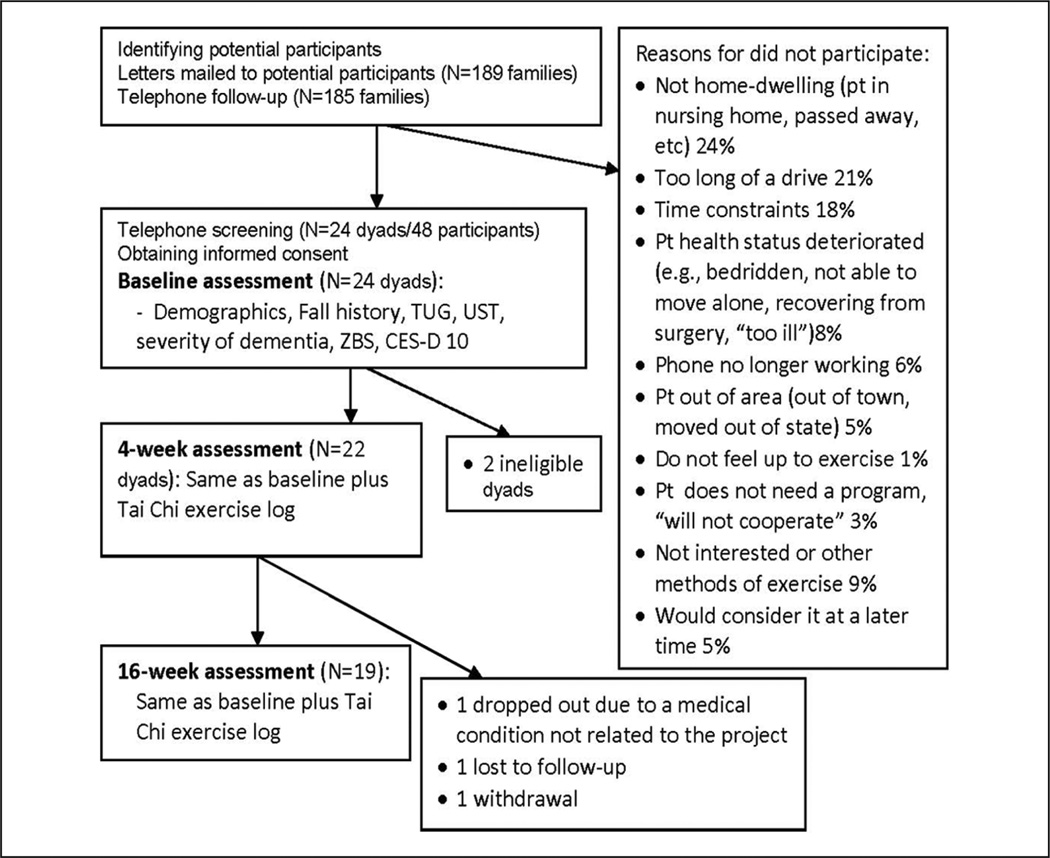
Community-dwelling AD-caregiver dyads were recruited from the Aging/Dementia Research Registry at the Michigan Alzheimer’s Disease Research Center (MADRC), a University-Affiliated Adult Day Care program and the Ann Arbor VA hospital. Recruitment criteria for AD patients included the following: probable and possible AD; age ≥60; Mini-Mental Status Examination (MMSE) ≤ 26 as was used by the MADRC (patients with MMSE > 24 were adjudicated as having AD by the MADRC based on behavioral presentation and diagnostic assessments); and ability to take at least one step without the assistance of a cane, frame, or another person. Patients who had other progressive medical conditions or contraindications to standing physical exercise (e.g., severe lumbar spine, hip, knee, or ankle arthritis) were excluded. Caregivers were family caregivers/friends who were actively involved with the patient (>10 hr/week), were willing to participate in a series of treatment sessions and facilitate the group and home exercise for the AD patient, and were free from cognitive impairments (see Figure 1 for recruitment information).
Figure 1.
