Abstract
OBJECTIVE
To describe the use and symptomatic outcomes of different therapies for lower urinary tract symptoms (LUTS) in a community-based population of men followed for 17 years.
PATIENTS AND METHODS
Data from a randomly selected cohort of 2184 men, aged 40–79 years in 1990, from Olmsted County, Minnesota, USA were included in the study. Participants completed a questionnaire similar to the American Urological Association Symptom Index (AUASI) and reported on incontinence. Men were followed biennially through 2007 (median follow-up: 13.7 years; Q1, Q3: 8.8, 15.7). Medical and surgical treatments for LUTS were reported on biennial questionnaires and abstracted from community medical records.
RESULTS
Overall, 610 (28%) men received medical or surgical therapy for treatment of LUTS. Patients undergoing vaporization and transurethral resection of the prostate (TURP) had the highest pre-intervention AUASI scores (P < 0.001) and the most rapid increase in scores over time (P = 0.002) compared with those treated with medications or no therapy. After intervention, symptom progression slowed in all treatment groups. However, the greatest improvement in AUASI score (median % change) was observed in the TURP group: − 27.45%. The TURP group also reported a significant decrease in incontinence after surgery (% change): TURP: − 22.58%.
CONCLUSION
All therapies were effective at slowing the progression of LUTS, but only TURP patients reported a significant decrease in both LUTS and incontinence after therapy.
Keywords: benign prostatic hyperplasia, urinary incontinence, prostatism
INTRODUCTION
One of the most common non-malignant conditions afflicting aging men is LUTS [1–3], which can significantly reduce quality of life and carries substantial healthcare costs. In the year 2000 alone, LUTS treatment was estimated to cost approximately 1.1 billion USD, with outpatient medication costing an additional 200 million USD [4]. The prevalence of LUTS and the associated healthcare costs are expected to increase as the aging population experiences greater longevity [3].
Patients may seek treatment because of LUTS severity, bladder outlet obstruction, direct urinary retention, renal insufficiency secondary to prostate enlargement, urinary tract infections, haematuria or bladder calculi [5]. Multiple clinical trials have shown the efficacy of pharmacological treatments, vaporization and surgical procedures for treating LUTS with and without prostate enlargement in clinical trial populations [5–10]. However, the factors that subsequently dictate treatment are multifactorial and include patient preference, cost, equipment availability and surgeon training [11]. As therapies for LUTS may be applied to patients that differ from clinical trial populations, the effectiveness of such therapies may be very different from what is observed during clinical trials [12,13]. The characteristics of clinical trial participants can differ substantially from the characteristics of the patients generally observed in the community setting. Therefore it is unclear if therapies that are successful in clinical trials will also be successful in individuals with different characteristics in the general population. Hence, the goal of our study was to describe the men who are actually receiving LUTS therapies in the community, and to determine whether these therapies are yielding effective relief of LUTS in the sub-populations receiving LUTS therapies.
METHODS
Details of the ‘Natural History of Prostatism: The Olmsted County Study’ have been published previously [14]. Briefly, a random sample of men aged 40–79 years in 1990, and residing in Olmsted County, MN, USA, was identified using the resources of the Rochester Epidemiology Project [15]. Men with a previous history of prostate cancer, prostatectomy, or other urological conditions (bladder cancer, bladder disorders or surgery, and urethral disorders or surgery) were excluded. Of the 3874 eligible men, 2115 (55%) agreed to participate and completed a detailed questionnaire that included questions similar to the American Urological Association Symptom Index (AUASI). In addition, the response categories in our questionnaire were worded as ‘I do not have the symptom, rarely, a few times, fairly often, usually, almost always, and always’. For this study, the top two categories were collapsed into a single category to match the highest AUASI category of ‘almost always’ [16]. The cohort was followed biennially thereafter (through 2007). To replace men who either died or dropped out of the study during the follow-up period, additional men were randomly sampled from the community and were invited to participate during the first 4 years of follow-up (n = 332). After that time, the study was maintained as a closed cohort.
