Abstract
Heat shock proteins (HSPs) are chaperones that are known to have important roles in facilitating protein synthesis, protein assembly and cellular protection. While HSPs are known to be induced by damaging exercise, little is known about how HSPs actually mediate skeletal muscle adaption to exercise. The purpose of this study was to determine the effects of a heat shock pretreatment and the ensuing increase in HSP expression on early remodeling and signaling (2 and 48 h) events of the soleus (Sol) muscle following a bout of downhill running. Male Wistar rats (10 weeks old) were randomly assigned to control, eccentric exercise (EE; downhill running) or heat shock + eccentric exercise (HS; 41°C for 20 min, 48 h prior to exercise) groups. Markers of muscle damage, muscle regeneration and intracellular signaling were assessed. The phosphorylation (p) of HSP25, Akt, p70s6k, ERK1/2 and JNK proteins was also performed. As expected, following exercise the EE group had increased creatine kinase (CK; 2 h) and mononuclear cell infiltration (48 h) compared to controls. The EE group had an increase in p-HSP25, but there was no change in HSP72 expression, total protein concentration, or neonatal MHC content. Additionally, the EE group had increased p-p70s6k, p-ERK1/2, and p-JNK (2 h) compared to controls; however no changes in p-Akt were seen. In contrast, the HS group had reduced CK (2 h) and mononuclear cell infiltration (48 h) compared to EE. Moreover, the HS group had increased HSP72 content (2 and 48 h), total protein concentration (48 h), neonatal MHC content (2 and 48 h), p-HSP25 and p-p70s6k (2 h). Lastly, the HS group had reduced p-Akt (48 h) and p-ERK1/2 (2 h). These data suggest that heat shock pretreatment and/or the ensuing HSP72 response may protect against muscle damage, and enhance increases in total protein and neonatal MHC content following exercise. These changes appear to be independent of Akt and MAPK signaling pathways.
Keywords: Heat shock, Heat shock proteins, Skeletal muscle
Introduction
Eccentric exercise is well known to cause cell membrane disruptions, damaged myofibrillar structures and increased intracellular calcium (Zhang et al. 2008; Hather et al. 1991; Tesch 1988; Butterfield and Best 2009; Spangenburg and McBride 2006) ultimately resulting in muscle damage. In order to recover from these stressors and eventually regenerate new muscle proteins, skeletal muscle cells undergo a host of biochemical and molecular changes intended to reduce muscle damage and trigger subsequent remodeling of the skeletal muscle fibers. As part of this stress response, muscle cells produce heat shock proteins (HSPs). HSPs are molecular chaperones that function to maintain homeostasis, facilitate protein synthesis and provide protection from future insults. Not surprisingly, it has been shown that HSPs are increased in rat skeletal muscle during functional overload (Huey 2006; Locke 2008; O’Neill et al. 2006; Oishi et al. 2005; Ogata et al. 2005) and in human skeletal muscle following eccentric contractions (Gjovaag et al. 2006; Paulsen et al. 2007b; Thompson et al. 2001, 2003). While functional overload and eccentric muscle actions have been shown to be a substantial inducer of HSPs, little progress has been made in understanding what role HSPs play in the adaptation of skeletal muscle.
Recent studies in rat have shown that heat shock can increase skeletal muscle: protein synthesis (Kojima et al. 2007), protein content (Ohno et al. 2010; Goto et al. 2003), and muscle weight (Ohno et al. 2010; Kobayashi et al. 2005). In addition, studies in the rat soleus (Sol) demonstrate that heat stress may aid in maintaining fiber size (Oishi et al. 2009) and enhancing the re-growth of muscle (Selsby et al. 2007). It is thought that these muscle adaptations are mediated in party by HSPs, which chaperone nascent peptides during translation (Beckmann et al. 1990; Locke 1997; Beck and De Maio 1994) and therefore facilitate muscle protein synthesis. In addition to these findings, it has been shown that HSPs can provide protection against muscle damage in cell culture (Maglara et al. 2003) and enhance the recovery force following lengthening contractions in mice (McArdle et al. 2004a). These data suggest that HSPs may play an important role in regulating the rate and efficiency of muscle adaptation, particularly following damaging contractions.
The subsequent recovery of damaged skeletal muscle is also mediated in part by the involvement of signal transduction pathways. Following contractile activity the Akt pathway and the MAPK family of proteins function to regulate protein translation, proliferation, differentiation, and cell survival (Kolch 2000; Sasai et al. 2010). The activation of Akt and its downstream target p70s6k have been shown to be critical mechanisms in regulating protein synthesis (Bodine et al. 2001; Baar and Esser 1999). In addition, MAPK signaling has recently been shown to be a critical regulator in the maintenance of skeletal muscle mass (Shi et al. 2009). While exercise has been clearly shown to activate these signaling pathways, the molecular mechanisms that govern adaptation to eccentric actions have not been fully elucidated. Moreover, it has recently been recognized that HSPs can directly interact with multiple key components of signaling pathways that regulate growth and development (Nollen and Morimoto 2002), including Akt (Sato et al. 2000) and MAPKs (Song et al. 2001). Therefore, the induction of HSPs prior to damage may alter exercise induced signaling responses and enhance the recovery of damaged skeletal muscle.
While data demonstrate that heat shock alone can increase protein synthesis and enhance the re-growth of muscle, limited information is available on the combined effect of heat stress and damaging exercise on skeletal muscle adaptation and signal transduction. Therefore, the purpose of this study was to determine the effects of a heat shock pretreatment and the ensuing increase in HSP expression on early remodeling and signaling (2 and 48 h) events of the Sol muscle following a bout of downhill running. We hypothesized that a single heat stress pretreatment 48 h prior to downhill running would increase markers of regeneration, increase activation of the Akt/MAPK, and reduce the severity of muscle injury.
Methods
Materials
Antibodies against p-Thr202/Tyr204 and total ERK1/2 (#9101 and #9102), p-Thr183/Tyr185 and total Jun NH2-terminal kinase (JNK; #9251 and #9252), p-Ser473 and total Akt (#9271 and #9272), p-Thr421/Ser424 and total p70s6k kinase (#9204 and #9202) and Β-Tubulin (#2146) were purchased from Cell Signaling (Beverly, MA). Anti-HSP72 (SPA-810), p-Ser82 and total HSP25 (SPA-524 and SPA-800) were purchased from Enzo Life Sciences (Victoria, BC, Canada). Anti-Myosin-neonatal (sc-53097) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The creatine kinase kit (C7522) was purchased from Pointe Scientific (Canton, MI, USA). Remaining reagents were purchased from Fisher Scientific (Pittsburgh, PA).
Animals
Ten-week-old male Wistar rats (~300–350 g) were used in this investigation. Rats were housed in pairs in a temperature-controlled facility (24 ± 1°C) with a 12:12 h light–dark cycle. All animals were given free access to chow and water ad libitum. Rats were randomly assigned into three groups: (1) a control group (n = 6), which did not exercise or receive a heat shock treatment (CON); (2) eccentric exercise only group (n = 9) (EE); (3) heat shock group (n = 9), whose members were heat-shocked 48 h prior to performing a bout of eccentric exercise (HS). The rats were fasted for 12 h overnight before receiving heat shock or sham treatments, and again before completing the bout of eccentric exercise. Before heat shock treatments and muscle dissection of skeletal muscles, the rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (5 mg/100 g bodyweight). All protocols were approved by the Animal Care and Use Committee of the University Kansas Medical Center.