Study design and participant recruitment
The study was approved by the University Institutional Review Board (IRBMed) and the IRB at the VA hospital. Potentially eligible AD participants, who had already agreed to be contacted for research opportunities and whose domiciles were within 1-hr driving distance from the university mobility research center, were identified by MADRC core/Adult Day Care/hospital staff and communicated to the research team. A letter and a brochure were then sent to the identified contact person for each AD patient. The letter described the study and informed the contact person that the research team would follow-up by telephone to discuss the project further. During the follow-up phone calls, caregiver and/or patient questions were answered. If volunteers remained interested, an appointment was scheduled to sign written consent forms and to complete a health screening at the mobility center. Consent forms were signed by the AD patient and the participating family caregiver and, if different, the person with medical power of attorney for the person with AD.
Measures
Fall-risk-relevant functional mobility performance
This was assessed using the Timed Up and Go test (TUG), a timed sequence of rising from a chair, walking 3 m, turning, and returning to the chair (Shumway-Cook, Brauer, & Woollacott, 2000). Participants were instructed to complete these sequenced movements quickly and as safely as possible. A score >14 s is a reliable and simple indicator for fall risk (Alexander & Goldberg, 2005; Shumway-Cook et al., 2000). Another robust test of balance and postural stability (van Iersel, Benraad, & Rikkert, 2007; van Iersel, Munneke, Esselink, Benraad, & Olde Rikkert, 2008; Vellas, Wayne, Garry, & Baumgartner, 1998) was the Unipedal Stance time (UST) where a participant was asked to stand on one leg for up to 30 s with eyes open and arms crossed over chest. Timing of standing balance was stopped when the participant moved his or her feet out of step, or grasped the handrail or other persons for support during the test, whichever came first. The inability to balance on one leg for 5 s or more without support is a risk factor for injurious falls (Vellas et al., 1998). Averaged TUG over three trials and the best UST of three trials (participants stood on one leg, followed by opposite leg, and then preferred leg), were taken as indicators for optimal functional mobility performance of the participant.
Severity of cognitive impairment
This was assessed at baseline by the Folstein MMSE scores. The MMSE is a quick way to evaluate cognitive function and is often used to screen for dementia or monitor its progression. It is scored on a 30-point scale. Severity of cognitive impairment is categorized as severe (<10 points), moderate (10–20 points), or mild (≥21 points; Folstein, Folstein, & McHugh, 1975; Mungas, 1991).
Procedure
As per our pilot program (Yao et al., 2008), a 2-stage dyadic Tai Chi exercise for AD patients were performed, which consisted of a 4-week Tai Chi training sessions with other dementia-caregiver pairs in a group of up to five dyads, and a 12-week follow-up home practice.
Main exercise movements included simplified Yang style Tai Chi-based moves adapted from a previous study conducted by the research team (Nnodim et al., 2006). Tai Chi demands body alignment and stance balance. Six forms that best exemplified these movements were extracted and further tailored to AD participants, which exemplify weight shifts in multiple directions, hip and ankle rotations, ankle and knee flexion, stepping motions forward, backward, and laterally, and one prolonged complete UST (Yao et al., 2008). All exercise groups began and ended with a 5 min warm-up and 5 min of cool down adapted Tai Chi movements, which emphasize breathing and relaxation.
Group training sessions were offered twice per week, with 1 to 3 business days between each session, to allow flexibility in scheduling and to promote attendance. The group sessions were led by an experienced exercise instructor who was accustomed to working with frail older adults, under the supervision of a certified Tai Chi instructor. Duration of each session was 60 min, which allowed questions, answers, and interaction between the instructor and participant dyads. After 2 weeks of group training, participants were asked to start an additional session each week at home to promote a smooth transition to the home exercise stage. During the home phase, caregivers facilitated patients practicing three 20-min Tai Chi session (plus warm-up and cooldown) each week for additional 12 weeks.
Caregivers played an important role in this intervention as they were the exercise partners and facilitators of the Tai Chi protocol for both group sessions and the 12-week follow-up home practice. Caregivers participated in the exercise with the AD patient and guided the patient while they practiced Tai Chi movements. To accomplish this, a cognitive approach was applied to interactions with caregivers. Caregivers were informed that patients’ agitation, apathy, and other behavioral problems were manifestations of their cognitive impairment. Reasoning and/or criticism is ineffective for patients with AD; however, rewards and encouragement work well as patients’ abilities to respond to positive emotion were well preserved. Caregivers were encouraged to optimize these spared emotional functions when interacting with the patient.
