Abstract
In oligodendrogliomas, 1p loss of heterozygosity (LOH) is a predictor of good prognosis and treatment response. In contrast, in uveal melanomas, LOH of chromosome 3 has been linked to poor prognosis and downregulation of Hsp27. In the present study, we have analyzed the expression of heat-shock proteins (Hsps) to characterize subtypes of gliomas and their histopathologic features and to correlate with other molecular markers including LOH of 1p. Biopsies from patients with primary gliomas (n = 65) were analyzed by immunohistochemistry, chromogenic in situ hybridization and fluorescent in situ hybridization and methylation-specific PCR (MSP). Elevated Hsp27 and total Hsp70 expression levels were associated with high-grade astrocytomas (p = 0.0001 and p = 0.01, respectively). In grade III oligodendrogliomas, the Hsp27 levels were significantly higher (p = 0.03). Low O6-methylguanine-DNA methyltransferase (MGMT) expression was associated with grade II astrocytomas. Elevated β-catenin expression was associated with grade III/IV astrocytomas (p = 0.003); p53 (+) tumors were more frequently found in grade III/IV astrocytomas (p = 0,001). LOH on 1p was associated with oligodendroglial tumours. In addition, a higher Hsp27 expression correlated with LOH of 1p (p = 0.017); this was also tested in two glioma cell lines. MSP was successful in only six samples. No significant correlations were found for the other markers. In conclusion, in oligodendroglial tumors, Hsp27 appeared as a surrogate marker of LOH of 1p which could also help to predict the disease prognosis. In gliomas, p53, Hsp27, Hsp70, MGMT, and β-catenin correlated with histopathological characteristics, suggesting that these markers could predict the disease outcome and the response to treatments.
Keywords: Gliomas, Heat-shock proteins, 1p status, Molecular markers
Introduction
Malignant gliomas comprise a wide range of neoplasms arising from astrocytes (astrocytomas) and from oligodendrocytes (oligodendrogliomas). When these two tumor types are well-differentiated, it is relatively easy to distinguish one from the other; however, the distinction may be difficult in more undifferentiated states or when mixed oligoastrocytomas are present. Diverse molecular pathways during the transformation process of these tumors may explain the different morphological characteristics, clinical behaviors, and responses to treatments (Louis and International Agency for Research on Cancer 2007; Jung et al. 2011).
Among the theories for cancer causation the concept of “obligate haploinsufficiency” is emerging, in which partial loss of important tumor-suppressor genes is more tumorigenic than the complete loss (Berger et al. 2011). The partial deletion of a chromosome arm causes loss of heterozygosity (LOH) which can produce alterations in the physiological balance between tumor-suppressor genes and oncogenes leading to genomic instability. Therefore, the LOH has important consequences for cancer predisposition, progression, diagnosis, and therapy. For example, the LOH of chromosome arms 1p and 19q in oligodendroglial tumors is useful for diagnosis and in predicting the prognosis and treatment response (Smith et al. 2000; Jha et al. 2011). An interesting link between LOH and protein expression level has emerged, in a previous study in uveal melanomas. Coupland et al. (2010) reported that the LOH of chromosome 3 is linked to a downregulation of a specific heat-shock protein (Hsp): Hsp27 (HSPB1 according to Kampinga et al. 2009). The partial or complete deletion of chromosome 3 (i.e., monosomy 3) in melanomas is a strong predictor of metastatic mortality. Then, the low expression of Hsp27 has been suggested as a surrogate molecular marker for the LOH of chromosome 3.
The purpose of the present study was to examine in gliomas the expression of Hsp27 and Hsp70 (both the stress-inducible (Hsp70Ind) and the constitutive (total Hsp70) forms). Hsps constitute a superfamily of molecular chaperones present in all cells and in all cell compartments, operating in a complex interplay with synergistic/overlapping multiplicity of functions, even though the common effect is cell protection. Hsps are implicated in the genesis as well as in the progression of cancer; many oncogenic agents/events generate in cancer cells stress-responsive proteins with chaperone activity, so they are being described as chaperones of tumorigenesis (Calderwood et al. 2006). In previous studies, relatively high Hsp levels have been reported in tumors arising from the central nervous system, particularly in gliomas where Hsp27 seems to be a marker of poor prognosis (Ciocca and Calderwood 2005; Graner et al. 2005).
We have particular interest in Hsp27 since this protein as been found in several human cancers; its expression has been correlated with disease progression and resistance to therapies (Ciocca and Calderwood 2005). Therefore, one of the aims of our study was to analyze Hsp27 in gliomas to know whether this protein can be used as a surrogate marker of LOH of chromosome 1p. In addition, we have explored Hsp27 and total Hsp70 (Hsp70t) to evaluate if they are useful molecular markers to characterize a particular type of astrocytoma. We investigated the expression of these two Hsps in 65 primary human gliomas, correlating their expression levels with 1p status, histologic grade, proliferating cell nuclear antigen (PCNA), β-catenin, p53, and O6-methylguanine-DNA methyltransferase (MGMT). MGMT was included in this study because the level of MGMT activity has been correlated with cellular resistance to alkylating therapeutic agents (Hegi et al. 2005).
Materials and methods
Clinical patient data
Sixty-five patients diagnosed with primary gliomas between June 2000 and November 2011 were included in this study (Table 1). Age and sex of all patients were noted; the patients came from the province of Mendoza, Argentina (ethnic group principally formed by descendants of native, and Spanish and Italian immigrants). This study has been reviewed and approved by the Ethic Committee of the Argentina Foundation for Cancer Research.
Table 1.
Main characteristics of the patients entered into this study
| Diagnosis | Numbers | Age | Gender |
|---|---|---|---|
| Astrocytoma GII | 15 | 46 (26–74) | f (5), m (10) |
| Astrocytoma GIII | 13 | 51 (29–65) | f (5), m (8) |
| Astrocytoma GIV | 20 | 63 (38–84) | f (9), m (11) |
| Oligodendroglioma GII | 11 | 49 (29–74) | f (5), m (6) |
| Oligodendroglioma GIII | 6 | 51 (18–75) | f (2), m (4) |
Histopathological examination
Sixty-five supratentorial gliomas with sufficient material available in paraffin blocks were selected for further analysis. The original hematoxylin and eosin slides were re-evaluated independently by our neuropathologist (P.L.). Detailed histopathological features were noted: cellularity, pleomorphism, presence of giant cells, mitotic activity, endothelial proliferation, including glomeruloid formation, and necrosis (confluent/palisading). The diagnosis was reconfirmed as per the recent WHO classification (Kleihues et al. 2007).