Study participants were asked to report all prescribed and over-the-counter medications that were taken on a daily basis at the time of each questionnaire. The questionnaire also included specific questions regarding use of α-adrenergic receptor inhibitors (α-ARs) and 5α-reductase inhibitors. Additionally, men were passively followed through community medical and surgical records to determine whether they required a procedure or surgery for treatment of LUTS.
Questions regarding urinary incontinence were incorporated into the follow-up questionnaire in 1992 and included biennially thereafter. Urinary incontinence was classified as answering yes to at least one question regarding urinary leakage, use of bladder control items, a diagnosis of incontinence, or a report to a physician regarding trouble controlling urination. Not all study participants participated in all rounds of follow-up. Of the 610 men with some treatment, 27 did not have an incontinence assessment before and after treatment, 139 only had an incontinence assessment after treatment, 156 only had an incontinence assessment before treatment, and 288 had an incontinence assessment before and after treatment. Only participants with data available both before and after treatment were included in analyses related to urinary incontinence.
All analyses were conducted using SAS (SAS Institute, Cary, NC, USA; Version 8.2). Men who developed prostate cancer (n = 259) were removed from the analysis. Four men received transurethral microwave thermotherapy in this population. Because of the small sample size, data for these men are not included in this study, leaving 2184 study participants. Men were categorized by most aggressive therapy received, with 5α-reductase inhibitors considered more aggressive than α-ARs. Linear mixed-effects regression models were used to estimate annual longitudinal changes in LUTS both before and after treatment. Symptom score was regressed on the time from initial measure to the last assessment before treatment and on the time from the first assessment after treatment to the last follow-up. Additional terms were added to assess 10-year age groups and type of treatment. Interaction terms were also included to allow for different slopes across age and treatment groups. The SAS mixed-effects model incorporates maximum-likelihood estimates of complete cases.
RESULTS
Overall, 610 (28%) men received a medical or surgical therapy for LUTS over a median follow-up period of 13.7 years (Q1, Q3: 8.8, 15.7). The most aggressive therapy received during follow-up is shown in Table 1, but many individuals had received other forms of therapy before their most aggressive treatment: n = 71 (36.4%) of 5α-reductase inhibitor users, n = 18 (78.3%) of men with laser vaporization, and n = 38 (44.7%) of men receiving TURP.
TABLE 1.
Baseline characteristics of study participants categorized by most aggressive therapy
|
Characteristic |
None n = 1574 Median (Q1, Q3) |
α-AR n = 307 Median (Q1, Q3) |
5-ARI n = 195 Median (Q1, Q3) |
Vaporization n = 23 Median (Q1, Q3) |
TURP n = 85 Median (Q1, Q3) |
P |
|---|---|---|---|---|---|---|
| Age (years) | 48.67 | 55.56 | 57.62 | 60.11 | 62.55 | < 0.001 |
| (43.89, 58.67) | (46.80, 64.42) | (50.31, 65.48) | (49.06, 65.86) | (55.53, 69.43) | ||
| BMI (kg/m2) | 28.09 | 28.31 | 27.75 | 26.63 | 27.70 | 0.32 |
| (25.56, 30.93) | (25.87, 31.10) | (25.31, 30.18) | (24.79, 28.47) | (25.76, 30.10) | ||
| Prostate volume* (mL) | 23.95 | 27.40 | 31.52 | 31.74 | 40.08 | < 0.001 |
| (19.49, 29.20) | (20.38, 32.99) | (24.19, 43.82) | (27.20, 37.73) | (34.87, 58.66) | ||
| Maximum flow rate* | 21.70 | 16.70 | 16.60 | 10.15 | 15.80 | < 0.001 |
| (mL/s) | (16.20, 27.60) | (12.40, 23.20) | (11.90, 22.10) | (8.55, 16.90) | (12.60, 18.00) | |
| Symptom score | 4 | 7 | 7 | 11 | 10 | < 0.001 |
| (2, 7) | (4, 10) | (3, 12) | (5, 17) | (5, 14) |
α-ARs, α-adrenergic receptor inhibitors; 5-ARI, 5α-reductase inhibitors; TURP, transurethral resection of the prostate.