Because the primary goal of the study was to assess the acceptability of the dyadic Tai Chi, no incentives for participation or transportation for Tai Chi classes were provided. A small incentive was provided for completing assessments and submitting home exercise logs. Make-up sessions for short-term absence during the group stage (i.e., up to a maximum of three consecutive absences) were allowed for appropriate reasons (e.g., caregiver commitment, mild illness).
Regarding intervention adherence, the research investigator completed a group class log and recorded each class’s date, time, movements completed, and whether participants were responsive to the class. In the home practice stage, caregivers documented practice time, date, individual patient’s response and acceptance to the program, and other issues on the weekly follow-up/home Tai Chi exercise log. A weekly phone call and a monthly refresher group class were provided to monitor the progress of home practice and to promote the fidelity of participants’ exercise.
Analysis
Descriptive data analyses and program acceptability and effects were carried out by SAS® 9.2. Patient demographics/characteristics, and program attendance and completion data were described first and comparisons were made between the completion group and attrition or less-adherent groups. To evaluate effect size of the trial effect on the outcomes of TUG and UST at the end of Week 4 (or Week 16), the following formula was used: [mean score for measurement at baseline − mean score for measurement at Week 4 (or Week 16)] / pooled standard deviation. The pooled standard deviation was calculated using paired t test (Rosenthal, 1991).
To explore time effects adjusted by age and severity of cognitive impairment, linear mixed model with Autoregressive (1), that is, “(ar(1))” covariance structure analyses were conducted using PROC MIXED. Ar(1) accounted for the correlation of multiple observations within the same participant, and accounted for the stronger relationship of two next time measurements than those measured further part. Because TUG and UST data were skewed, log transformation was applied to carry out the analyses. Then the adjusted means and standard errors (SE) were transformed back using delta approximation method, which used Taylor series expansion of g(x) (the exponential of x) around X = μ to the first order E(Y) ≅ g(μ) and var(Y) ≅ [g’(μ)]2 ×Var(X).
Results
Participant Characteristics and Program Adherence
Twenty-two community-dwelling persons with probable or possible AD and their caregivers participated in the study. Participant characteristics are presented in Table 1. Participants were dominantly Caucasian (84.1%), with the rest being Asian (6.8%), Hispanic/Latino (4.5%), and ethnicity unreported (4.5%). Mean age of the participants was 80.6 ± 6.2 (range 63–90). Mean MMSE score was 17.9 ± 7.2 (range 0–25). Caregivers were spouses (n = 13), daughters (n = 8), and one paid caregiver (22 years old), with a mean age of 66.4 ± 15 years.
Table 1.
Sample Characteristics of Patients
| Demographic Characteristics | M ± SD Or % (n = 22) |
|---|---|
| Age | 80.6 ± 6.2 |
| 61 to 80 | 10 (45.5%) |
| 81+ | 12 (54.5%) |
| Gender | |
| Male | 14 (63.6%) |
| Female | 8 (36.3%) |
| Education (in years) | 14.6 ± 5.1 |
| BMI | |
| Normal/underweight | 7 (31.8%) |
| Over weight | 11 (50.0%) |
| Obesity | 4 (18.2%) |
| Baseline MMSE | 17.9 ± 7.2 |
| Mild (MMSE ≥ 21) | 12 (54.5%) |
| Moderate (MMSE 10–20) | 6 (27.3%) |
| Severe (MMSE < 10) | 4 (18.2%) |
| Baseline UST | 4.5 ± 5.3 |
| At no/low risk for falls (UST ≥ 5) | 7 (31.8%) |
| At high risk for falls (UST < 5) | 15 (68.2%) |
| Baseline TUG | 13.1 ± 5.8 |
| At no/low risk for falls (TUG ≤ 14) | 15 (68.2%) |
| At high risk for falls (TUG > 14) | 7 (31.8%) |
Note: BMI = body mass index; MMSE = Mini-Mental State Examination; UST = Unipedal Stance Time; TUG = Timed Up and Go test.