Immunohistochemistry
Hematoxylin-and-eosin-stained tissue sections (5 μm thickness) were used for histopathological studies. Serial 5-μm-thick sections were mounted onto 3-aminopropyltrietoxysilane (Sigma-Aldrich, St. Louis, MO)-coated slides for subsequent immunohistochemistry (IHC) analysis. The primary antibodies and the dilutions used were: (1) anti Hsp27 (rabbit polyclonal, recognizes recombinant Hsp25/Hsp27) (Fanelli et al. 2008), at 1:1,000 dilution, and anti Hsp27 (mouse monoclonal, Stressgen Biotech, Corp. Victoria, Canada), at 1:500 dilution; (2) anti total Hsp70 (clone BRM22, Sigma-Aldrich, which recognizes total Hsp70 including the Hsp70Ind), 1:1,000 dilution; (3) anti-inducible Hsp70 (Hsp70Ind) (Santa Cruz, CA, USA), 1:800 dilution; (4) anti-β-catenin (Zymed, San Francisco, CA), 1:400 dilution; (5) anti-MGMT (clone MT23.2, Zymed), 1:50 dilution; (6) anti-PCNA (Dako, Carpinteria, CA) 1:700; and (7) anti-p53 (clone DO7, Dako), 1:50 dilution. The specificity of these antibodies has been assessed by Western blot in our laboratory (Fanelli et al. 2008).The antigen retrieval protocol with heat was used to unmask the antigens (30 min in citrate buffer 0.01 M, pH 6.0). Tissue sections were incubated with the primary antibodies overnight at 4 °C in humidity chambers at the dilutions shown above. A commercial kit to detect mouse and rabbit primary antibodies was used (Dako EnVision system, horseradish peroxidase, diaminobenzidine (DAB), from Dako, Carpinteria, CA). Slides were lightly counterstained with hematoxylin to reveal nuclei, and they were observed and photographed with a Nikon Eclipse E200 microscope (Japan). Non-specific mouse IgG1 antibody and purified rabbit pre-immune serum (Dako, Kingsgrove, NSW, Australia) were used as isotype controls. The immunostaining was evaluated in the whole sections; the extent and intensity of immunostaining were assessed independently by two experienced immunopathologists blinded regarding histological diagnosis, and the disagreements (<10 %, often relating to the level of staining intensity) were resolved by consensus. We used a score as reported previously (Gago et al. 1998). Briefly, intensity score—0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining; proportion score—0 = no staining, 1 = staining in less than 1/10 of the cells, 2 = 1/10 to <1/3 of the cells, 3 = 1/3 to <2/3 of the cells, and 4 = > 2/3 of the cells. The total score was obtained combining both intensity and proportion scores; they were independently recorded for membrane, cytoplasmic, and nuclear cell compartments. The cut-off was: (a) Hsp27: cytoplasmic and/or membrane staining ≥3; (b) total Hsp70 (BRM22) cytoplasmic and/or nuclear and/or membrane ≥4; (c) inducible Hsp70 cytoplasmic and/or nuclear ≥3; (d) β-catenin: cytoplasmic ≥3; (e) MGMT: nuclear >3; (f) PCNA nuclear >30 % of total cells analyzed; and (g) p53 nuclear >30 % of total cells analyzed.
Immunocytochemistry
U87 and DBTRG.05MG cell lines (generously provided by Dr. Martin Radrizzani, Buenos Aires, Argentina) were fixed in 10 % buffered formalin at room temperature and smeared for immunocytochemistry. The antibodies used were: rabbit polyclonal antibody against Hsp25/27, provided by Dr. M. Gaestel (Max-Delbrück Center for Molecular Medicine, Berlin, Germany), and mouse monoclonal antibody against Hsp27 (Stressgen Biotech, Corp. Victoria, Canada). For cell permeabilization, the slides were immersed 5 min in Triton X 0.5 % in phosphate-buffered saline (pH 7.4) at 4 °C. Antigen unmasking was carried out in 0.01 M citrate buffer (pH 6.0) at 100 °C for 25 min. The cells were incubated with the primary antibodies overnight at 4 °C in humidity chambers at the following dilutions—Hsp25/27, 1:1,000, and Hsp27, 1:500. A commercial kit to detect mouse and rabbit primary antibodies was used (Dako EnVision system, horseradish peroxidase, DAB, from Dako, Carpinteria, CA). Slides were lightly counterstained with hematoxylin to reveal nuclei, and they were observed and photographed with a Nikon Eclipse E200 microscope (Japan). Non-specific mouse IgG1 antibody and purified rabbit pre-immune serum (Dako, Kingsgrove, NSW, Australia) were used as isotype controls.
Immunoblotting
The immunoblotting analysis of U87 and DBTRG.05MG cell lines was performed as previously described (Fanelli et al. 2008).
Chromogenic in situ hybridization
Chromogenic in situ hybridization (CISH) analysis was performed in 63 formalin-fixed paraffin-embedded sections of the gliomas (two samples did not have enough material) for detection of 1p deletion (1p36 LOH). Tissues sections were deparaffinized, hydrated, treated with citrate buffer 0.01 M (pH 6.0), at 98 °C for 15 min, rinsed in saline sodium citrate (SSC) twice at room temperature for 5 min, digested in pepsin solution (10 mg/mL in 0.2 N HCl pH 2.0) for 15 min at 37 °C, rinsed in wash buffer once for 5 min, dehydrated, and then air dried. Hybridization was performed using a digoxigenin-labeled locus-specific 1p36 probe (SPOT-Light Chromosome 1p36 deletion, Zymed). Probe and target DNA were denatured simultaneously in a humidity chamber at 98 °C for 20 min, followed by overnight incubation at 37 °C. Slides were then washed in blocking buffer (2.5 % of non-fat dry milk in 0.2× SSC) at 45 °C for 10 min and incubated with monoclonal anti-digoxin, biotin conjugate antibody (dilution 1:50 clone DI-22 from Sigma-Aldrich). The probe was detected using a commercial kit Dako Cytomation GenPoint (Tyramide signal amplification system for biotinylated probes). Nuclei were lightly counterstained with hematoxylin.
The tissue sections were examined with a Nikon Eclipse E200 microscope (Japan) using oil immersion lens (final magnification ×1,000). CISH results were independently evaluated by two pathologists and were found to be reproducible. A minimum of 100 nuclei were counted. Deletion was defined when a single signal was observed in 50 % of the counted nuclei.
Fluorescent in situ hybridization
Tissue
Fluorescent in situ hybridization (FISH) analysis was performed in 63 formalin-fixed paraffin-embedded sections of 3–5 μm (two samples did not have enough material). Tissues sections were baked for 2 h at 56 °C and deparaffinized in xylol (15 min × 2) followed by 100 % ethanol (ETOH; 10 min). Permeabilization was done in microwave with citrate buffer 0.01 M (pH 6.0), at 98 °C for 15 min, rinsed twice in SSC at room temperature for 5 min, followed by pepsin digestion (4 mg pepsin/L 0.5 % NaCl for 15 min at 37 °C). The slides (to reduce autofluorescence) were then immersed in 30 % sodium bisulfite twice in SSC for 20 min at 45 °C and then dehydrated in ETOH at increasing concentrations, and then drying at 45 °C. Each section was hybridized using a bacterial artificial chromosome probes RP11-671C15 (chromosome 1p, labeled red) and RP11-438F14 (chromosome 1q, labeled green). Both probes were labeled by nick translation with rhodamine and FITC. All probe pairs were co-denatured with the tissue sections and hybridized overnight at 37 °C in separate slides. After hybridization, the slides were washed on 2×SSC/0.3 % Tween 20 for 2 min at 72 °C, counterstained with 4′,6′-diamidino-2-phenylindole dihydrochloride, and then cover-slipped. The tissue sections were examined with a Nikon Eclipse E200 microscope (Japan) using an oil immersion lens (final magnification ×1,000). At least 100 cells were scored for each probe set. Relative copy numbers for 1p/1q was counted, and a ratio of 0.7 or less for 1p:1q was considered a loss.