In vivo heat shock
Wistar rats were randomized into CON, EE or HS groups. The CON and EE groups had their core temperature maintained at 37°C, while the HS group had their core temperature elevated to 41°C. Preliminary experiments in our laboratory established that one heat treatment per week maintains an increase in HSP72 expression in skeletal muscles and avoids potential HSP inhibition by repeated heat treatment (Lee et al. 2005). The heat shock treatment was performed as previously described (Gupte et al. 2009). Briefly, HS rats were supported in a hot water bath in 43°C water, while animals had their core temperature elevated and maintained between 41 and 41.5°C for 20 min. Core temperature was monitored with a thermometer (ThermoWorks: TH-5A; Braintree Scientific, Braintree, MA, USA) and animal probe (ThermoWorks: RET-2; Braintree Scientific) to ensure a heat shock effect. The thermometers calibration conforms to National Bureau of Standards tables, and has an accuracy of 0.1°C ± 1 digit. Controls underwent sham treatment and maintained core temperature at 37°C. After treatment, 5 ml of 0.9 % saline was administered to prevent dehydration. Preliminary experiments in our laboratory established that this heat shock treatment increased HSP72 expression that peaked at 48 h in the Sol (Fig. 1). After 48 h, both the EE and HS animals performed a bout of downhill treadmill running.
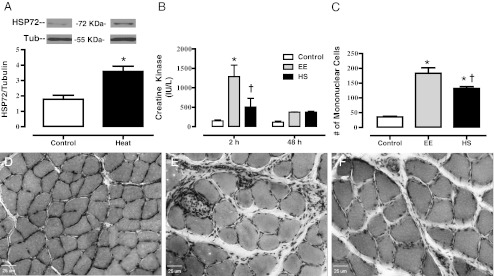
Fig. 1.
HSP72 expression in Sol muscle following heat treatment and downhill running induced muscle damage. a Average increases in HSP72 expression 48 h following the heat stress treatments in the Sol muscle. Overnight-fasted rats underwent a control bath (sham) of 37°C or a heat shock bath (Heat) of 43°C. Average colonic temperature gradually increased, reaching a peak temperature of 41.4 ± 0.3°C upon competition of the heating period. Muscles were dissected and frozen and homogenates were separated with SDS-PAGE. Western blots were analyzed for HSP72, then stripped and probed for Tubulin (n = 3). b Plasma CK (IU/L) response to downhill running in Control, eccentric exercised (EE) and heat-shock + eccentric exercised (HS) rats 2 and 48 h after exercise (n = 6–9 animals/group). c Quantified mononuclear cell infiltration counted from Control, EE and HS rats 48 h after exercise (n = 6–9 animals/group). Mononuclear cells were counted in H&E-stained Sol muscle cross-sections from control (d), EE (e) and HS (f) groups 48 h post-exercise. Note that the control group (d) had normal morphology. Both EE (f) and HS (f) show muscle damage (fiber swelling and edema); however, HS (f) had less immune cell infiltration. Bar, 25 μm. Data are presented as means ± SE. *Statistically greater than control. †Statistically less than EE
Downhill running
Downhill running is a common injurious exercise model and was chosen as it stimulates muscle remodeling and myogenic processes (Smith et al. 1999, 2001; Armand et al. 2003; Parise et al. 2008). Previous research has shown downhill running to induce upregulation of MAPK signaling such as the phosphorylation (p) of JNK, and ERK1/2 (Boppart et al. 2006). Recently, a similar protocol was used to examine the effect of heat shock preconditiong in Sol muscle (Shima et al. 2008). Overall there is sufficient data showing that downhill running induces muscle damage and subsequent regulation of myogenesis and hypertrophic signaling. Downhill treadmill running was performed as outlined by Armstrong et al. (1983). The exercise bout consisted of running 18 m/min down a −16 % grade. Rats ran for 5 min, and then were allowed a 2-min recovery. All animals were able to complete the prescribed bout of exercise.
Muscle dissection and storage
Muscles were removed 2 and 48 h post-exercise. These time points were chosen to investigate the signaling responses and were based on data from previous literature (Carlson et al. 2001; Lee et al. 2002). Muscles were rapidly clamp frozen in liquid nitrogen and stored at −80°C until further analysis. The Sol muscle is a postural muscle that is recruited during standing, locomotion and exercise (Roy et al. 1991; Tsivitse et al. 2003; Bombardier et al. 2009), and it has been used in previous studies involving heat stress (Milne and Noble 2002; Naito et al. 2000; Oishi et al. 2002; Selsby et al. 2007; Smolka et al. 2000; Uehara et al. 2004).
Protein extraction, protein quantification, and Western immunoblotting
50 mg pieces of clamp-frozen muscles were homogenized in a 12:1 (volume/weight) ratio of ice-cold cell extraction buffer (Invitrogen) with protease inhibitor cocktail (Invitrogen), sodium fluoride (200 mM), sodium orthovanodate (200 mM) and phenylmethanesulphonylfluoride (200 mM) added. Homogenized samples were transferred to 1.7 ml tubes and mixed on a laboratory rotator for 30 min at 4°C and then centrifuged for 2 min at 3,000×g at 4°C. Samples were diluted (1:1800), and the total protein concentration of the samples was determined using the micro bicinchoninic acid protein assay (Pierce, Rockford, IL). Samples were prepared in 5× Laemmli buffer containing 100 mM dithiothreitol and heated at 85°C for 15 min. Then, 90 μg of protein was subjected to SDS-PAGE (8 %, 10 %, or 12 % gel) followed by a wet transfer to a PVDF membrane for 90 min (200 mA/tank). Membranes were blocked for 1 h at room temperature in Tris-buffered saline, 0.1 % Tween 20 (TBST)–5 % nonfat dry milk followed by an overnight incubation with the appropriate primary antibodies at a concentration of 1:1,000, in TBST–5 % BSA. Blots were incubated in appropriate HRP-conjugated secondary antibody at a concentration of 1:10,000, in TBST 1 %–nonfat dry milk for 1 h at room temperature. Bands were visualized by enhanced chemiluminescence and quantified using densitometry. Visualization and quantification of the protein bands were performed using densitometry (Alpha Innotech, San Leandro, CA, USA). Densitometry was performed on each protein of interest five times and then averaged. To limit blot to blot variability, all Western blots were normalized to each other by adjusting the signal strength to a standard (eccentric exercised triceps muscle) run on every blot. Samples were normalized within each blot to time-matched controls.
Blood collection and creatine kinase analysis
Blood samples were obtained by cardiac puncture and the plasma was analyzed for creatine kinase (CK) following manufacturer’s instructions. Duplicate samples were averaged (IU/l).
Histopathological analysis of muscles
Frozen Sol muscles from each group at the 48 h time point were sectioned (10 μm) from the midbelly and fixed in 1.5 % formalin. Sections were then stained with hematoxylin and eosin (H&E). Images were visualized using an inverted microscope (Olympus). Digital images were captured (originally at 200× magnification) and analyzed (>100 fibers/group) for cross-sectional area using slidebook software (Intelligent Imaging Innovations). The number of mononucleated immune cells that infiltrated the muscles was averaged in three random fields in each section by an observer blinded to the treatment group.