All participants (n = 22 dyads) attended 8 group sessions at the university mobility research center in full (60 min) and completed the assessment at the end of Week 4. A total of 19/22 patients (86.4%) and 18/22 (81.8%) caregivers completed the final assessment at the end of Week 16. Nineteen caregivers submitted their Tai Chi exercise logs. Participant attrition rate was 13.6%. All three dropouts occurred at the home practice stage. One participant stopped participation due to changes in a medical condition unrelated to the study, one caregiver withdrew due to change of mind, and the 3rd dyad did not return calls for final follow-up (see Figure 1). As reported in the Tai Chi exercise log filled out by participating caregivers, the shortest weekly home Tai Chi practice time was 0 min, whereas the longest was 185 min. The mean weekly practice time for the group was 73 ± 12 min (range 58–97). No obvious change in mean practice time was observed from Week 1 to Week 12. Mean Tai Chi practice sessions completed at home was 27 ± 8 (range 5–36), which was 2.3 session per week on average, over the entire 12 weeks of home practice for the group. No falls, injuries, or other adverse experience were observed or found throughout the study for participants. Because the home practice target is 3 times per week, 20 min minimum per session, for 12 weeks, total practicing time of 720 min (3 × 20 × 12 = 720) was used as a general criterion for home practice adherence. Using 720 min as the cut-off, 16 dyads (84%) were considered as adherent participants. Shortfalls in home practicing time were related to patient or caregiver illness, including a caregiver’s preexisting depression. No statistically significant differences were found in patient MMSE or in patient or caregiver demographics between the adherent (n = 16) and the less-adherent group (n = 3), or between the dropouts versus the rest of the participants. Patients in the less-adherent group had better baseline (ns, p > .05) but poorer post-intervention fall risk-relevant mobility performances, compared with those in the adherent group (ns, p > .05). Due to the pilot nature of the study, these three patients were included in further analyses for a more conservative estimate.
Trial Effect Size on TUG and UST Outcomes
Table 2 shows that a 2-s average improvement was observed in patients’ TUG performances (16% improvement from baseline). Effects size on TUG and UST were small (~0.2–0.4).
Table 2.
Mobility Performance at Baseline, 4 Weeks and 16 Weeks
| Effect Size | ||||
|---|---|---|---|---|
| Percentage | ||||
| n | M (SD) | a – b(c) / pooled SD |
||
| Change (%) | ||||
| TUG | ||||
| Baseline | 22 | 13.2 (5.8) | ||
| Week 4 | 22 | 11.4 (5.0) | 0.3 | 13.5 |
| Week 16 | 19 | 11.0 (4.5) | 0.4 | 16.1 |
| UST | ||||
| Baseline | 22 | 4.5 (5.3) | ||
| Week 4 | 22 | 5.7 (5.6) | 0.2 | 26.8 |
| Week 16 | 19 | 5.8 (5.8) | 0.2 | 27.4 |
Note: TUG = Timed Up and Go test; UST = Unipedal Stance Time.
a, b, c are mean scores for measurement at baseline, at Week 4, and at Week 16, respectively.
Age, Baseline Cognitive Function, and TUG and UST Outcomes
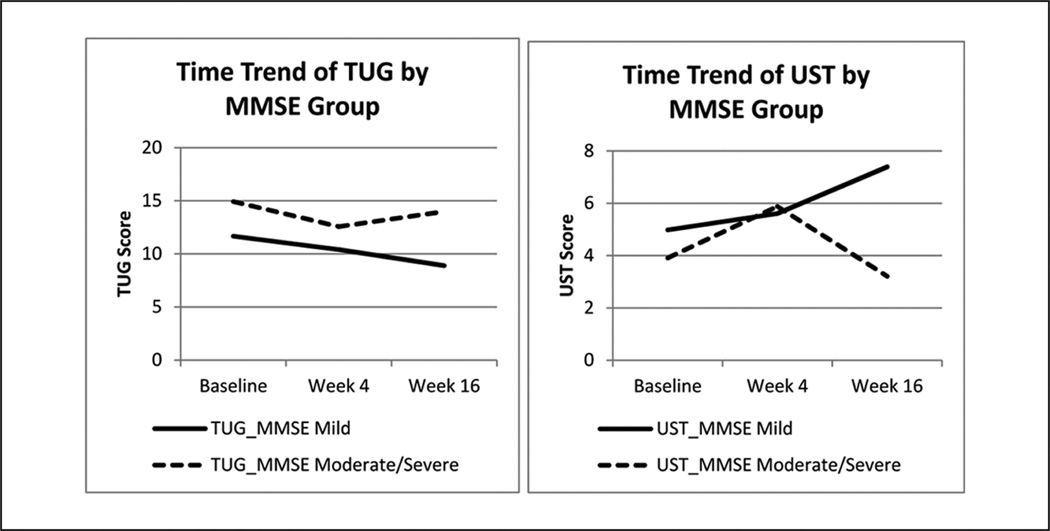
Age and cognitive status may be moderators to mobility performance. Line chart (see Figure 2) shows that baseline cognition may have affected post-intervention TUG and UST scores: Patients with mild cognitive impairment had larger improvement than those who were more impaired post-intervention, which agrees with results in Table 3. Overall, TUG and UST scores from more impaired patients improved after the group training (Week 4), but relapsed after the home practice period (Week 16).