Prostate volume and maximum flow rate are only available for the 552 men evaluated as an in-clinic subset (399 None, 69 α-AR, 47 5- ARI, 12 vaporization, and 25 TURP).
Baseline characteristics of the men differed across therapy categories. For example, men who received TURP were the oldest and had the largest prostates at baseline (Table 1). Additionally, men who received laser vaporization or TURP had the worst baseline symptom scores (Table 1).
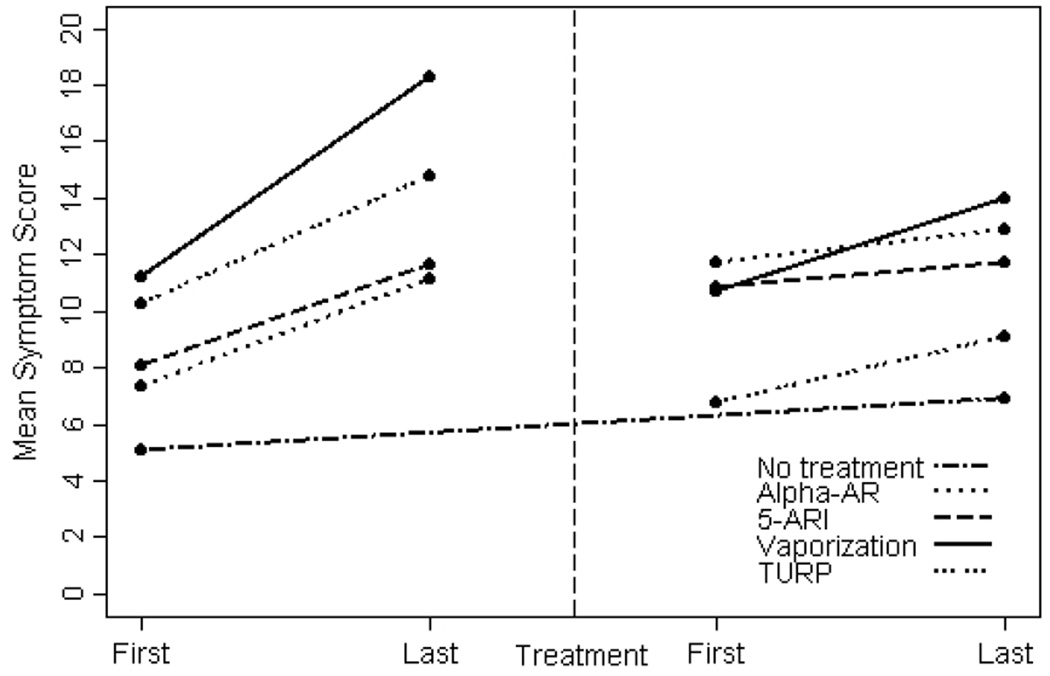
We next examined changes in symptom scores both before and after treatment. The median duration of follow-up from baseline assessment to last assessment before treatment was 8.2 years (25th, 75th centile: 5.1, 11.8) and from first assessment after treatment to last follow-up was 5.9 years (25th, 75th centile: 3.7, 8.6). Before therapeutic intervention, symptom scores increased significantly in all groups, and most rapidly in men who later received laser vaporization or TURP (Fig. 1). However, after all treatments, symptom scores stabilized. Symptom scores continued to rise over time, but more gradually in all groups. Additionally, there was no difference in rate of change in scores over time among the different treatment groups after intervention (Fig. 1). While symptom scores stabilized in all groups, median symptom scores improved significantly only in men treated with TURP (decline of − 4.31 points/year (25th, 75th centile: − 6.03, − 0.83) and laser vaporization − 2.84 points/year (25th, 75th percentile: − 3.75, − 0.95). Patients treated with α-ARs and 5α-reductase inhibitors did not have a significant improvement in symptom scores (Table 2).
FIG. 1.
Change in mean symptom scores over time.
TABLE 2.