Statistical analysis
All statistical procedures and graphs were performed with GraphPad Prism 5 (La Jolla, CA, USA). Data are presented as means ± SEM. Significance was set at the p ≤ 0.05 level, unless otherwise noted by appropriate post hoc testing when necessary. Our sample size was selected prior to beginning this study using a statistical power analysis. Based on previous data from our laboratory involving the analysis of signaling proteins via Western blot, five animals per group were required to detect significant difference (95 % confidence intervals) among the treatment groups. Our preliminary data on the effectiveness of heat shock on HSP72 expression was analyzed with a one-way analysis of variance (ANOVA). A two-way ANOVA was employed to detect differences in the two factors: time (2 and 48 h) or treatment (CON, EE, HS). When the one-way or the two-way ANOVA was statistically significant, Bonferroni posttests were conducted.
Results
Animal mass
The mass of the animals used for the 2-h time point were as follows: control (322.3 ± 6.6 g), EE (318.3 ± 7.3 g) and HS (321.6 ± 6.3 g). The masses at 2 h were not statistically different between groups (p = 0.91). The mass of the animals at 48 h between control (323.8 ± 3.0 g), EE (321.6 ± 5.1 g) and HS (327.9 ± 5.1 g) groups at 2 h and were not statistically different (p = 0.62).
Heat shock
The average colonic temperature of the HS group started at 36.1 ± 0.15°C gradually increased, reaching a mean peak temperature of 41.4 ± 0.06°C upon competition of the heating period (p < 0.01). The HS preconditioning protocol caused an elevation in HSP72 expression (Fig. 1a) from control (p < 0.01) in the Sol muscle 48 h following hyperthermia. These results show that our HS protocol increased HSP72 levels, demonstrating the effectiveness of our heat shock therapy.
Eccentric damage
Figure 1b shows the average CK responses between the groups at 2 and 48 h post-eccentric exercise. EE increased (p < 0.01) CK content as compared to control 2 h following exercise. In addition, at the 2-h time point the HS group was not significantly elevated from control, and had reduced (p < 0.01) CK when compared to EE. CK values of both the EE and HS had returned to baseline by the 48-h time point. Quantitative analysis of mononucleated immune infiltration 48 h following exercise obtained via H&E staining is presented in Fig. 1c. Downhill running increased (p < 0.01) the number of immune cells infiltrating the muscle cross-section in both the EE and HS groups when compared to controls; however, the number of immune cells in the HS group was reduced (p < 0.05) when compared to the EE group. Qualitative analysis of muscle injury via H&E staining of muscle cross-sections demonstrated evidence of gross injury 48 h following downhill running. When compared to controls (Fig. 1d) the EE group demonstrated cell swelling (+28.4 %) and the infiltration of many mononucleated cells to the muscle cross-section (Fig. 1e). The HS group (Fig. 1f) also presented with cell swelling (+35.6 %) when compared to controls; however, the fibers were not statistically larger than those seen in the EE group.
Heat shock protein responses to eccentric exercise
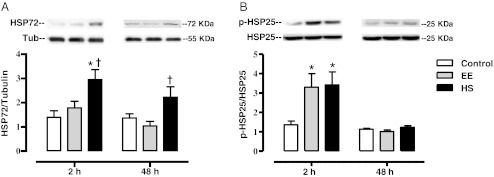
Figure 2 summarizes the data from Western blots of the Sol homogenates for both HSP72 and p-HSP25. Two hours following exercise, HSP72 content was increased in the HS group when compared to controls (p < 0.01) and EE (p < 0.05) groups. Forty-eight hours following exercise, HSP72 content remained elevated in the HS group when compared to the EE group (p < 0.05), but not control. No increases above control were seen as a result of EE at either time point. Two hours following exercise, p-HSP25 (Ser82) increased in both the EE (p < 0.05) and HS (p < 0.05) groups, as compared to control. This elevated p-HSP25 returned to base line in both the EE and HS groups by 48 h post-exercise.
Fig. 2.
HSP responses to eccentric exercise with and without heat shock pretreatment. Sol muscles were dissected from control, EE and HS rats 2 and 48 h after exercise. All animals were fasted overnight and muscles were snap-frozen in liquid nitrogen. Homogenates were subjected to Western blot analysis for HSP72/Tubulin (a) and p-HSP25/HSP25 (b). Data are presented as means ± SE (6–9 muscles/group). *Statistically greater than control; †statistically greater than EE
Total protein concentration and MHCneo Content
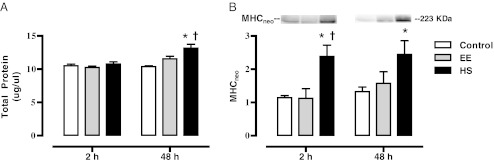
To begin to assess the effects of EE and HS on muscle regeneration, we looked at two common markers: total protein and MHCneo. Figure 3 summarizes the data from total protein expression and Western blots of the Sol homogenates for MHCneo. Two hours following exercise there were no differences in total protein content among the treatment groups; however, 48 h protein content was elevated in the HS group when compared to controls (p < 0.001) and the EE group (p < 0.01). We also measured an additional maker of regeneration using a MHCneo antibody. Two hours following exercise, there was an increase in MHCneo content in the HS group when compared to controls (p < 0.05) and the EE only group (p < 0.05). Forty-eight hours following exercise, there was an increase in the MHCneo content in the HS group when compared to control (p < 0.05).
Fig. 3.
Total protein concentration and MHCneo content following eccentric exercise, with and without heat shock pretreatment. Sol muscles were dissected from control, EE, and HS rats 2 and 48 h after exercise. All animals were fasted overnight and muscles were snap frozen in liquid nitrogen. The total protein concentrations of homogenates were quantified (a). Western blot analysis for neonatal myosin heavy chain is also shown (b). Data are presented as means ± SE (6–9 muscles/group). *Statistically greater than control; †statistically greater than EE
Intracellular signaling
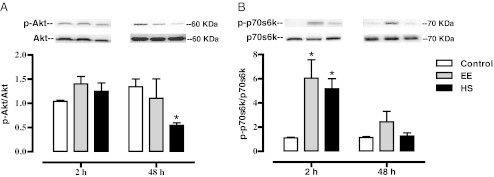
Because of the enhanced total protein and MHCneo content in the HS group, we investigated two signaling pathways strongly associated with regulating skeletal muscle mass and the initiation of protein synthesis following mechanical stimuli: Akt/p70s6k and MAPK’s (ERK1/2/JNK). Figure 4 summarizes the data from Western blots of the Sol homogenates for both p-Akt and p-p70s6k. These results indicate that exercise performed by either group (EE or HS) did not increase p-Akt at the 2- or 48-h time point in the Sol muscle. However, HS treatment did significantly reduce (p < 0.05) p-Akt compared to control 48 h following exercise. Because p70s6k has been shown to be critical regulator of protein synthesis we also measured its phosphorylation. Figure 4 also shows the average results of the p70s6k densitometry. Two hours following exercise, p-p70s6k increased in the EE group (p < 0.01) and HS group (p < 0.01) when compared to control. There were no statistical differences in p-p70s6k from control at the 48-h time point.
Fig. 4.