Figure 2.
Time trend of TUG and UST means (unadjusted) by severity of cognitive impairment
Note: TUG = Timed Up and Go; UST = Unipedal Stance Time. MMSE mild n = 12; MMSE moderate/severe n = 10.
Table 3.
TUG and UST at Three Time Points Controlling for Age and Severity of Cognitive Impairment
| DV: TUG | DV: UST | |||
|---|---|---|---|---|
| Adjusted Means (SE) |
p Value | Adjusted Means (SE) |
p Value | |
| Age | ||||
| 61 to 80 | 10.6 (0.9) | <.05* | 6.1 (1.2) | >.05 |
| 80+ | 14.0 (1.1) | Ref | 3.8 (0.7) | Ref |
| Time | ||||
| Baseline | 13.2 (0.9) | Ref | 4.0 (0.6) | Ref |
| Week 4 | 11.6 (0.8) | <.05* | 5.1 (0.8) | <.05* |
| Week 16 | 11.6 (0.8) | >.05 | 5.6 (0.9) | <.05* |
| MMSE groups | ||||
| Mild | 10.2 (0.8) | <.01** | 5.9 (1.0) | >.05 |
| Moderate/severe | 14.4 (1.2) | Ref | 4.0 (0.8) | Ref |
Note: TUG = Timed Up and Go test; UST = Unipedal Stance Time; MMSE = Mini-Mental State Examination.
p < .05, two-tailed.
p < .01, two-tailed.
As is seen in Table 3, after controlling for age and severity of cognitive impairment, patients’ adjusted mean (SE) TUG performance improved from 13.2 (0.9) at baseline to 11.6 (0.8) at 4 weeks (p < .05), and to 11.6 (0.8) at 16 weeks (p = .055). Likewise, improvements in adjusted mean UST performance were also observed from 4.0 (0.6) at baseline to 5.1 (0.8; 4 weeks, p < .05) and 5.6 (0.9; 16 weeks, p < .05) post-intervention.
Discussion
These data showed that the majority of the participants, nearly half of which were moderately to severely impaired, completed the 2-stage 16-week program. As is shown in Figure 2, the time trend of TUG and UST indicates a promising improvement in fall-relevant mobility post-intervention. All participants attended the eight group classes and were actively practicing the Tai Chi movements during classes and at home with caregivers’ support. The participant dropout rate of 13.6% was relatively low, considering the reported dropout rate for physical activity trials (~8.3%–44.2%) in dementia (Forbes et al., 2008). No falls, injuries, or other adverse experience agrees with other studies on cognitively intact participants. These data indicate that Tai Chi is a safe form of exercise for frail older persons, improves TUG and UST, and thus likely reduces the risks for falling (Gatts & Woollacott, 2006; Li et al., 2005; Murphy & Singh, 2008).
Table 3 shows that mildly impaired patients have larger UST and TUG gains post-intervention compared with those who are moderately and severely affected. The severity of cognitive impairment likely affected the patients’ response during the home stage. Although no correlations between home Tai Chi practice time and patients’ severity of cognitive impairment were found (probably due to the small sample size), it is likely that caregivers needed additional support to help severely demented patients to engage in and adhere to the Tai Chi program at home. Three of our participating caregivers stated in the anonymous exit interview that additional group sessions would be more helpful. Group exercise and classes provide external stimulation and motivation to participants to engage in regular physical activities (Resnick & D’Adamo, 2011). Providing longer group practice time or more group sessions may thus be particularly useful for those with moderate and severe levels of cognitive impairment.