Cell lines
FISH analysis was performed on two glioma cells lines (U87 and DBTRG.05MG). The cells were grown in DMEN + 10 % fetal bovine serum (FBS) and RPMI 1640 + 10 % FBS, respectively at 37 °C in 5 % CO2; when the cells arrived at 80 % confluence, they were harvested by trypsinization, re-suspended in hypotonic solution (0.075 M KCl) at 37 °C for 10 min, and then fixed with fixative solution (methanol/glacial acetic acid, 3:1, v/v). Then, the cells were centrifuged at 500×g for 5 min; the supernatant was removed; the cellular sediment was shaken and added drop by drop 5–10 ml of fresh fixative solution (at 4 °C for 10 min). The last two steps were repeated twice. Then, the cells were centrifuged at 500×g for 5 min; finally, the cell pellet is resuspended in a small volume of fresh 0.5–1 ml fixative, dropped onto a clean slide, and then air-dried. The slides were left at room temperature for at least 24 h, then were immersed in 70 % formamide (in 2×SSC at pH 7) at 72 C for 2 to 5 min, then dehydrated in ethanol at increasing concentrations, and air-dried. Each cell suspension was then hybridized and revealed as described for tissue samples.
Isolation of nucleic acids
DNA from each of 59 paraffin-embedded tissues (five samples did not have enough material) was extracted using a phenol–chloroform modified protocol (using five serial tissue sections).
Methylation-specific PCR
The methylation status of the MGMT promoter was determined by the method of methylation-specific PCR (MSP) (Herman et al. 1996) with some modifications. We performed a nested polymerase chain reaction (PCR), two-stage PCR approach, as previously described by Palmisano et al. (2000). DNAs were subjected to bisulfite modification using the EZ DNA Methylation-Direct Kit (Zymo Research, Orange, California). In the external round, the primers recognize the bisulfite-modified template but do not discriminate between methylated and unmethylated alleles. The PCR amplification protocol for stage 1 was as follows: Master Mix: PCR 1× Buffer, MgCl2 [2 mM], dNTPs [0.8 mM], primers [5–2.5 pmol/μL], 1 U Taq Pol, DNA template. Conditions—95 °C for 10 min, then denatured 95 °C for 15 s, annealed 52 °C for 30 s, extension at 72 °C for 30 s for 40 cycles, then followed by a 10-min final extension. Primers to selectively amplify unmethylated or methylated alleles of the MGMT gene in the internal round were used as previously described (Esteller et al. 1999). Annealing temperature was increased to 62 °C, and all of the cycling times were reduced to 15 s for a total of 35 cycles. Forward and reverse primers used are listed in Table 2. DNA extracted from whole blood of healthy individuals and cell lysate of MDA-MB 231 breast cancer cell line (previously known to have the methylated gene) (Wojdacz et al. 2007) were used as unmethylated and methylated controls, respectively. The PCR products were analyzed by electrophoresis (2 % agarose gels) and stained in ethidium bromide solution (0.5 μg/ml).
Table 2.
PCR primers and product sizes
| Purpose | Primer | Product (bp) |
|---|---|---|
| External round | Fw 5´-GGATATGTTGGGATAGTT-3´ | 289 |
| Rv 5´-CCAAAAACCCCAAACCC-3 | ||
| Internal round | ||
| Methylated | Fw 5′-TTTCGACGTTCGTAGGTTTTCGC-3′ | 81 |
| Rv 5′-GCACTCTTCCGAAAACGAAACG-3′ | ||
| Unmethylated | Fw 5′TTTGTGTTTTGATGTTTGTAGGTTTTTGT-3′ | 93 |
| Rv 5′-AACTCCACACTCTTCCAAAAACAAAACA-3′ | ||
Statistical analysis
Correlations between tumor type, histological characteristics, and the different markers were performed using the Prism computer program (Graph Pad Software, San Diego, CA). Contingency tables (Fisher’s exact tests or the Chi-square tests) were used; a p < 0.05 was considered significant.
Results
Hsp expression in gliomas
Among the Hsp family members, two Hsps were selected (Hsp27 and Hsp70) because they are frequently de-regulated and expressed at high levels in cancer tissues. These proteins were detected by IHC in gliomas but not in “normal” cells adjacent to tumor tissues. Figure 1 shows examples of the immunoreactions in both normal and neoplastic tissues.
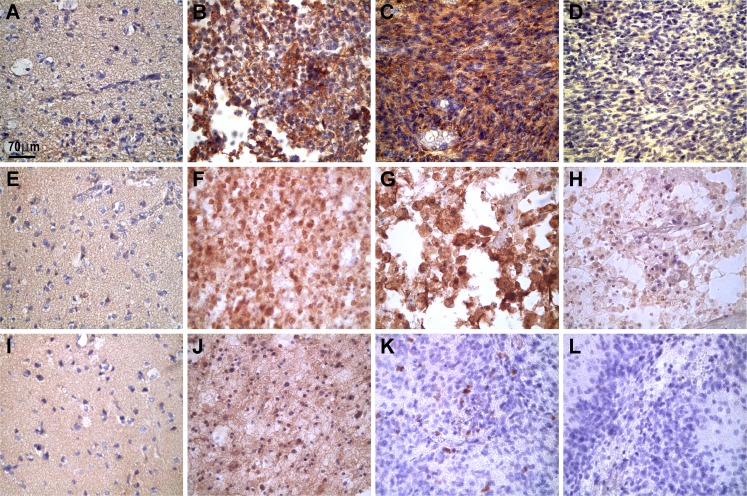
Fig. 1.
Representative photographs showing the microscopic expression of Hsp27, Hsp70total (recognized by monoclonal antibody BRM22), and Hsp70Ind in normal and tumor biopsy samples. In “normal” brain tissues, the three Hsps were practically absent (a, e, and i). In contrast, in tumor cells, Hsp27 was expressed in the cytoplasm of grade II oligodendroglioma (b) and in grade IV astrocytoma (c). Hsp70total was expressed in the nuclei and cytoplasm of grade II oligodendrogliomas (f) and in grade IV astrocytomas (g). In oligodendrogliomas (grade II) (j), Hsp70Ind was expressed with weak immunostaining intensity in some nuclei and in the background while, in grade IV astrocytomas (k), the Hsp70Ind protein appeared in a few nuclei. Negative controls using isotype antibodies are shown in d, h, and l (grade IV astrocytomas). The images were captured with a Nikon Eclipse E200 microscope (×40 objective). The positive immunoreaction appears as brown deposits; the slides were lightly counterstained with hematoxylin to reveal nuclei
The markers analyzed in each sample were considered positive if the total score equalled or exceeded the score previously established (see “Materials and methods”); then, we obtained the percentages of positive versus negative for each marker. Table 3 shows the relationship between the positive expression of Hsp27, total Hsp70, Hsp70Ind, and the number of tumors/subgroups/degrees. In oligodendrogliomas, where we found a significant correlation between 1pLOH and Hsp27 expression (see below), Hsp27 expression was analyzed using two different specific antibodies (rabbit polyclonal antibody against Hsp25/27 and a mouse monoclonal antibody against Hsp27). Identical results were found using both antibodies (Fig. 2). Positive expression of Hsp27 was considerably more elevated (p = 0.0001) in grade III/IV gliomas (90 % of analyzed cases) than grade II gliomas (21 %), and a similar result was observed in oligodendrogliomas (grade III compared with grade II, p = 0.03) (Table 3).
Table 3.