Effect of eccentric exercise on Akt and p70S6K phosphorylation with and without heat shock pretreatment. Sol muscles were dissected from control, EE, and HS rats 2 and 48 h after exercise. All animals were overnight-fasted and muscles were snap-frozen in liquid nitrogen. Homogenates were subjected to Western blot analysis for p-Akt/Akt (a) and p-p70/p70 (b). Data are presented as means ± SE (6–9 muscles/group). *Statistically greater than control
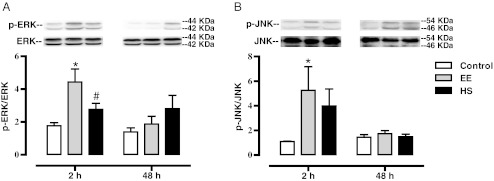
Next, we explored the effect of HS on MAPK phosphorylation following EE exercise. Figure 5 summarizes the data from Western blots of the Sol homogenates for both p-ERK1/2 and p-JNK. These results indicate that exercise performed in the EE group (p < 0.01) increased p-ERK1/2 at the 2-h time point, when compared to control. This increase returned to baseline levels by 48 h. A Pearson correlation was performed between the increased p-ERK1/2 at the 2-h time point and the expression of HSP72. We found a large negative correlation between increased pERK1/2 and HSP72/Tubulin ratios (r2 = −0.61; p < 0.05). The HS group had significantly lower p-ERK1/2 than the EE group (p < 0.05), and was not statistically different from controls 2 h following exercise. In addition, no changes in p-ERK1/2 were noted in the HS group at 48 h. Figure 5 also shows the average results of the JNK densitometry. At the 2-h time point only the EE group had an increase (p < 0.05) in p-JNK when compared to control. In the HS group, JNK phosphorylation was not statistically greater than control, less than the EE group, and was not correlated with HSP72 expression. At the 48 h post-exercise time point p-JNK had returned to control levels and there were no differences among the groups.
Fig. 5.
Effect of eccentric exercise on ERK1/2 and JNK activation with and without heat shock pretreatment. Sol muscles were dissected from control, EE, and HS rats 2 and 48 h after exercise. All animals were overnight-fasted and muscles were snap-frozen in liquid nitrogen. Homogenates were subjected to Western blot analysis for p-ERK/ERK (a) and p-JNK/JNK (b). Data are presented as means ± SE (6–9 muscles/group). *Statistically greater than control; #statistically less than EE
Discussion
Eccentric damage can be a potent stimulus to induce protein synthesis, HSP expression and stimulate muscle regeneration. Over time, these responses ultimately culminate in increased skeletal muscle hypertrophy. Because HSPs are known to provide protection from damaging stressors, facilitate protein synthesis and regulate intracellular signaling, we determined if heat shock pretreatment and the ensuing increase in HSP expression could improve early remodeling and signaling (2 and 48 h) events following a bout of damaging exercise. The novel finding of this study is that heat stress 48 h prior to damaging exercise may reduce markers of muscle damage, increase total protein concentration and MHCneo protein content. Interestingly, these enhanced protein responses in the HS group do not appear to be accompanied by increased Akt/p70s6k or MAPK signaling.
Heat shock treatment
Hyperthermic treatment in this investigation was applied to the whole body using methods that we have previously shown to be effective in inducing HSPs (Gupte et al. 2009). As previously reported, we were able to gradually increase and maintain body temperature between 41.0 and 41.5°C for 20 min. We have established that this heat treatment induces HSP72 expression 48 h following heat stress in the Sol muscle.
Muscle damage
The downhill running model we used in this investigation is well established and results in damage to the muscles of the high limb, including the Sol muscle (Tsivitse et al. 2003; Bombardier et al. 2009; Komulainen et al. 1994). Not surprisingly, we show that downhill running increased plasma levels of CK immediately (2 h) following exercise. Importantly, the HS group had significantly reduced CK (2 h) when compared to the EE group. Our results agree with previous literature in cell culture (Maglara et al. 2003) and human skeletal muscle (Skurvydas et al. 2008) that demonstrated heat shock prior to injury reduces CK release from damaged muscle fibers. In contrast to our findings, a previous study in mice found (McArdle et al. 2004b) that damaging contractions performed after heat shock (4 and 12 h) did not reduce CK release and argued against a role for HSPs as a protective countermeasure. Our results may differ from McArdle et al (2004b), as we allowed greater time for HSP accumulation prior to the exercise stressor. We also noted an increase in muscle damage via H&E staining in the EE and HS groups. Our quantitative data show significant increases in cell swelling in both the EE and HS groups. However, the HS group had a reduction in the number of mononuclear cells infiltrating the muscle cross-sections. Our histopathological findings are in agreement with previous literature demonstrating reduced immune-cell infiltration when heat shock was used prior to damaging exercise (Shima et al. 2008). The mechanism by which heat shock protects skeletal muscle from damage is currently unknown. The involvement of HSPs as a mechanism for protection against eccentric exercise has been previously suggested (Koh 2002; McHugh 2003). It is thought that HSP25 phosphorylation triggers HSP25 to translocate to damaged sacromeric proteins and stabilize or repair the cytoskeletal elements (Koh 2002; Koh and Escobedo 2004). While we observed increase p-HSP25 in both the EE and HS groups following exercise, we did not see a difference in p-HSP25 between the EE and HS groups. In addition, skeletal muscle protection has been reported in mice overexpressing HSP70 (McArdle et al. 2004a; Miyabara et al. 2006). In contrast to our p-HSP25 data, HSP72 was elevated in the HS group 2 and 48 h following exercise. This increase in HSP72 was not observed in the EE group. Collectively these data suggests that HSP72 or another heat sensitive protein (i.e., alphaB-crystallin) may play a role in mediating cytoprotection of skeletal muscle cells.
HSP expression
HSP72 has been shown to increase following eccentric damage in mouse EDL (Koh and Brooks 2001; Koh and Escobedo 2004) and human skeletal muscle (Gjovaag et al. 2006; Thompson et al. 2001). Our data show that total HSP72 expression in the EE group did not change from controls (2 and 48 h) in the Sol muscle. It is currently unclear why there were no increases in HSP72 following damaging exercise in the EE group; however, it may be related to our exercise selection. Downhill running has previously been shown to not change HSP70 protein levels in human skeletal muscle (Thompson et al. 2003). This effect may be linked to the exercise intensity (Liu et al. 2000, 2004), muscle recruitment and treadmill speed (Milne and Noble 2002) used during downhill running. The reduced HSP72 response in the EE group may also be related to an increase in p-ERK1/2 (2 h). Several studies have shown that p-ERK1/2 mediates the transcriptional suppression of HSP70 via the phosphorylation of Heat shock factor 1 (Chu et al. 1996; Knauf et al. 1996). Our data show a significant increase in p-ERK1/2 in the EE group was correlated to the decrease in HSP72 (2 h). As expected, the HS group did have increased HSP72 expression (2 and 48 h) following exercise. Given that the EE group showed no changes in HSP72 protein expression, we conclude that HSP72 induction in the HS group is principally from the heat shock treatment 48 h prior to exercise.
In addition, both the EE and HS groups increased p-HSP25 2 h after exercise. These findings are in agreement with previous data that has shown that both overload (Huey 2006) and heat stress increase p-HSP25 (van Ginneken et al. 2006). Increased p-HSP25 is seen in the soluble (cytoplasmic) fraction of muscle during muscle remodeling (Huey 2006; Vissing et al. 2009; Paulsen et al. 2009); however what role p-HSP25 plays in muscle remodeling is currently unknown. While we did not distinguish between soluble and insoluble fractions, our data further supports a role for HSP25 in muscle adaptation during loading. Because we did not see a difference in p-HSP25 between the EE and HS groups, we are unable to elucidate a role for HSP25 in regulating muscle remodeling when exercise is used in combination with heat shock. Thus, it remains possible that the roles of HSP25 and HSP72 in mediating muscle repair and regrowth are independent of each other. Finally, we did not investigate whether these changes in p-HSP25 were mediated by exercise itself or the damaging nature of the downhill running. Future studies should consider the inclusion of a level running control in an effort to address this question.