This study joins a growing body of research suggesting that exercise programs for patients with cognitive impairment and dementia need to be adapted and are more likely to be successful if they are individualized and enjoyable, and involve caregivers (McCurry et al., 2010; Teri, Logsdon, & McCurry, 2008). In the Resources and Activities for Life Long Independence (RALLI) program (Logsdon et al., 2009), behavioral and social learning theory was used as the guiding principle (Teri et al., 2008). Goal setting, self-monitoring, problem solving, and the provision of feedback and positive reinforcement were provided to make exercise more accessible to patients with mild cognitive impairment. For persons with more significant cognitive impairment, exercise training likely should include a family caregiver, friend, or staff person who can assist dementia patient with remembering how to perform the exercises safely and consistently (Teri et al., 2008). Caregivers are able to direct patients to follow scheduled exercise activities (Logsdon, McCurry, & Teri, 2005; Teri et al., 1998). Optimization of program adherence may thus depend not only on the patient but also on the caregiver (such as participation of the spouse vs. another caregiver) and the effect of the intervention on the caregiver (such as perception of caregiving burden; McCurry et al., 2010).
This dyadic Tai Chi program focuses on the use of PEMs and kinesthetic facilitation to reduce cognitive load/demand (Yao et al., 2008). It directly addresses patients’ motivation and attention deficit, which is considered necessary for older adults to positively respond to rehabilitation (Rentz, 1991). Emotion and attention have been considered to be theoretically related to one another because they both deal with information processing priorities (Compton, 2003). Research has shown that the emotional significance of stimuli is first evaluated pre-attentively, and then, only stimuli with emotional significance are prioritized to guide the selective attention mechanisms that operate within a limited-capacity system (Compton; Yao & Algase, 2008). In addition, emotional stimuli preferentially influence selective attention, where individual differences play an important role (Compton). Partnering the demented patients with their caregivers in the dyadic Tai Chi program with the use of patient-sensitive PEMs and kinesthetic facilitation thus provides a means to optimize attention and motivation.
Although TUG and UST are robust measures of fall risk, adaptations for mobility testing may be necessary in patients with dementia (Hauer & Oster, 2008). Timed motor testing requires that test participants are able to comprehend test commands, develop an adequate motor plan and have adequate motivation and attention. In addition, test performance depends heavily on the person’s willingness to test his or her utmost performance (Hauer & Oster, 2008). Strategies utilized in the present study included data collector training and using the same data collector for each participant. We also applied strategies to encourage the patient to complete the tests. Similar to other researchers (Brill, Drimmer, Morgan, & Gordon, 1995), a traffic cone was placed at end points, and a staff member or family caregiver walked alongside for safety and to encourage the demented person to cross over the end point. The participant was positioned next to a wall with handrails when UST was tested. Despite the efforts, intraindividual variability is present in UST and TUG results.
Limitations of the study include the small sample size, the relatively high education level of participants, and the highly motivated caregivers who volunteered, which may limit the generalizability of the results. In addition, the findings are limited due to lack of a control intervention. It is also possible that the intervention effect was due to type of caregiver interaction, regardless of the use of Tai Chi though specific effects of caregiver attention solely on fall-related outcomes is not likely. Future studies should consider other types of caregivers, for example, those who may have more limited contact with the patient, such as a relative or hired staff or companions, to evaluate whether the present study effects are solely associated with the type of the caregiver. Finally, the sample size was probably too small and follow-up time too short to meaningfully assess actual fall reduction.
In conclusion, exercise is a promising intervention for older adults with cognitive impairment. More research is needed to explain factors associated with exercise (Quindry, Yount, O’Bryant, & Rudisill, 2011; Resnick & D’Adamo, 2011), especially in older adults with dementia as our understanding of this subject remains inadequate. The absence of recommendations for demented patients in the 2010 AGS/BGS fall prevention guidelines underscores the inherent challenges to design, implement, engage, and/or retain dementia patients in an exercise program, and justifies the need to conduct further research in this population. A dyadic program can facilitate Tai Chi participation and adherence and result in functionally relevant improvement but needs further study, particularly under more rigorous randomized controlled conditions.