Median symptom score for all treatment groups a different time points relative to enrolment, treatment and last follow-up
| Baseline to last assessment before treatment |
Last assessment before treatment to first assessment after treatment |
First assessment after treatment to last assessment |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline Median (Q1, Q3) |
Last Median (Q1, Q3) |
P† | Last Median (Q1, Q3) |
First Median (Q1, Q3) |
P† | First Median (Q1, Q3) |
Last Median (Q1, Q3) |
P | |
| None* | 4 (2, 7) | 6 (2, 10) | <0.001 | - | - | - | - | - | - |
| α-AR | 7 (4, 10) | 11 (6, 15) | <0.001 | 11 (7, 15) | 12 (6, 17) | 0.16 | 11 (6, 16) | 13 (8, 17) | < 0.001 |
| 5-ARI | 7 (3, 12) | 12 (7, 17) | <0.001 | 10 (6, 16) | 10 (6, 15) | 0.35 | 10 (6, 15) | 11 (6, 17) | 0.03 |
| Vaporization | 11 (5, 17) | 18 (14, 22) | <0.001 | 15 (13, 20) | 9 (6, 11) | 0.001 | 10 (8, 12) | 15 (8, 20) | 0.84 |
| TURP | 9 (4, 14) | 15 (10, 19) | <0.001 | 16 (11, 19) | 6 (2, 10) | <0.0001 | 7 (2, 12) | 8 (3, 13) | 0.07 |
α-ARs, α-adrenergic receptor inhibitors; 5-ARI, 5α-reductase inhibitors; TURP, transurethral resection of the prostate.
Median symptom score from baseline to last follow-up assessment.
t test P value.
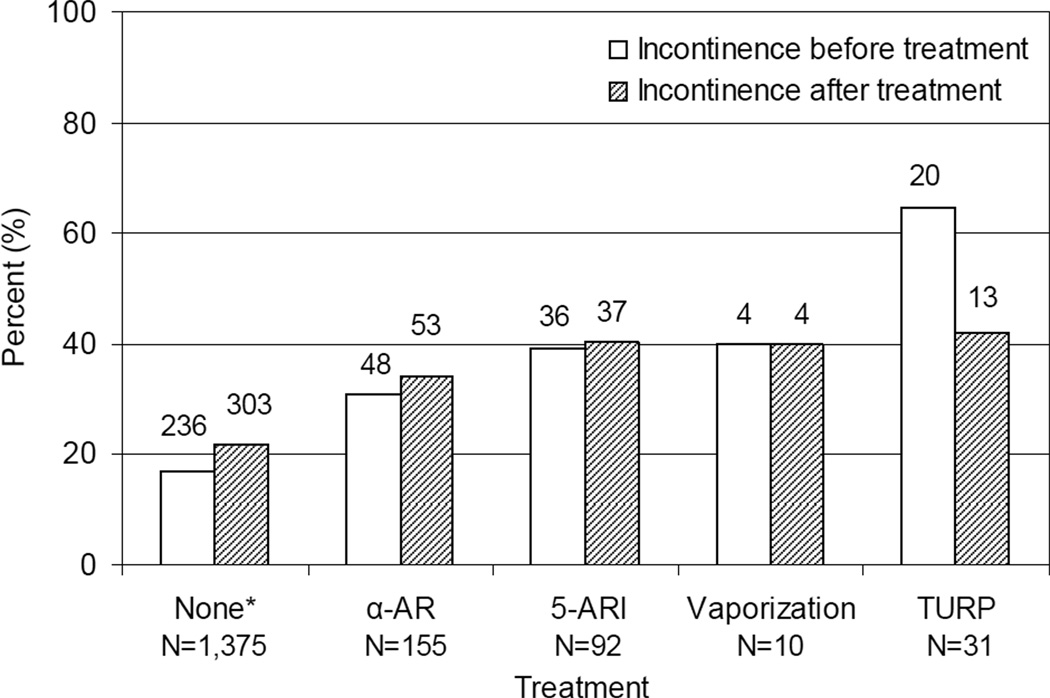
Urinary incontinence was also assessed in this cohort. Men undergoing TURP had the highest percentage of incontinence before surgery, but were also the only group to show a significant improvement in incontinence after therapeutic intervention (Fig. 2).
FIG. 2.
Incontinence in each group before and after therapeutic intervention. Only the 288 men receiving treatment who also had an incontinence assessment before and after treatment were evaluated. Incontinence assessments in the ‘None’ group are the first and last assessments because this group did not have treatment.