Protein concentration and MHCneo content
To begin to investigate whether the EE and HS groups had enhanced muscle remodeling, we analyzed the total protein concentrations of our muscle lysates. We noted no difference in protein concentration between the treatment groups at the 2-h time point. At this early time point, we did not expect to see increases in total protein. Because of the extent of muscle damage, it was not unreasonable to expect a decline in total protein content after exercise, as some proteins (i.e., CK) are thought to be able to diffuse out of the damaged cell. It is possible that increases in protein synthesis offset the net loss of proteins out of the cell. It is also possible that we would have seen large loses in protein content at an intermediate time point. However, one must also consider that the cytoplasm of a cell is a hydrogel (Trevors and Pollack 2005). One would not expect that membrane disruption would result in global diffusivity of proteins from an intracellular gel to an aqueous extracellular environment. In addition, diffusion of protein is complicated by steric hindrance, molecular size and weight. Moreover, it has been shown that “diffusible” proteins are co-compartmentalized and tethered to scaffolding proteins which impairs their diffusion (Kellermayer et al. 1986; Cameron et al. 1996). In addition, the myofilamental lattice has been shown to strongly impair sarcoplasmic protein diffusion (Papadopoulos et al. 2000, 2001). Lastly, it has been known for quite some time that damaged muscle fibers are able to quickly seal membrane disruptions via recruitment of dysferlin and several other proteins including Mitsugumin 53, annexin and calpain (Han 2011). Collectively, with these facts in mind, we are not surprised that there was not a dramatic decline in total protein concentrations 2 h following exercise. As muscle membranes heal and protein synthesis increases after exercise (Hernandez et al. 2000), we expected to see an increase in muscle protein concentration in the EE and HS groups. While there was a trend towards increased total protein in the EE group following exercise (48 h), it did not reach statistical significance. Although we were surprised by this finding, previous studies have shown that increased protein content can be delayed for up to 7–14 days following damage (Morioka et al. 2008; Kojima et al. 2007). Interestingly, we observed increases in total protein in the HS group (48 h). These data may suggest that heat shock prior to muscle damage increases protein synthesis, and/or prevents the loss of intracellular proteins following damage. For example, Goto et al. (2003) reported cellular protein concentrations were increased in L6 muscle cells that were preconditioned with heat stress prior to mechanical stress, suggesting that heat shock increases protein synthesis. In addition, it has been shown that HSPs immediately respond to eccentric exercise by binding to cytoskeletal/myofibrillar proteins, which may stabilize the sarcomere (Paulsen et al. 2007a, b, 2009) and ultimately stop the global diffusion of proteins out of damaged myofibrils.
To further investigate whether the EE and HS groups had enhanced muscle remodeling, we analyzed MHCneo content. MHCneo is induced by contractile activity (Cerny and Bandman 1986) and is a standard marker for regeneration and muscle hypertrophy (Paul and Rosenthal 2002). We were unable to observe changes in MHCneo content in the EE group (2 and 48 h). Previous work has shown that increases in other myosin isoforms are delayed until 3 to 7 days after muscle loading (Smith et al. 1999; Frier and Locke 2007). Thus it is possible that we needed to extend our time course to see these changes in the EE group. In contrast, we report for the first time that heat stress prior to eccentric exercise significantly enhances the expression of MHCneo. We did not study the isolated effect of heat shock alone on skeletal muscle protein concentration and MHCneo content. Therefore it remains possible that heat shock treatment itself induced these changes in protein concentration and MHCneo content, independent of the interaction with exercise. Future studies would benefit from the inclusion of a heat shock only treatment group to control for these issues.
It has been shown in cell culture that combing heat stress with mechanical stress results in greater protein changes than using either stressor alone (Goto et al. 2003). It is possible that the Sol muscles in the HS group may have a more efficient rate of regeneration due to an increased expression of HSP72. It has been suggested that the upregulation of HSP72 has hypertrophic effects on skeletal muscle (Goto et al. 2007) and that HSF-1 null mice with reduced HSP72 expression have impaired regrowth of skeletal muscle (Yasuhara et al. 2011). Interestingly, we report increased markers of regeneration in the HS group that had elevated HSP72, and see little evidence of regeneration in the EE group that did not have significant elevations in HSP72. Collectively, these data support the hypothesis that heat stress, and/or the ensuing increase in HSP expression can contribute to muscle remodeling (Kojima et al. 2007). In contrast these findings, it has shown that heat shock can reduce protein concentration and Type I MHC content accumulation 3, 5, and 7 days following functional overload (Frier and Locke 2007). The different results of these studies strongly suggest a need for more work specifically directed at understanding the role of HSP induction in muscle recovery.
Protein signaling (Atk and MAPK pathways)
The Akt/p70s6k pathway plays an important role in regulating skeletal muscle protein synthesis and thus hypertrophy (Bodine et al. 2001). In the present study, p-Akt 2 h was not increased in the EE group (at 2 or 48 h). These findings confirm previously published studies showing p-Akt was unaffected by eccentric exercise (Eliasson et al. 2006; Zou et al. 2011). Interestingly, while there was no change in p-Akt at 2 h, by 48 h the HS group had a significant reduction in p-Akt. Previous studies have shown that acute heat stress can immediately increase signaling through the Akt pathway (Wei and Vander Heide 2008; Jurivich et al. 1991; Oehler-Janne et al. 2008; Lin et al. 1997). Akt is a major anti-apoptotic/pro-survival kinase, which is known to be under the regulation of HSP72 (Koren et al. 2010). It is possible that since the animals in the HS group had reduced markers of muscle damage, they also had a reduced need to maintain activation of a pro-survival kinase.
Next, we looked at the downstream effector p70s6k, a key regulator of translation initiation and protein synthesis. Two hours following exercise, both the EE and HS groups had an increase in p-p70s6k, which returned to control levels by 48 h. Our results agree with previous data showing that eccentric contractions increase p-p70s6k (Zou et al. 2011; Spangenburg and McBride 2006). It has previously been shown that heat shock during a bout of resistance training does increase the phosphorylation of p70s6k anymore that exercise alone in human skeletal muscle (Kakigi et al. 2011). Together, these findings suggest that Akt and p70s6k may not be important regulators of heat shock induced muscle regrowth following damaging exercise.