Expression of the Hsps in gliomas by immunohistochemistry
| Diagnosis | Hsp27 + (TS ≥ 3) | Hsp70t + (TS ≥ 4) | Hsp70ind + (TS ≥ 3) |
|---|---|---|---|
| Astrocytoma GII | 3/14 (21 %) | 9/13 (69 %) | 4/12 (33 %) |
| Astrocytoma GIII | 11/12 (92 %) | 11/12(92 %) | 5/12 (42 %) |
| Astrocytoma GIV | 16/18 (89 %) | 18/18 (100 %) | 9/18 (50 %) |
| Oligodendroglioma GII | 4/11 (36 %) | 7/10 (70 %) | 4/10 (40 %) |
| Oligodendroglioma GIII | 6/6 (100 %) | 3/6 (50 %) | 3/6 (50 %) |
TS total store
Hsp27: astrocytomas grade III/IV versus grade II, p = 0.0001. Oligodendrogliomas: grade III versus grade II, p = 0.03. Hsp70t: astrocytomas grade III/IV versus grade II, p = 0.01. Oligodendrogliomas, p = NS Hsp70Ind: astrocytomas and oligodendrogliomas, p = NS
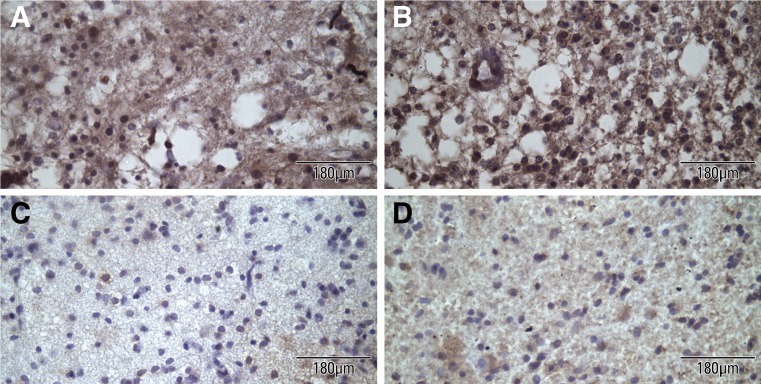
Fig. 2.
Representative photographs of grade II oligodendrogliomas showing the microscopic expression of Hsp27 using two different specific antibodies. Positive expression of Hsp27 using the mouse monoclonal antibody (a) and using the rabbit polyclonal antibody against the chimeric Hsp25/27 protein (b). Negative expression of Hsp27 using the mouse monoclonal antibody (c) and using the rabbit polyclonal antibody against the chimeric Hsp25/27 protein (d)
On the other hand, total Hsp70 (recognized by monoclonal antibody BRM22) was expressed in almost all grade III/IV astrocytomas (97 %) while it was expressed in 69 % of grade II astrocytomas (p = 0.01). In patients with grade II oligodendrogliomas, total Hsp70 expression appeared in 70 % of the patients while in grade III oligodendrogliomas it appeared in 50 % of the cases (Table 3).
Hsp70Ind positive expression was relatively lower than Hsp27 and total Hsp70 (BRM22+), ranging from 33 % to 50 % of positive in the tested samples (Table 3).
Expression of other molecular markers
The determination of MGMT methylation status by MSP was successfully achieved in only six of 59 patients (from the total of 65 patients, six samples did not have enough material to be studied by MSP). Hypermethylation was found in three patients; two patients exhibited an unmethylated MGMT promoter, and one patient had both statuses. Figure 3 shows the state of the MGMT gene promoter in the patient with a grade III astrocytoma that had both unmethylated and methylated status. The MDA-MB 231 cell line used as control had 67 % of MGMT hypermethylation (Paz et al. 2003). MGMT expression evaluated by IHC was seen about 50 % in grade III and IV astrocytomas, while grade II astrocytomas showed lower expression (20 %). In patients with grade II oligodendrogliomas, the MGMT levels were the same as those in grade III (50 %) (Table 4). Figure 4 shows examples of the immunoreactions for MGMT and β-catenin.
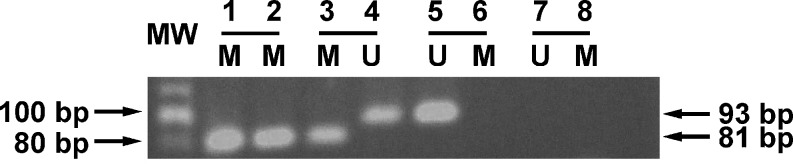
Fig. 3.
Methylation analysis of the MGMT in primary gliomas by nested PCR methylation-specific (MSP). Molecular weight markers are shown at the left. Lanes 1 and 2: controls of methylation (M, approximately 81 bp) using MDA-MB-231 cells. These cells have two states (methylated and unmethylated), but only the methylated state (duplicate loading) was used as control using the primer to amplify the methylated state. Lanes 3 and 4: two statuses of methylation of MGMT; methylated (lane 3), and unmethylated (U) (lane 4, 93 bp) from a patient with grade III astrocytoma. Lanes 5 and 6: blood used as control; in the blood, there is only unmethylated (U) state. Lanes 7 and 8: negative control (duplicate loading containing polymerase chain reaction mix, primers, and water, but not sample)
Table 4.
Astrocytomas: relationship of the markers with tumor grade
| Diagnosis | MGMT + (TS > 3) | β-catenin + (TS > 3) | PCNA + (TS > 30 %) | p53 + (TS > 30 %) |
|---|---|---|---|---|
| Astrocytoma GII | 2/11 (18 %) | 3/11 (40 %) | 7/11 (64 %) | 3/12 (25 %) |
| Astrocytoma GIII | 6/12 (50 %) | 10/12 (83 %) | 11/12 (92 %) | 9/11 (82 %) |
| Astrocytoma GIV | 8/18 (44 %) | 13/18 (72 %) | 16/18 (89 %) | 14/18 (78 %) |
| Oligodendroglioma GII | 5/10 (50 %) | 7/10 (70 %) | 5/7 (71 %) | 2/8 (25 %) |
| Oligodendroglioma GIII | 3/6 (50 %) | 3/6 (50 %) | 5/6 (83 %) | 1/9 (11 %) |
TS total store
β- catenin: astrocytomas grade III/IV versus grade II, p = 0.003. Oligodendrogliomas, p = NS
p53: astrocytomas grade III/IV versus grade II, p = 0.001. Oligodendrogliomas, p = NS
MGMT and PCNA, p = NS
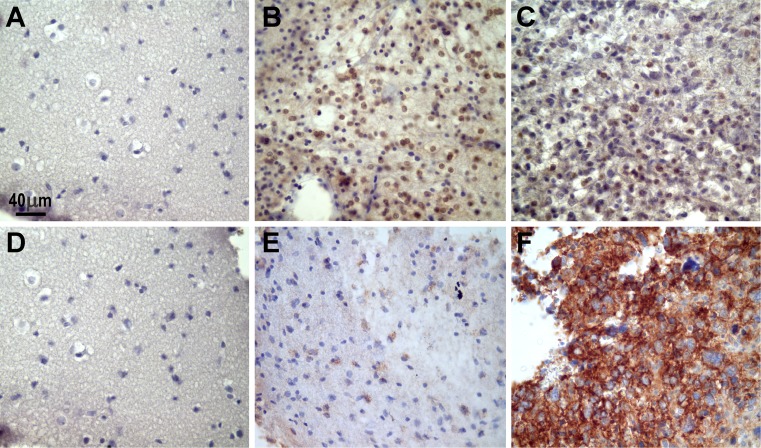
Fig. 4.