DISCUSSION
In our community-based observational study, we found that about 28% of the population eventually received a therapy for LUTS. All therapies in this population were effective at slowing progression of symptoms, but declines in pretreatment symptoms were observed only among men who ultimately underwent TURP or laser vaporization. Additionally, pre-intervention urinary incontinence (any leakage) was present in all groups, including over 20% of the men that did not receive treatment for BPH. Although pre-intervention incontinence was greatest in patients treated with TURP, this group was the only treatment group to report improvement in their incontinence symptoms. In this study, men treated with TURP and vaporization had significant reductions in symptoms after treatment. TURP, the only intervention with physical removal of prostate tissue, is still considered the standard surgical treatment for prostates less than 100 g [5]. However, there are potential disadvantages to TURP, including the possibility of significant blood loss and operative hyponatraemia, need for hospitalization and possible prolonged catheterization, and low but real risks of urinary incontinence, erectile dysfunction, bladder neck contractures and urethral stricture disease [17]. In an attempt to limit potential complications associated with TURP, multiple minimally invasive treatment options that ablate or vaporize tissue have been developed. These treatments might be expected to cause reductions in symptoms and incontinence similar to those for TURPs; however, these treatments were not widely applied in this population during the time-frame studied (n = 23). Our results in this small sample do suggest that laser vaporization therapies were also effective at reducing symptoms in treated men.
Our results also indicate that significant reductions in symptoms were not observed among men treated with α-ARs or finasteride. The lack of change in symptom scores among men treated with medical therapies differs from the current literature. A Cochrane review and large randomized trial found α-ARs to be superior to placebo in decreasing symptoms in men with prostate enlargement [6,7]. However, in our study there was no significant improvement in symptom scores in either the α-AR or the 5α-reductase inhibitor groups. This result could be because men included in the Cochrane review of clinical trials had more severe symptoms than the men in our population cohort (mean AUASI score ≥ 18 points vs median AUASI score of 11 points before treatment). Our data indicate that men with mild to moderate symptoms are also receiving these medical therapies. However, these men may not benefit from the medications to the same extent as men selected for the clinical trials. In particular, men with mild symptoms may not see a significant decline in their symptoms.
However, while symptom scores did not decline significantly after treatment, their symptoms did stabilize, and did not increase at the same rate as before treatment. For example, we compared the rate at which symptoms changed in the α-AR group before treatment with the rate at which symptoms changed within the α-AR group after treatment. In Fig. 2, the slope of the α-AR line on the left side of the figure indicates the rate at which symptoms are changing in that particular population. The assumption is that, without intervention, symptoms in that group of men would keep increasing at a rate of about 0.43 points per year (slope of that line). However, after treatment, the slope of the line flattens out (symptoms are only increasing at 0.20 points per year in the line on the right side of the figure). Hence, symptoms still increased over time in that group, but at a slower rate than before treatment with α-ARs. We repeated this analysis within every treatment group, and, in each case, the slopes of the lines after treatment were significantly lower than the slopes of the lines before treatment. Therefore, in each case, men who received any of the therapies (medical or surgical) had a slow-down in symptom progression, regardless of the type of therapy received. In summary, men with mild to moderate symptoms may not see significant improvement in symptom scores with medical treatment. However, these men may still benefit from these medications, as these data suggest that the medical therapies slow symptom progression.
Self-reported urinary incontinence was high in this cohort, with up to 22% of the men not receiving therapeutic intervention and 65% of men before TURP reporting some degree of leakage. These rates of incontinence are much higher than in the reported literature. The rate of urinary incontinence in men not undergoing surgical intervention or in the community setting is reported as from 0.3% to 24% [6,18,19]. McConnell et al. [20] have previously reported that urinary incontinence either secondary to overflow or caused by detrusor instability/urge affect up to half of men undergoing surgical intervention. Our definition of incontinence was broad, and may account for the higher rates in this population compared with previously reported incontinence rates. However, our definition of incontinence also used self-reported data, and self-reported rates may more accurately reflect incontinence than provider-reported rates [21].