Acknowledgments
Funding
This study was supported by the Building Academic Geriatric Nursing Capacity Award program and NINR K01 NR011164 grants. We thank Eric Pear, Brad Grincewicz for their assistance with participant screening, data collection and testing, and Wendy Champoux, Debbie Strasburg for their assistance in the intervention protocol development. We especially thank Ayowale Oladeji for his service in Tai Chi training. The authors would like to acknowledge the support of National Institute on Aging (NIA) grant AG024824 (University of Michigan Claude D. Pepper Older Americans Independence Center), the Office of Research and Development, Medical Service and Rehabilitation Research, the Development Service of the Department of Veterans Affairs, the Dorothy and Herman Miller Fund for Mobility Research in Older Adults, and the Michigan Alzheimer’s Disease Research Center. Dr. Alexander is also a recipient of the K24 Mid-Career Investigator Award in Patient-Oriented Research AG109675 from NIA.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- The American Geriatrics Society and the British Geriatrics Society Guideline for the Prevention of Falls in Older Persons. Clinical practice guideline: Prevention of falls in older persons, summary of recommendations. 2010 Retrieved from http://www.americangeriatrics.org/health_care_professionals/clinical_practice/clinical_guidelines_recommendations/2010/ [Google Scholar]
- Alexander NB, Goldberg A. Gait disorders: Search for multiple causes. Cleveland Clinic Journal of Medicine. 2005;72:586. doi: 10.3949/ccjm.72.7.586. [DOI] [PubMed] [Google Scholar]
- Brill PA, Drimmer AM, Morgan LA, Gordon NF. The feasibility of conducting strength and flexibility programs for elderly nursing home residents with dementia. Gerontologist. 1995;35:263–266. doi: 10.1093/geront/35.2.263. [DOI] [PubMed] [Google Scholar]
- Buchner DM, Larson EB. Falls and fractures in patients with Alzheimer-type dementia. Journal of the American Medical Association. 1987;257:1492–1495. [PubMed] [Google Scholar]
- Compton RJ. The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behavioral and Cognitive Neuroscience Reviews. 2003;2:115–129. doi: 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forbes D, Forbes S, Morgan DG, Markle-Reid M, Wood J, Culum I. Physical activity programs for persons with dementia. Cochrane Database of Systematic Reviews. 2008;3 doi: 10.1002/14651858.CD006489.pub2. CD006489. [DOI] [PubMed] [Google Scholar]
- Gatts SK, Woollacott MH. Neural mechanisms underlying balance improvement with short term Tai Chi training. Aging Clinical and Experimental Research. 2006;18:7–19. doi: 10.1007/BF03324635. [DOI] [PubMed] [Google Scholar]
- Hauer K, Oster P. Measuring functional performance in persons with dementia. Journal of the American Geriatrics Society. 2008;56:949–950. doi: 10.1111/j.1532-5415.2008.01649.x. [DOI] [PubMed] [Google Scholar]
- Li F, Harmer P, Fisher KJ, McAuley E, Chaumeton N, Eckstrom E, Wilson NL. Tai Chi and fall reductions in older adults: A randomized controlled trial. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60:187–194. doi: 10.1093/gerona/60.2.187. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, McCurry SM, Pike KC, Teri L. Making physical activity accessible to older adults with memory loss: A feasibility study. Gerontologist. 2009;49:S94–S99. doi: 10.1093/geront/gnp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon RG, McCurry SM, Teri L. A home health care approach to exercise for persons with Alzheimer’s disease. Care Management Journals. 2005;6:90–97. doi: 10.1891/cmaj.6.2.90. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, Teri L. The Pleasant Events Schedule-AD: Psychometric properties and relationship to depression and cognition in Alzheimer’s disease patients. Gerontologist. 1997;37:40–45. doi: 10.1093/geront/37.1.40. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Pike KC, Logsdon RG, Vitiello MV, Larson EB, Teri L. Predictors of short- and long-term adherence to a daily walking program in persons with Alzheimer’s disease. American Journal of Alzheimer’s Disease and Other Dementias. 2010;25:505–512. doi: 10.1177/1533317510376173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Rubin EH, Morris EJ, Mandel SA. Senile dementia of the Alzheimer’s type: An important risk factor for serious falls. Journals of Gerontology. 1987;42:412–417. doi: 10.1093/geronj/42.4.412. [DOI] [PubMed] [Google Scholar]