We also examined the phosphorylation state of the MAPK’s, including ERK1/2 and JNK as MAPK signaling is critical regulator of skeletal muscle mass (Shi et al. 2009). The levels of phosphorylated ERK1/2 and JNK have been shown to increase following exercise (Thompson et al. 2003; Williamson et al. 2003) and these MAPK’s are important mediators of the heat shock response (Kline and Morimoto 1997). We noted a strong attenuation of p-ERK1/2 in the HS group at 2 h. Importantly, ERK1/2 is known to be a repressor of HSP synthesis via an association with heat shock transcription factor 1 (HSF1) (Wang et al. 2004). This may help explain the differences in HSP72 expression between EE and HS groups at the 2-h time point. In this case, an increase in p-ERK1/2 in the EE group correlated with an impaired HSP72 response in the EE group (2 h). Conversely, significant attenuation of p-ERK1/2 in the HS group corresponded with increased HSP72 expression in the HS group (2 h). Exercise alone increased p-JNK, but we noted no significant treatment effect in the HS group. We have previously shown that heat shock, particularly the induction of HSP72, is able to inhibit JNK activation (Gupte et al. 2011). It is plausible that elevated levels of HSP72 in the HS group prevented an increase in p-JNK similar to those seen in the EE group. These findings suggest that ERK1/2 phosphorylation may influence HSP72 expression; however, activation of the MAPK signaling pathway does not explain our increases in total protein concentration or MHCneo content in the HS group.
After analysis of critical portions of signaling pathways that mediate muscle remodeling and protein synthesis, we are currently unable to provide a mechanism for these changes in protein concentration and MHCneo content. We hypothesize that the recruitment of satellite cells can help explain our changes. Heat stress has been shown to trigger activation of satellite cells (Oishi et al. 2009; Halevy et al. 2001). In fact, it has been suggested that the liberation of satellite cells could serve as the source of MHCneo (Singh et al. 1999). Therefore, future studies are needed to elucidate the role of satellite cells, and other heat shock sensitive pathways (NF-KappaB and calcineurin) during hyperthermic treatment of damaged skeletal muscle.
Conclusion
In summary, our conclusions fall in line with the majority of studies that have demonstrated elevation of HSPs can have a positive effect on skeletal muscle. Our data lend strength to the hypothesis that increased HSP expression prior to muscle damage may protect muscle fibers from injury. In addition, we suggest that heat shock prior to damaging exercise may facilitate recovery from exercise by increasing the total protein concentration and the expression of MHCneo. Interestingly, these increases in protein content were not associated with increased signaling through the Akt or MAPK pathways. Our findings are consistent with the idea that heat stress prior to damaging exercise may facilitate recovery and/or accelerate the repair of injured muscle fibers. Although we hypothesize that heat shock prior to damaging exercise is a useful aid to improve skeletal muscle recovery, further research is needed to uncover the mechanisms by which heat shock and HSPs may contribute to skeletal muscle regrowth and regeneration.
Acknowledgments
We thank Dr. Michael J. Wacker for technical assistance and useful scientific discussions during the preparation of this manuscript.
Footnotes
P.C. Geiger and P.M. Gallagher served as principal investigators for this study.
References
- Armand AS, Launay T, Gaspera BD, Charbonnier F, Gallien CL, Chanoine C. Effects of eccentric treadmill running on mouse soleus: degeneration/regeneration studied with Myf-5 and MyoD probes. Acta Physiol Scand. 2003;179(1):75–84. doi: 10.1046/j.1365-201X.2003.01187.x. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54(1):80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276(1 Pt 1):C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Beck SC, Maio A. Stabilization of protein synthesis in thermotolerant cells during heat shock. Association of heat shock protein-72 with ribosomal subunits of polysomes. J Biol Chem. 1994;269(34):21803–21811. [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bombardier E, Vigna C, Iqbal S, Tiidus PM, Tupling AR. Effects of ovarian sex hormones and downhill running on fiber-type-specific HSP70 expression in rat soleus. J Appl Physiol. 2009;106(6):2009–2015. doi: 10.1152/japplphysiol.91573.2008. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Burkin DJ, Kaufman SJ. Alpha7beta1-integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol. 2006;290(6):C1660–C1665. doi: 10.1152/ajpcell.00317.2005. [DOI] [PubMed] [Google Scholar]
- Butterfield TA, Best TM. Stretch-activated ion channel blockade attenuates adaptations to eccentric exercise. Med Sci Sports Exerc. 2009;41(2):351–356. doi: 10.1249/MSS.0b013e318187cffa. [DOI] [PubMed] [Google Scholar]
- Cameron IL, Hardman WE, Fullerton GD, Miseta A, Koszegi T, Ludany A, Kellermayer M. Maintenance of ions, proteins and water in lens fiber cells before and after treatment with non-ionic detergents. Cell Biol Int. 1996;20(2):127–137. doi: 10.1006/cbir.1996.0017. [DOI] [PubMed] [Google Scholar]
- Carlson CJ, Fan Z, Gordon SE, Booth FW. Time course of the MAPK and PI3-kinase response within 24 h of skeletal muscle overload. J Appl Physiol. 2001;91(5):2079–2087. doi: 10.1152/jappl.2001.91.5.2079. [DOI] [PubMed] [Google Scholar]
- Cerny LC, Bandman E. Contractile activity is required for the expression of neonatal myosin heavy chain in embryonic chick pectoral muscle cultures. J Cell Biol. 1986;103(6 Pt 1):2153–2161. doi: 10.1083/jcb.103.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271(48):30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- Eliasson J, Elfegoun T, Nilsson J, Kohnke R, Ekblom B, Blomstrand E. Maximal lengthening contractions increase p70 S6 kinase phosphorylation in human skeletal muscle in the absence of nutritional supply. Am J Physiol Endocrinol Metab. 2006;291(6):E1197–E1205. doi: 10.1152/ajpendo.00141.2006. [DOI] [PubMed] [Google Scholar]
- Frier BC, Locke M. Heat stress inhibits skeletal muscle hypertrophy. Cell Stress Chaperones. 2007;12(2):132–141. doi: 10.1379/CSC-233R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjovaag TF, Vikne H, Dahl HA. Effect of concentric or eccentric weight training on the expression of heat shock proteins in m. biceps brachii of very well trained males. Eur J Appl Physiol. 2006;96(4):355–362. doi: 10.1007/s00421-005-0084-6. [DOI] [PubMed] [Google Scholar]
- Goto K, Kojima A, Morioka S, Naito T, Akema T, Matsuba Y, Fujiya H, Sugiura T, Ohira Y, Yoshioka T. Geranylgeranylaceton induces heat shock protein 72 in skeletal muscle cells. Biochem Biophys Res Commun. 2007;358(1):331–335. doi: 10.1016/j.bbrc.2007.04.129. [DOI] [PubMed] [Google Scholar]