The BPH/LUTS treatments examined in our study would not necessarily be expected to improve incontinence. However, men treated with TURP had a significant reduction in reported incontinence after surgery. One obvious explanation for this finding is the elimination of overflow incontinence, but another possible explanation for this finding is through an improvement of urge incontinence. Upregulation of bladder cholinergic receptors are thought to occur with bladder outlet obstruction [22]. After maximally debulking the prostate to eliminate the obstruction, it is possible that over the ensuing 6–9 months the cholinergic receptors in the bladder downregulate, resulting in less bladder irritability and a subsequent decrease in urge incontinence. In support of this theory, no continence improvement was seen in the medical therapy groups, where prostatic tissue is not physically removed. However, we had a limited number of men with incontinence data available both before and after therapy. Therefore, further studies are necessary to determine whether physical removal of the prostate can significantly improve incontinence.
Potential limitations of the study should be recognized. First, the cohort of men studied was predominantly white. As previous studies have shown that there is a racial variation in the incidence and treatment of LUTS, the results of the current study may not be generalized to other racial groups [23–27]. Second, data were based on a combination of patient survey and chart review and are potentially subject to reporting bias. Additionally, as the study is not a randomized controlled trial, but rather an observational study, treatments were not randomly assigned to each group. Hence, data on prostate size, prostate-specific antigen levels, or urodynamic abnormalities were not available for all patients, and we cannot directly compare treatments between different groups. In particular, we had incontinence data available both before and after TURP for only 31 patients. Our results were significant, but this small sample size makes it possible that this group does not adequately reflect the incontinence experience of most men who receive TURP. Therefore, further studies are necessary to determine whether TURP effectively relieves incontinence in most patients. Despite these limitations, this study offers a unique opportunity to describe LUTS and treatment outcomes in a community setting.
In this study, we found that 28% of the male population eventually received a medical or surgical therapy for LUTS. All treatments reduced the rate at which symptoms progressed; however, a reduction in symptoms was observed only in patients treated with laser vaporization and TURP. These results suggest that men with characteristics similar to our community population and who have significant baseline LUTS may experience maximal therapeutic outcomes with surgical prostate tissue removal.
What is known on the subject? and What does the study add?
It is known that benign prostatic hyperplasia is a common condition affecting most men by the age of 80 years. There are multiple treatment options available, including both medical and surgical interventions. However, what is not known is how affective the different types of interventions are in the general population. Previous studies have focused on centre-specific data. What is unique about our study is that it is a prospective cross-section analysis of a community cohort of men. Through this study we were able to assess the outcomes in the general population as opposed to in a high-volume surgical centre. Our findings show that in this community medical management was poor at symptomatic improvement, whereas surgical intervention produced the best improvement.
ACKNOWLEDGMENTS
The initial cohort was established through funding from Merck Research Laboratories. These analyses were funded in part by the United States National Institutes of Health (DK058859, AG034676 and RR024150).