Representative photographs of the microscopic expression of MGMT and β-catenin in normal and tumor biopsy samples. “Normal” brain tissues did not show positive immunoreaction for MGMT (a) or β-catenin (d). In contrast, MGMT was noted mainly in the nuclei of oligodendroglioma cells (b), and the expression decreased in this grade II astrocytoma (c). β-catenin appeared as granular deposits in the cytoplasm in this grade II astrocytoma (e). This protein notably increased in a grade IV astrocytoma (note the cytoplasmic immunostaining) (f). The images were captured with a Nikon Eclipse E200 microscope (×40 objective). The positive immunoreaction appears as brown deposits; the slides were lightly counterstained with hematoxylin to reveal nuclei
β-catenin expression was relatively high in astrocytomas ranging from 40 % (grade II astrocytomas) to 80 % (in grade III/IV astrocytomas). This difference was statistically significant (p = 0.003) (Table 4). In oligodendrogliomas, the β-catenin expression ranged from 50–70 % (p = NS). PCNA levels were also relatively high in all tumor types (with no significant differences), while p53 positive tumors were more frequently found in astrocytomas grade III/IV compared with grade II (p = 0.001). In oligodendrogliomas (GII and GIII) p53 levels were very low (Table 4).
Loss of heterozygosity for 1p
FISH and CISH analyses for 1p deletion were successfully performed in 63 out of 66 cases. Table 5 shows the LOH for 1p in the tumors; as expected, this anomaly was more frequently found in oligodendrogliomas (11/17) than in astrocytomas (3/46) (p < 0.0001). Therefore, our interest was then to correlate the LOH for 1p with the expression of the molecular markers under study. In oligodendrogliomas (subdivided according to the presence or absence of LOH 1p), the expression of Hsp27 correlated with LOH of 1p. That is, more Hsp27-positive tumors were found among those that showed LOH 1p36, and almost all oligodendroglial tumors that showed Hsp27-negative expression did not present LOH 1p36 (p = 0.017) (Table 6). No significant correlations were found for the other markers.
Table 5.
Relationship between tumor type and loss of heterozygosity 1p36
| 1p36 LOH | 1p36LOH | Total | p value | |
|---|---|---|---|---|
| − | + | |||
| Oligodendrogliomas, grades II and III | 6 (35 %) | 11 (65 %) | 17 | <0.0001 |
| Astrocytomas, grades II, III, and IV | 43 (93 %) | 3 (7 %) | 46 |
Table 6.
Relationship between expression of Hsp27 and loss of heterozygosity 1p36 in oligodendrogliomas
| Hsp27 | 1p36 LOH | 1p36LOH | Total | p value |
|---|---|---|---|---|
| − | + | |||
| Positive | 1 (10 %) | 9 (90 %) | 10 | 0.017 |
| Negative | 5 (71 %) | 2(29 %) | 7 |
Oligodendrogliomas, n = 17
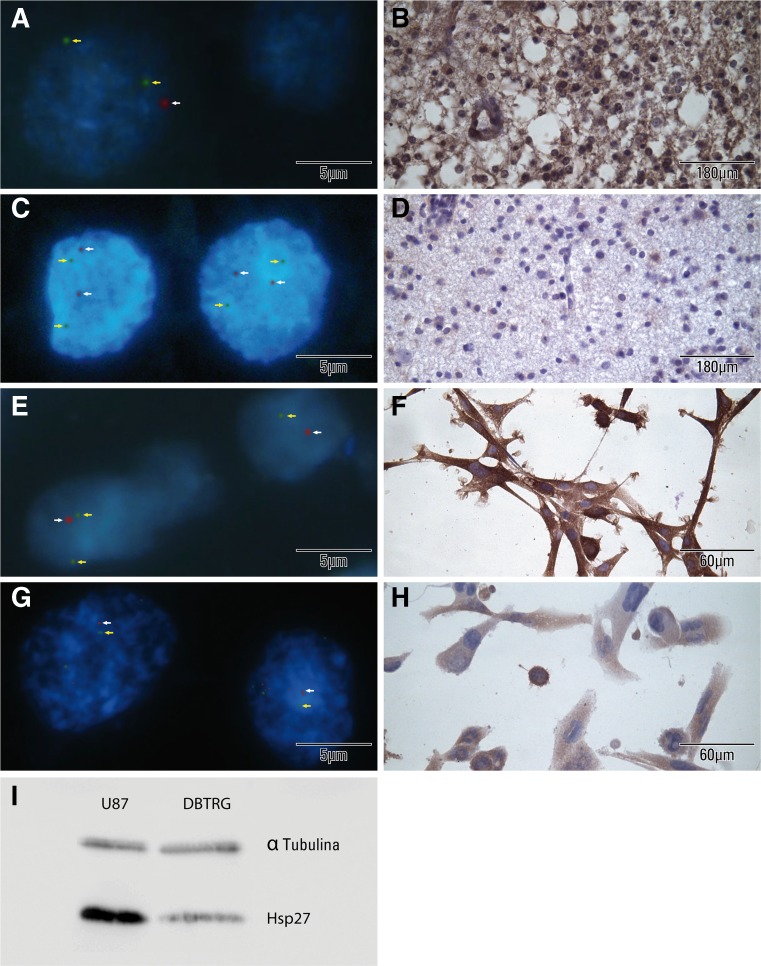
Figure 5 shows the relationship between the expression of Hsp27 (immunohistochemistry) and LOH of 1p (FISH) in oligodendroglial tumors and cell lines (U87, DBTRG.05MG). To validate the data found in vivo, we analyzed the expression of Hsp27 in two glioma cell lines (U87 and DBTRG.05MG) and evaluated the status of 1p by FISH. U87 cell line showed positive LOH of 1p and positive expression of Hsp27 (total score = 4). DBTRG.05MG cell line was negative for LOH of 1p and had negative expression of Hsp27 (total score = 2; this low expression level was considered negative according to our cut-off). In this figure, we also added the Hsp27 expression as revealed in Western blot analysis in the two cell lines.
Fig. 5.
Representative FISH and IHC images of oligodendrogliomas and glioma cell lines comparing 1pLOH with Hsp27 expression. Oligodendrogliomas, a nucleus with 1pLOH [using BACs probes 1p (red)/1q (green)]; b in the same tissue, the strong IHC staining for Hsp27; c nuclei with intact 1p [using BACs probes 1p (red)/1q (green)]; d in the same tissue, the negative IHC staining for Hsp27. Glioma cell lines, e nuclei of U87 with 1pLOH [using BACs probes 1p (red)/1q (green)]; f in the same cell line, the strong IHC staining for Hsp27, g nuclei of DBTRG with multiple copies of 1p [using BACs probes 1p (red)/1q (green)]; h in the same cell line, the weak IHC staining for Hsp27. i Immunoblots showing the Hsp27 content in the two cell lines under study. Note that the U87 cells have higher Hsp27 levels compared with the DBTRG cells
Discussion
In this study, we found a significant expression of Hsp27 and total Hsp70 in gliomas; the expression was not selective; for this reason, these markers were not useful in distinguishing astrocytomas from oligodendrogliomas. However, in astrocytomas, Hsp27 was found mainly in grade III and IV tumors (92 % and 89 %, respectively), while the protein was expressed in 21 % of grade II astrocytomas. This result is in agreement with previous studies reporting that this Hsp is related with the degree of malignancy (Ciocca and Calderwood 2005). In oligodendrogliomas, Hsp27 was expressed in 36 % of grade II tumors and in 100 % of grade III oligodendrogliomas. To our knowledge, this is one of the few reports on the expression of this Hsp27 in oligodendrogliomas (Hitotsumatsu et al. 1996). Regarding total Hsp70, in astrocytomas, it was expressed in almost all grade III/IV tumors (96 %), and although levels in grade II astrocytomas (69 %) were also relatively high, significant differences were observed (p = 0.01, Table 3). This relationship with the degree of malignancy is a novel report. In contrast, in oligodendrogliomas, high total Hsp70 expression levels were found in grade II (70 %), even higher than those with grade III (50 %), suggesting that this marker is not related to tumor anaplasia in oligodendrogliomas. No clear relationships with the tumor type and tumor grade were found for Ind Hsp70.