- Goto K, Okuyama R, Sugiyama H, Honda M, Kobayashi T, Uehara K, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflugers Arch. 2003;447(2):247–253. doi: 10.1007/s00424-003-1177-x. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58(3):567–578. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol. 2011;110(2):451–457. doi: 10.1152/japplphysiol.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O, Krispin A, Leshem Y, McMurtry JP, Yahav S. Early-age heat exposure affects skeletal muscle satellite cell proliferation and differentiation in chicks. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R302–R309. doi: 10.1152/ajpregu.2001.281.1.R302. [DOI] [PubMed] [Google Scholar]
- Han R. Muscle membrane repair and inflammatory attack in dysferlinopathy. Skelet Muscle. 2011;1(1):10. doi: 10.1186/2044-5040-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiol Scand. 1991;143(2):177–185. doi: 10.1111/j.1748-1716.1991.tb09219.x. [DOI] [PubMed] [Google Scholar]
- Hernandez JM, Fedele MJ, Farrell PA. Time course evaluation of protein synthesis and glucose uptake after acute resistance exercise in rats. J Appl Physiol. 2000;88(3):1142–1149. doi: 10.1152/jappl.2000.88.3.1142. [DOI] [PubMed] [Google Scholar]
- Huey KA. Regulation of HSP25 expression and phosphorylation in functionally overloaded rat plantaris and soleus muscles. J Appl Physiol. 2006;100(2):451–456. doi: 10.1152/japplphysiol.01022.2005. [DOI] [PubMed] [Google Scholar]
- Jurivich DA, Chung J, Blenis J. Heat shock induces two distinct S6 protein kinase activities in quiescent mammalian fibroblasts. J Cell Physiol. 1991;148(2):252–259. doi: 10.1002/jcp.1041480210. [DOI] [PubMed] [Google Scholar]
- Kakigi R, Naito H, Ogura Y, Kobayashi H, Saga N, Ichinoseki-Sekine N, Yoshihara T, Katamoto S. Heat stress enhances mTOR signaling after resistance exercise in human skeletal muscle. J Physiol Sci. 2011;61(2):131–140. doi: 10.1007/s12576-010-0130-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer M, Ludany A, Jobst K, Szucs G, Trombitas K, Hazlewood CF. Cocompartmentation of proteins and K+ within the living cell. Proc Natl Acad Sci U S A. 1986;83(4):1011–1015. doi: 10.1073/pnas.83.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline MP, Morimoto RI. Repression of the heat shock factor 1 transcriptional activation domain is modulated by constitutive phosphorylation. Mol Cell Biol. 1997;17(4):2107–2115. doi: 10.1128/mcb.17.4.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf U, Newton EM, Kyriakis J, Kingston RE. Repression of human heat shock factor 1 activity at control temperature by phosphorylation. Genes Dev. 1996;10(21):2782–2793. doi: 10.1101/gad.10.21.2782. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Goto K, Kojima A, Akema T, Uehara K, Aoki H, Sugiura T, Ohira Y, Yoshioka T. Possible role of calcineurin in heating-related increase of rat muscle mass. Biochem Biophys Res Commun. 2005;331(4):1301–1309. doi: 10.1016/j.bbrc.2005.04.096. [DOI] [PubMed] [Google Scholar]
- Koh TJ. Do small heat shock proteins protect skeletal muscle from injury? Exerc Sport Sci Rev. 2002;30(3):117–121. doi: 10.1097/00003677-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Brooks SV. Lengthening contractions are not required to induce protection from contraction-induced muscle injury. Am J Physiol Regul Integr Comp Physiol. 2001;281(1):R155–R161. doi: 10.1152/ajpregu.2001.281.1.R155. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Escobedo J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am J Physiol Cell Physiol. 2004;286(3):C713–C722. doi: 10.1152/ajpcell.00341.2003. [DOI] [PubMed] [Google Scholar]
- Kojima A, Goto K, Morioka S, Naito T, Akema T, Fujiya H, Sugiura T, Ohira Y, Beppu M, Aoki H, Yoshioka T. Heat stress facilitates the regeneration of injured skeletal muscle in rats. J Orthop Sci. 2007;12(1):74–82. doi: 10.1007/s00776-006-1083-0. [DOI] [PubMed] [Google Scholar]
- Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. doi: 10.1042/0264-6021:3510289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komulainen J, Kytola J, Vihko V. Running-induced muscle injury and myocellular enzyme release in rats. J Appl Physiol. 1994;77(5):2299–2304. doi: 10.1152/jappl.1994.77.5.2299. [DOI] [PubMed] [Google Scholar]
- Koren J, 3rd, Jinwal UK, Jin Y, O’Leary J, Jones JR, Johnson AG, Blair LJ, Abisambra JF, Chang L, Miyata Y, Cheng AM, Guo J, Cheng JQ, Gestwicki JE, Dickey CA. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J Biol Chem. 2010;285(4):2498–2505. doi: 10.1074/jbc.M109.057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Bruce CR, Spurrell BE, Hawley JA. Effect of training on activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase pathways in rat soleus muscle. Clin Exp Pharmacol Physiol. 2002;29(8):655–660. doi: 10.1046/j.1440-1681.2002.03713.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Preheating accelerates mitogen-activated protein (MAP) kinase inactivation post-heat shock via a heat shock protein 70-mediated increase in phosphorylated MAP kinase phosphatase-1. J Biol Chem. 2005;280(13):13179–13186. doi: 10.1074/jbc.M410059200. [DOI] [PubMed] [Google Scholar]
- Lin RZ, Hu ZW, Chin JH, Hoffman BB. Heat shock activates c-Src tyrosine kinases and phosphatidylinositol 3-kinase in NIH3T3 fibroblasts. J Biol Chem. 1997;272(49):31196–31202. doi: 10.1074/jbc.272.49.31196. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lormes W, Baur C, Opitz-Gress A, Altenburg D, Lehmann M, Steinacker JM. Human skeletal muscle HSP70 response to physical training depends on exercise intensity. Int J Sports Med. 2000;21(5):351–355. doi: 10.1055/s-2000-3784. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lormes W, Wang L, Reissnecker S, Steinacker JM. Different skeletal muscle HSP70 responses to high-intensity strength training and low-intensity endurance training. Eur J Appl Physiol. 2004;91(2–3):330–335. doi: 10.1007/s00421-003-0976-2. [DOI] [PubMed] [Google Scholar]
- Locke M. The cellular stress response to exercise: role of stress proteins. Exerc Sport Sci Rev. 1997;25:105–136. doi: 10.1249/00003677-199700250-00007. [DOI] [PubMed] [Google Scholar]
- Locke M. Heat shock protein accumulation and heat shock transcription factor activation in rat skeletal muscle during compensatory hypertrophy. Acta Physiol (Oxf) 2008;192(3):403–411. doi: 10.1111/j.1748-1716.2007.01764.x. [DOI] [PubMed] [Google Scholar]
- Maglara AA, Vasilaki A, Jackson MJ, McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J Physiol. 2003;548(Pt 3):837–846. doi: 10.1113/jphysiol.2002.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18(2):355–357. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004;561(Pt 1):233–244. doi: 10.1113/jphysiol.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MP. Recent advances in the understanding of the repeated bout effect: the protective effect against muscle damage from a single bout of eccentric exercise. Scand J Med Sci Sports. 2003;13(2):88–97. doi: 10.1034/j.1600-0838.2003.02477.x. [DOI] [PubMed] [Google Scholar]
- Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol. 2002;93(2):561–568. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- Miyabara EH, Martin JL, Griffin TM, Moriscot AS, Mestril R. Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am J Physiol Cell Physiol. 2006;290(4):C1128–C1138. doi: 10.1152/ajpcell.00399.2005. [DOI] [PubMed] [Google Scholar]