- Mungas D. In-office mental status testing: A practical guide. Geriatrics. 1991;46:54–58. [PubMed] [Google Scholar]
- Murphy L, Singh BB. Effects of 5-Form, Yang Style Tai Chi on older females who have or are at risk for developing osteoporosis. Physiotherapy Theory and Practice. 2008;24:311–320. doi: 10.1080/09593980701884790. [DOI] [PubMed] [Google Scholar]
- Nnodim JO, Strasburg D, Nabozny M, Nyquist L, Galecki A, Chen S, Alexander NB. Dynamic balance and stepping versus tai chi training to improve balance and stepping in at-risk older adults. Journal of the American Geriatrics Society. 2006;54:1825–1831. doi: 10.1111/j.1532-5415.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- Oleske DM, Wilson RS, Bernard BA, Evans DA, Terman EW. Epidemiology of injury in people with Alzheimer’s disease. Journal of the American Geriatrics Society. 1995;43:741–746. doi: 10.1111/j.1532-5415.1995.tb07042.x. [DOI] [PubMed] [Google Scholar]
- Patience T’ai Chi Association. Tai Chi push hands and Wing Chun sticky hands: A comparison. 2011 Retrieved from http://www.patiencetaichi.com/ [Google Scholar]
- Quindry JC, Yount D, O’Bryant H, Rudisill ME. Exercise engagement is differentially motivated by age-dependent factors. American Journal of Health Behavior. 2011;35:334–345. doi: 10.5993/ajhb.35.3.7. [DOI] [PubMed] [Google Scholar]
- Rentz D. The assessment of rehabilitation potential: Cognitive factors. Gaithersburg, MD: Aspen: 1991. [Google Scholar]
- Resnick B, D’Adamo C. Factors associated with exercise among older adults in a continuing care retirement community. Rehabilitation Nursing. 2011;36:47–53. doi: 10.1002/j.2048-7940.2011.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal R. Meta-analytic procedures for social research. Newbury Park, CA: SAGE; 1991. [Google Scholar]
- Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Physical Therapy. 2000;80:896–903. [PubMed] [Google Scholar]
- Teri L, Logsdon RG, McCurry SM. Exercise interventions for dementia and cognitive impairment: The Seattle Protocols. Journal of Nutrition Health and Aging. 2008;12:391–394. doi: 10.1007/BF02982672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teri L, McCurry SM, Buchner DM, Logsdon RG, LaCroix AZ, Kukull WA, Larson EB. Exercise and activity level in Alzheimer’s disease: A potential treatment focus. Journal of Rehabilitation Research and Development. 1998;35:411–419. [PubMed] [Google Scholar]
- van Iersel MB, Benraad CE, Rikkert MG. Validity and reliability of quantitative gait analysis in geriatric patients with and without dementia. Journal of the American Geriatrics Society. 2007;55:632–634. doi: 10.1111/j.1532-5415.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- van Iersel MB, Munneke M, Esselink RA, Benraad CE, Olde Rikkert MG. Gait velocity and the Timed-Up-and-Go test were sensitive to changes in mobility in frail elderly patients. Journal of Clinical Epidemiology. 2008;61:186–191. doi: 10.1016/j.jclinepi.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Vellas BJ, Wayne SJ, Garry PJ, Baumgartner RN. A two-year longitudinal study of falls in 482 community-dwelling elderly adults. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1998;53:M264–M274. doi: 10.1093/gerona/53a.4.m264. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: An investigation of Tai Chi and computerized balance training. Journal of the American Geriatrics Society. 1996;44:489–497. doi: 10.1111/j.1532-5415.1996.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Yao L, Algase D. Emotional intervention strategies for dementia-related behavior: A theory synthesis. Journal of Neuroscience Nursing. 2008;40:106–115. doi: 10.1097/01376517-200804000-00010. [DOI] [PubMed] [Google Scholar]
- Yao L, Giordani B, Alexander NB. Developing a positive emotion-motivated Tai Chi (PEM-TC) exercise program for older adults with dementia. Research and Theory for Nursing Practice. 2008;22:241–255. [PMC free article] [PubMed] [Google Scholar]