Methylation status of the MGMT promoter in diffusely infiltrating gliomas (grades II–IV) has been associated with the response to treatment with alkylating agents and with the overall clinical outcome (Vassella et al. 2010). MGMT expression has a central role in resistance to alkylating agents such as temozolomide, and it is an ideal potential target for biochemical modulation of drug resistance (Bignami et al. 2000; Pegg 2000; Gerson 2004). Previous studies have assessed the MGMT gene methylation status by the MSP technique with successful results (Herman et al. 1996; Esteller et al. 2000; Cankovic et al. 2007; Jung et al. 2010); however, the optimal method for MGMT analysis remains a matter of debate. Contradicting results have been reported regarding the correlation between MGMT methylation status, mRNA expression, and MGMT protein expression (Preusser et al. 2009; Vassella et al. 2010; Kreth et al. 2011). In our study, we performed a nested PCR as previously described by Palmisano et al. 2000, with some modifications in cycling conditions. Unfortunately, we obtained good results in only six of 59 tumors; approximately 90 % of the tumor blocks failed to provide results because of insufficient material, extensive tumor necrosis or fixation, and inclusion conditions that adversely affected the quality of the DNA. This result highlights an important limitation of the clinical implementation of the MSP assay when formalin-fixed and paraffin-embedded tumor tissues are used. Our results are consistent with previous reports (Preusser et al. 2008a; Coupland et al. 2010) which have also shown a low MSP success rate using fixed biopsy material, suggesting technical difficulties. This emphasizes that it will be necessary to use fresh or frozen tissues to improve the success of DNA extraction and MGMT promoter methylation testing.
We have also analyzed MGMT expression by IHC which we considered the most appropriate method when small quantities of tumor material are present in the stereotaxic biopsies. A controversial utility of IHC to determine MGMT activity has been reported (Preusser et al. 2008b). We found low MGMT expression levels in grade II astrocytomas (18 %) compared with high-grade astrocytomas (∼50 %). This could indicate that MGMT is methylated in low-grade tumors and that the protein is not expressed. According to these results, grade II astrocytomas should show better response compared with high-grade astrocytomas. Further studies are needed to confirm this assumption. This is in agreement with previous studies where a prognostic significance of MGMT expression examined by IHC in glioblastomas has been reported (Capper et al. 2007; Chinot et al. 2007; Parkinson et al. 2008). No correlations for MGMT protein and Hsp expression were found; future studies are required to correlate our data with the disease outcome.
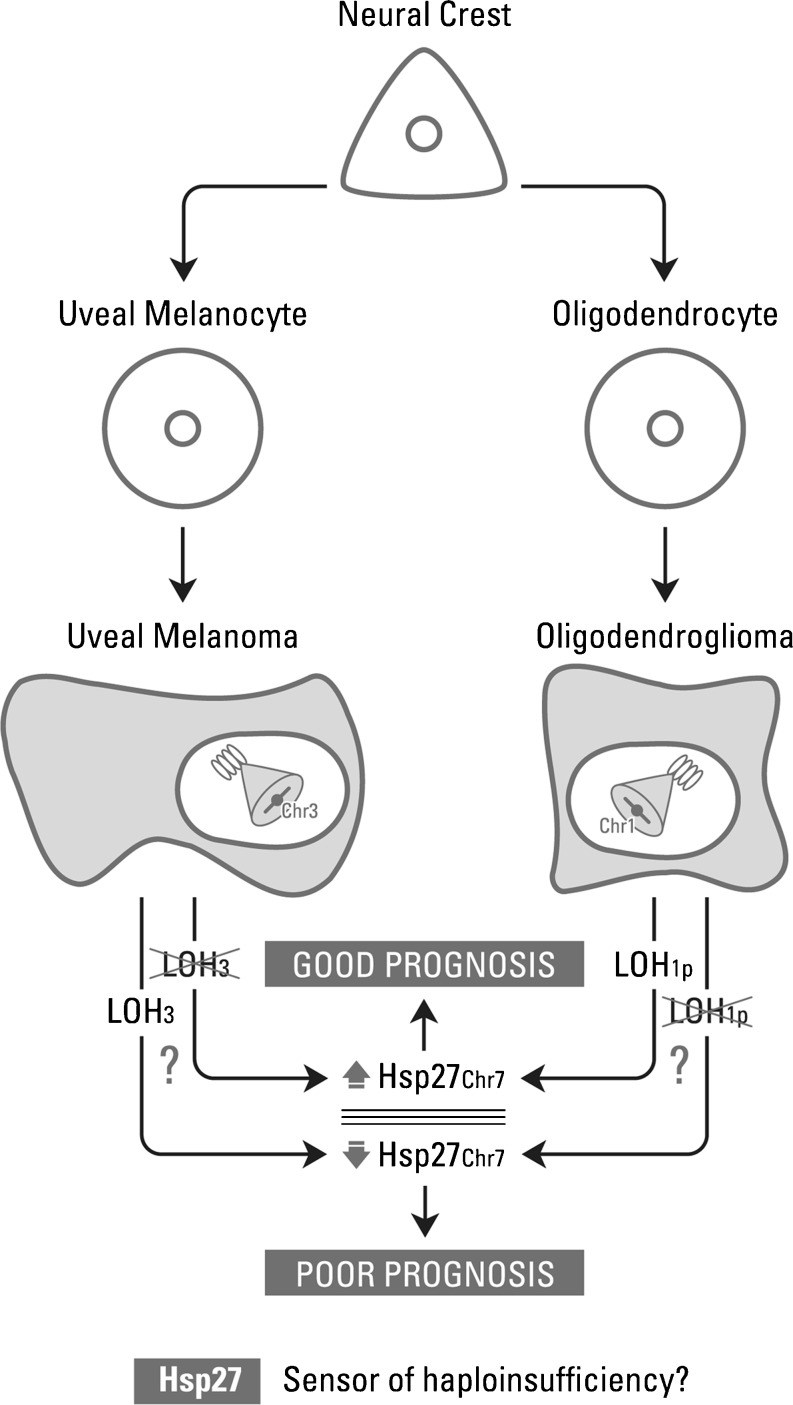
LOH of chromosome arms 1p and 19q is associated with the tumor phenotype, chemosensitivity, and prolonged overall survival in oligodendroglial tumors (Smith et al. 2000; Felsberg et al. 2004; Kim et al. 2005; Huang et al. 2009). Our study agreed with the literature since 1p LOH helped to characterize oligodendroglial tumors. Furthermore, we were interested in the possible correlations of LOH for 1p with the expression of Hsp27, Hsp70, MGMT, PCNA, β-catenin, and p53. One of the objectives of our study was to find a surrogate molecular marker(s) of 1p LOH using immunohistochemical staining, which is relatively simple and appropriate when the sample is limited and the hybridization technique is not possible. We were encouraged because, in uveal melanomas, a previous study revealed a high degree of correlation between low expression of Hsp27 and monosomy of chromosome 3, suggesting that Hsp27 is a possible surrogate marker of chromosome 3 loss. LOH of chromosome 3 has been linked to a downregulation of Hsp27; partial or complete deletion of chromosome 3 (i.e., monosomy 3) is a strong predictor of metastatic mortality (Coupland et al. 2010). In our study, in oligodendrogliomas, we observed that the expression of Hsp27 correlated with LOH of 1p; that is, more Hsp27-positive tumors were found among those showing LOH 1p. Almost all oligodendroglial tumors that showed low Hsp27 levels did not present LOH 1p (p = 0.017). In order to strengthen the data found in vivo, we analyzed the expression of Hsp27 in two glioma cell lines (U87 and DBTRG.05MG) and evaluated the status of 1p by FISH. U87 cell line showed positive LOH of 1p (in concordance with previous results, Law et al. 2005) and positive expression of Hsp27 (total score = 4). DBTRG.05MG cell line showed negative LOH of 1p and negative expression of Hsp27 (total score = 2). These results validate our findings in vivo. This analysis could complement the tumor grade because, although grade II oligodendrogliomas have in general better survival than grade III, it is important to study molecular pathways, and here, we suggest that high Hsp27 levels are associated with LOH of 1p which, as mentioned earlier, is a well-known marker of good response to treatment and better prognosis. The molecular pathways linking the LOH with the expression of Hsp27 are unknown. However, we observe that the final result leading to an increase in Hsp27 is then reflected in a good prognosis. We propose that Hsp27 may be a sensor of genetic imbalances in the cell (Fig. 6). Then, in oligodendroglial tumors, Hsp27 could be used as a surrogate marker of 1pLOH, when the genetic study is not accessible.