Abbreviations
- AUASI
American Urological Association Symptom Index
- α-ARs
α-adrenergic receptor inhibitors
REFERENCES
- 1.Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen SJ, Girman CJ, Guess HA, et al. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol. 1996;155:595–600. doi: 10.1016/s0022-5347(01)66461-9. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen SJ, Guess HA, Panser L, et al. A population-based study of health care-seeking behavior for treatment of urinary symptoms. The Olmsted County Study of Urinary Symptoms and Health Status Among Men. Arch Fam Med. 1993;2:729–735. doi: 10.1001/archfami.2.7.729. [DOI] [PubMed] [Google Scholar]
- 4.Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005;173:1256–1261. doi: 10.1097/01.ju.0000155709.37840.fe. [DOI] [PubMed] [Google Scholar]
- 5.AUA guideline on management of benign prostatic hyperplasia 2003. Chapter 1: Diagnosis and treatment recommendations. J Urol. 2003;170:530–547. doi: 10.1097/01.ju.0000078083.38675.79. [DOI] [PubMed] [Google Scholar]
- 6.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 7.Wilt TJ, Howe RW, Rutks IR, et al. Terazosin for benign prostatic hyperplasia. Cochrane database of systematic reviews (Online) 2002:CD003851. doi: 10.1002/14651858.CD003851. [DOI] [PubMed] [Google Scholar]
- 8.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 9.Roehrborn CG, Siami P, Barkin J, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol. 2008;179:616–621. doi: 10.1016/j.juro.2007.09.084. discussion 21. [DOI] [PubMed] [Google Scholar]
- 10.Ruszat R, Wyler SF, Seitz M, et al. Comparison of potassium-titanyl-phosphate laser vaporization of the prostate and transurethral resection of the prostate: update of a prospective non-randomized two-centre study. BJU Int. 2008;102:1432–1438. doi: 10.1111/j.1464-410X.2008.07905.x. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 11.Black L, Naslund MJ, Gilbert TD, Jr, et al. An examination of treatment patterns and costs of care among patients with benign prostatic hyperplasia. Am J Manag Care. 2006;12:S99–S110. [PubMed] [Google Scholar]
- 12.Godwin M, Ruhland L, Casson I, et al. Pragmatic controlled clinical trials in primary care: the struggle between external and internal validity. BMC Med Res Methodol. 2003;3:28. doi: 10.1186/1471-2288-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Girman CJ, Guess HA, et al. Natural history of prostatism: factors associated with discordance between frequency and bother of urinary symptoms. Urology. 1993;42:663–671. doi: 10.1016/0090-4295(93)90530-n. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Su L, Guess HA, Girman CJ, et al. Adverse effects of medications on urinary symptoms and flow rate: a community-based study. J Clin Epidemiol. 1996;49:483–487. doi: 10.1016/0895-4356(96)00567-7. [DOI] [PubMed] [Google Scholar]
- 17.Wasson JH, Reda DJ, Bruskewitz RC, et al. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med. 1995;332:75–79. doi: 10.1056/NEJM199501123320202. [DOI] [PubMed] [Google Scholar]
- 18.Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatrics Soc. 1998;46:467–472. doi: 10.1111/j.1532-5415.1998.tb02468.x. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ, Berra-Unamuno A, Martin-Gordo A. Prevalence of urinary symptoms and other urological conditions in Spanish men 50 years old or older. J Urol. 1996;155:1965–1970. [PubMed] [Google Scholar]
- 20.McConnell JD, Barry MJ, Bruskewitz RC, et al. Benign prostatic hyperplasia: Diagnosis and treatment. Rockville: Public Health Service, U.S. Department of Health and Human Services; 1994. [Google Scholar]
- 21.Burgio KL, Ives DG, Locher JL, et al. Treatment seeking for urinary incontinence in older adults. J Am Geriatrics Soc. 1994;42:208–212. doi: 10.1111/j.1532-5415.1994.tb04954.x. [DOI] [PubMed] [Google Scholar]
- 22.Nitti VW, Blaivas JG. Urinary incontinence: epidemiology, pathophysiology, evaluation, and management overview. Chapt 60. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell-Walsh Urology. 9th edn. Philadelphia: Saunders; 2007. [Google Scholar]
- 23.Kristal AR, Arnold KB, Schenk JM, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395–1400. doi: 10.1016/j.juro.2006.11.065. quiz 591. [DOI] [PubMed] [Google Scholar]
- 24.Sarma AV, Wallner L, Jacobsen SJ, et al. Health seeking behavior for lower urinary tract symptoms in black men. J Urol. 2008;180:227–232. doi: 10.1016/j.juro.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarma AV, Wei JT, Jacobson DJ, et al. Comparison of lower urinary tract symptom severity and associated bother between community-dwelling black and white men: the Olmsted County Study of Urinary Symptoms and Health Status and the Flint Men's Health Study. Urology. 2003;61:1086–1091. doi: 10.1016/s0090-4295(03)00154-7. [DOI] [PubMed] [Google Scholar]
- 26.Kupelian V, Wei JT, O'Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Arch Int Med. 2006;166:2381–2387. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 27.Platz EA, Kawachi I, Rimm EB, et al. Race, ethnicity and benign prostatic hyperplasia in the health professionals follow-up study. J Urol. 2000;163:490–495. [PubMed] [Google Scholar]