Fig. 6.
Proposed relationships between the loss of heterozygosity (LOH) with the expression of Hsp27. The molecular pathways linking the LOH with the expression of Hsp27 are unknown. However, we observe that the final result leading to an increase or decrease in Hsp27 is then reflected in a good or bad prognosis. Hsp27 is emerging as a sensor of genetic imbalances (haploinsufficiency) in the cell
Previous reports have shown a correlation between promoter methylation of MGMT gene, low expression of this protein, and LOH of chromosome arms 1p in oligodendrogliomas (Huang et al. 2009; Vassella et al. 2010). In contrast to the results published by Huang et al. (2009), we did not observe a relationship between low MGMT expression and LOH on 1p in GII oligodendrogliomas. In agreement with Huang et al., in astrocytomas, we found no association between 1p LOH and MGMT.
β-catenin, involved in cell–cell adhesion complexes and in Wnt/β-catenin signalling, has been reported to be an important player in a broad range of tumors. Fanelli et al. 2008 have demonstrated that, in breast cancer patients, 84 % of the tumors had abnormal β-catenin expression (mainly in cytoplasmic granules) and that this aberrant localization was associated with poor prognosis. β-catenin expression is known to be altered in gliomas, and its overexpression may be an important contributing factor to glioma progression (Sareddy et al. 2009; Liu et al. 2010, 2011). In the present study, we found relatively high β-catenin expression in all tumor types (astrocytomas, 65 %; oligodendrogliomas, 60 %) and a significant higher expression in grade III/IV astrocytomas (78 %). No significant correlations were found between β-catenin and the other markers examined here.
Another of the markers tested was p53; this protein is mutated/inactivated in approximately 60 % of diffuse gliomas (or low grade) and is associated with short survival (Ohgaki et al. 2011). We found p53 over-expression in 25 % of GII astrocytomas; this proportion was higher in high-grade tumors (GIII and GIV) about 80 % (p = 0.001). Our findings correlated with previous data reported in WHO classification of tumors of the central nervous system (Cavenee et al. 2000) and other studies (Huang et al. 2009). They have shown that loss of normal function of p53 increases the genomic instability of the cells causing tumor progression. Then, p53 apparently plays a key role in the formation of low-grade gliomas and in the progression towards glioblastomas.
In conclusion, we propose that in oligodendroglial tumors Hsp27 could be a surrogate marker of 1p LOH helping to predict the prognosis of the disease. In astrocytomas, Hsp27, Hsp70, β-catenin, and p53 are more frequently expressed in high-grade astrocytomas. We are following the clinical evolution of the patients to know whether the molecular markers analyzed here are useful in terms of prognosis and to predict the response to treatments.
Acknowledgments
The authors would like to thank National Research Council of Argentina (CONICET) (PIP 2428), the National Agency for scientific and Technological Promotion of Argentina (PICT 1047, 2007, préstamo BID), and the Argentina Foundation for Cancer Research.
Funding
National Research Council of Argentina (CONICET) (PIP 2428), the National Agency for scientific and Technological Promotion of Argentina (PICT 1047, 2007, préstamo BID), and the Argentine Foundation for Cancer Research.
Conflict of interest statement
None declared.
References
- Berger AH, Knudson AG, Pandolfi PP. A continuum model for tumour suppression. Nature. 2011;476:163–169. doi: 10.1038/nature10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami M, O’Driscoll M, Aquilina G, Karran P. Unmasking a killer: DNA O(6) methylguanine and the cytotoxicity of methylating agents. Mutat Res. 2000;462:71–82. doi: 10.1016/S1383-5742(00)00016-8. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque A, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cankovic M, Mikkelsen T, Rosenblum ML, Zarbo RJ. A simplified laboratory validated assay for MGMT promoter hypermethylation analysis of glioma specimens from formalin-fixed paraffin-embedded tissue. Lab Invest. 2007;87:392–397. doi: 10.1038/labinvest.3700520. [DOI] [PubMed] [Google Scholar]
- Capper D, Mittelbronn M, Meyermann R, Schittenhelm J. Pitfalls in the assessment of MGMT expression and in its correlation with survival in diffuse astrocytomas: proposal of a feasible immunohistochemical approach. Acta Neuropathol (Berl) 2007;115:249–259. doi: 10.1007/s00401-007-0310-x. [DOI] [PubMed] [Google Scholar]
- Cavenee WK, Furnari FB, Nagane M, Huang H, Newcomb EW, Bigner DD, Weller M, Berens ME, Plate KH, Israel MA, Noble MD, Kleihues P. Diffusely infiltrating astrocytomas. In: Kleihues P, Cavenee WK, editors. WHO classification of tumours of the central nervous system. Lyon: IARC Press; 2000. pp. 15–21. [Google Scholar]
- Chinot OL, Barrié M, Fuentes S, Eudes N, Lancelot S, Metellus P, Muracciole X, Braguer D, Ouafik L, Martin PM, Dufour H, Figarella-Branger D. Correlation between O6-methylguanine-DNA methyltransferase and survival in inoperable newly diagnosed glioblastoma patients treated with neoadjuvant temozolomide. J Clin Oncol. 2007;25:1470–1475. doi: 10.1200/JCO.2006.07.4807. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive and treatment implications. Cell Stress & Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland SE, Vorum H, Mandal N, Kalirai H, Honoré B, Urbak SF, Lake SL, Dopierala J, Damato B. Proteomics of uveal melanomas suggests HSP-27 as a possible surrogate marker of chromosome 3 loss. Invest Ophthalmol Vis Sci. 2010;51:12–20. doi: 10.1167/iovs.09-3913. [DOI] [PubMed] [Google Scholar]
- Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59:793–797. [PubMed] [Google Scholar]
- Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- Fanelli MA, Montt-Guevara M, Diblasi AM, Gago FE, Tello O, Cuello-Carrión FD, Callegary E, Bausero MA, Ciocca DR. P-cadherin and β-catenin are useful prognostic markers in breast cancer patients; β-catenin interacts with heat shock protein Hsp27. Cell Stress and Chaperones. 2008;13:207–220. doi: 10.1007/s12192-007-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsberg J, Erkwoh A, Sabel MC, Kirsch L, Fimmers R, Blaschke B, Schlegel U, Schramm J, Wiestler OD, Reifenberger G. Oligodendroglial tumors: refinement of candidate regions on chromosome arm 1p and correlation of 1p/19q status with survival. Brain Pathol. 2004;14:121–130. doi: 10.1111/j.1750-3639.2004.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago FE, Tello OM, Diblasi AM, Ciocca DR. Integration of estrogen and progesterone receptors with pathological and molecular prognostic factors in breast cancer patients. J Steroid Biochem Mol Biol. 1998;67:431–437. doi: 10.1016/S0960-0760(98)00140-X. [DOI] [PubMed] [Google Scholar]
- Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- Graner MW, Bigner DD. Chaperone proteins and brain tumors: potential targets and possible therapeutics. Neuro-Oncology. 2005;7:260–277. doi: 10.1215/S1152851704001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, Tribolet N, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Hitotsumatsu T, Iwaki T, Fukui M, Tateishi J. Distinctive immunohistochemical profiles of small heat shock proteins (heat shock protein 27 and alpha B-crystallin) in human brain tumors. Cancer. 1996;77:352–361. doi: 10.1002/(SICI)1097-0142(19960115)77:2<352::AID-CNCR19>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Huang L, Jiang T, Yuan F, Li GL, Cui Y, Liu EZ, Wang ZC. Correlation of chromosomes 1p and 19q status and expressions of O6-methylguanine DNA methyltransferase (MGMT), p53 and Ki-67 in diffuse gliomas of World Health Organization (WHO) grades II and III: a clinicopathological study. Neuropathol Appl Neurobiol. 2009;35:367–379. doi: 10.1111/j.1365-2990.2008.01002.x. [DOI] [PubMed] [Google Scholar]
- Jha P, Sarkar C, Pathak P, Sharma MC, Kale SS, Gupta D, Chosdol K, Suri V. Detection of allelic status of 1p and 19q by microsatellite-based PCR versus FISH: limitations and advantages in application to patient management. Diagn Mol Pathol. 2011;20:40–47. doi: 10.1097/PDM.0b013e3181e961e9. [DOI] [PubMed] [Google Scholar]
- Jung TY, Jung S, Moon KS, Kim IY, Kang SS, Kim YH, Park CS, Lee KH. Changes of the O6-methylguanine-DNA methyltransferase promoter methylation and MGMT protein expression after adjuvant treatment in glioblastoma. Oncol Rep. 2010;5:1269–1276. doi: 10.3892/or_00000760. [DOI] [PubMed] [Google Scholar]
- Jung CS, Unterberg AW, Hartmann C. Diagnostic markers for glioblastoma. Histol Histopathol. 2011;26:1327–1341. doi: 10.14670/HH-26.1327. [DOI] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Chetham ME, Chen B, Hightower LE. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14:105–111. doi: 10.1007/s12192-008-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim H, Kim TS. Clinical, histological, and immunohistochemical features predicting 1p/19q loss of heterozygosity in oligodendroglial tumors. Acta Neuropathol. 2005;110:27–38. doi: 10.1007/s00401-005-1020-x. [DOI] [PubMed] [Google Scholar]
- Kleihues P, Burger PC, Aldape KD, Brat DJ, Biernat W, Bigner DD. Glioblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. Lyon: IARC Press; 2007. pp. 33–49. [Google Scholar]
- Kreth S, Thon N, Eigenbrod S, Lutz J, Ledderose C, Egensperger R, Tonn JC, Kretzschmar HA, Hinske LC, Kreth FW. O-Methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation. PLoS One. 2011;6:e17156. doi: 10.1371/journal.pone.0017156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law ME, Templeton KL, Kitange G, Smith J, Misra A, Feuerstein BG, Jenkins RB. Molecular cytogenetic analysis of chromosomes 1 and 19 in glioma cell lines. Cancer Genet Cytogenet. 2005;160:1–14. doi: 10.1016/j.cancergencyto.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Liu C, Tu Y, Sun X, Jiang J, Jin X, Bo X, Li Z, Bian A, Wang X, Liu D, Wang Z, Ding L. Wnt/beta-Catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med. 2011;11:105–112. doi: 10.1007/s10238-010-0110-9. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang L, Zhao S, Ji X, Luo Y, Ling F. Beta-Catenin overexpression in malignant glioma and its role in proliferation and apoptosis in glioblastma cells. Med Oncol. 2010;28:608–614. doi: 10.1007/s12032-010-9476-5. [DOI] [PubMed] [Google Scholar]
- WHO classification of tumours of the central nervous system. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- Ohgaki H, Kleihues P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol. 2011;28:177–183. doi: 10.1007/s10014-011-0029-1. [DOI] [PubMed] [Google Scholar]
- Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–5958. [PubMed] [Google Scholar]
- Parkinson JF, Wheeler HR, Clarkson A, McKenzie CA, Biggs MT, Little NS, Cook RJ, Messina M, Robinson BG, McDonald KL. Variation of O(6)-methylguanine-DNA methyltransferase (MGMT) promoter methylation in serial samples in glioblastoma. J Neurooncol. 2008;87:71–78. doi: 10.1007/s11060-007-9486-0. [DOI] [PubMed] [Google Scholar]
- Paz MF, Fraga MF, Avila S, Guo M, Pollan M, Herman JG, Esteller M. A systematic profile of DNA methylation in human cancer cell lines. Cancer Res. 2003;63:1114–1121. [PubMed] [Google Scholar]
- Pegg AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res. 2000;462:83–100. doi: 10.1016/S1383-5742(00)00017-X. [DOI] [PubMed] [Google Scholar]
- Preusser M, Charles Janzer R, Felsberg J, Reifenberger G, Hamou MF, Diserens AC, Stupp R, Gorlia T, Marosi C, Heinzl H, Hainfellner JA, Hegi M. Anti-O6-methylguanine-methyltransferase (MGMT) immunohistochemistry in glioblastoma multiforme: observer variability and lack of association with patient survival impede its use as clinical biomarker. Brain Pathol. 2008;18:520–532. doi: 10.1111/j.1750-3639.2008.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusser M, Elezi L, Hainfellner JA. Reliability and reproducibility of PCR-based testing of O6-methylguanine-DNA methyltransferase gene (MGMT) promoter methylation status in formalin-fixed and paraffin-embedded neurosurgical biopsy specimens. Clin Neuropathol. 2008;27:388–390. doi: 10.5414/npp27388. [DOI] [PubMed] [Google Scholar]
- Preusser M. MGMT analysis at DNA, RNA and protein levels in glioblastoma tissue. Histol Histopathol. 2009;24:511–518. doi: 10.14670/HH-24.511. [DOI] [PubMed] [Google Scholar]
- Sareddy GR, Panigrahi M, Challa S, Mahadevan A, Babu PP. Activation of Wnt/beta-catenin/Tcf signaling pathway in human astrocytomas. Neurochem Int. 2009;55:307–317. doi: 10.1016/j.neuint.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Smith JS, Perry A, Borell TJ, Lee HK, O'Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- Vassella E, Vajtai I, Bandi N, Arnold M, Kocher V, Mariani L. Primer extension based quantitative polymerase chain reaction reveals consistent differences in the methylation status of the MGMT promoter in diffusely infiltrating gliomas (WHO grade II-IV) of adults. J Neurooncol. 2010;104:293–303. doi: 10.1007/s11060-010-0490-4. [DOI] [PubMed] [Google Scholar]
- Wojdacz TK, Dobrovic A. Methylation-sensitive high resolution melting (MS-HRM): a new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007;35:e41. doi: 10.1093/nar/gkm013. [DOI] [PMC free article] [PubMed] [Google Scholar]