- Morioka S, Goto K, Kojima A, Naito T, Matsuba Y, Akema T, Fujiya H, Sugiura T, Ohira Y, Beppu M, Aoki H, Yoshioka T. Functional overloading facilitates the regeneration of injured soleus muscles in mice. J Physiol Sci. 2008;58(6):397–404. doi: 10.2170/physiolsci.RP004008. [DOI] [PubMed] [Google Scholar]
- Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88(1):359–363. doi: 10.1152/jappl.2000.88.1.359. [DOI] [PubMed] [Google Scholar]
- Nollen EA, Morimoto RI. Chaperoning signaling pathways: molecular chaperones as stress-sensing ‘heat shock’ proteins. J Cell Sci. 2002;115(Pt 14):2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- O’Neill DE, Aubrey FK, Zeldin DA, Michel RN, Noble EG. Slower skeletal muscle phenotypes are critical for constitutive expression of Hsp70 in overloaded rat plantaris muscle. J Appl Physiol. 2006;100(3):981–987. doi: 10.1152/japplphysiol.00831.2005. [DOI] [PubMed] [Google Scholar]
- Oehler-Janne C, Bueren AO, Vuong V, Hollenstein A, Grotzer MA, Pruschy M. Temperature sensitivity of phospho-Ser(473)-PKB/AKT. Biochem Biophys Res Commun. 2008;375(3):399–404. doi: 10.1016/j.bbrc.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Ogata T, Oishi Y, Roy RR, Ohmori H. Effects of T3 treatment on HSP72 and calcineurin content of functionally overloaded rat plantaris muscle. Biochem Biophys Res Commun. 2005;331(4):1317–1323. doi: 10.1016/j.bbrc.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Yamada S, Sugiura T, Ohira Y, Yoshioka T, Goto K. A possible role of NF-kappaB and HSP72 in skeletal muscle hypertrophy induced by heat stress in rats. Gen Physiol Biophys. 2010;29(3):234–242. doi: 10.4149/gpb_2010_03_234. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Hayashida M, Tsukiashi S, Taniguchi K, Kami K, Roy RR, Ohira Y. Heat stress increases myonuclear number and fiber size via satellite cell activation in rat regenerating soleus fibers. J Appl Physiol. 2009;107(5):1612–1621. doi: 10.1152/japplphysiol.91651.2008. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Ogata T, Ohira Y, Taniguchi K, Roy RR. Calcineurin and heat shock protein 72 in functionally overloaded rat plantaris muscle. Biochem Biophys Res Commun. 2005;330(3):706–713. doi: 10.1016/j.bbrc.2005.03.049. [DOI] [PubMed] [Google Scholar]
- Oishi Y, Taniguchi K, Matsumoto H, Ishihara A, Ohira Y, Roy RR. Muscle type-specific response of HSP60, HSP72, and HSC73 during recovery after elevation of muscle temperature. J Appl Physiol. 2002;92(3):1097–1103. doi: 10.1152/japplphysiol.00739.2001. [DOI] [PubMed] [Google Scholar]
- Papadopoulos S, Endeward V, Revesz-Walker B, Jurgens KD, Gros G. Radial and longitudinal diffusion of myoglobin in single living heart and skeletal muscle cells. Proc Natl Acad Sci U S A. 2001;98(10):5904–5909. doi: 10.1073/pnas.101109798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos S, Jurgens KD, Gros G. Protein diffusion in living skeletal muscle fibers: dependence on protein size, fiber type, and contraction. Biophys J. 2000;79(4):2084–2094. doi: 10.1016/S0006-3495(00)76456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise G, McKinnell IW, Rudnicki MA. Muscle satellite cell and atypical myogenic progenitor response following exercise. Muscle Nerve. 2008;37(5):611–619. doi: 10.1002/mus.20995. [DOI] [PubMed] [Google Scholar]
- Paul AC, Rosenthal N. Different modes of hypertrophy in skeletal muscle fibers. J Cell Biol. 2002;156(4):751–760. doi: 10.1083/jcb.200105147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Lauritzen F, Bayer ML, Kalhovde JM, Ugelstad I, Owe SG, Hallen J, Bergersen LH, Raastad T. Subcellular movement and expression of HSP27, alphaB-crystallin, and HSP70 after two bouts of eccentric exercise in humans. J Appl Physiol. 2009;107(2):570–582. doi: 10.1152/japplphysiol.00209.2009. [DOI] [PubMed] [Google Scholar]
- Paulsen G, Vissing K, Kalhovde JM, Ugelstad I, Bayer ML, Kadi F, Schjerling P, Hallen J, Raastad T (2007a) Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol [DOI] [PubMed]
- Paulsen G, Vissing K, Kalhovde JM, Ugelstad I, Bayer ML, Kadi F, Schjerling P, Hallen J, Raastad T. Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R844–R853. doi: 10.1152/ajpregu.00677.2006. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70(6):2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Sasai N, Agata N, Inoue-Miyazu M, Kawakami K, Kobayashi K, Sokabe M, Hayakawa K. Involvement of PI3K/Akt/TOR pathway in stretch-induced hypertrophy of myotubes. Muscle Nerve. 2010;41(1):100–106. doi: 10.1002/mus.21473. [DOI] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97(20):10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL. Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol. 2007;102(4):1702–1707. doi: 10.1152/japplphysiol.00722.2006. [DOI] [PubMed] [Google Scholar]
- Shi H, Scheffler JM, Zeng C, Pleitner JM, Hannon KM, Grant AL, Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 2009;296(5):C1040–C1048. doi: 10.1152/ajpcell.00475.2008. [DOI] [PubMed] [Google Scholar]
- Shima Y, Kitaoka K, Yoshiki Y, Maruhashi Y, Tsuyama T, Tomita K. Effect of heat shock preconditioning on ROS scavenging activity in rat skeletal muscle after downhill running. J Physiol Sci. 2008;58(5):341–348. doi: 10.2170/physiolsci.RP004808. [DOI] [PubMed] [Google Scholar]
- Singh MA, Ding W, Manfredi TJ, Solares GS, O’Neill EF, Clements KM, Ryan ND, Kehayias JJ, Fielding RA, Evans WJ. Insulin-like growth factor I in skeletal muscle after weight-lifting exercise in frail elders. Am J Physiol. 1999;277(1 Pt 1):E135–E143. doi: 10.1152/ajpendo.1999.277.1.E135. [DOI] [PubMed] [Google Scholar]
- Skurvydas A, Kamandulis S, Stanislovaitis A, Streckis V, Mamkus G, Drazdauskas A. Leg immersion in warm water, stretch-shortening exercise, and exercise-induced muscle damage. J Athl Train. 2008;43(6):592–599. doi: 10.4085/1062-6050-43.6.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HK, Maxwell L, Rodgers CD, McKee NH, Plyley MJ. Exercise-enhanced satellite cell proliferation and new myonuclear accretion in rat skeletal muscle. J Appl Physiol. 2001;90(4):1407–1414. doi: 10.1152/jappl.2001.90.4.1407. [DOI] [PubMed] [Google Scholar]
- Smith HK, Plyley MJ, Rodgers CD, McKee NH. Expression of developmental myosin and morphological characteristics in adult rat skeletal muscle following exercise-induced injury. Eur J Appl Physiol Occup Physiol. 1999;80(2):84–91. doi: 10.1007/s004210050562. [DOI] [PubMed] [Google Scholar]
- Smolka MB, Zoppi CC, Alves AA, Silveira LR, Marangoni S, Pereira-Da-Silva L, Novello JC, Macedo DV. HSP72 as a complementary protection against oxidative stress induced by exercise in the soleus muscle of rats. Am J Physiol Regul Integr Comp Physiol. 2000;279(5):R1539–R1545. doi: 10.1152/ajpregu.2000.279.5.R1539. [DOI] [PubMed] [Google Scholar]
- Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat Cell Biol. 2001;3(3):276–282. doi: 10.1038/35060068. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, McBride TA. Inhibition of stretch-activated channels during eccentric muscle contraction attenuates p70S6K activation. J Appl Physiol. 2006;100(1):129–135. doi: 10.1152/japplphysiol.00619.2005. [DOI] [PubMed] [Google Scholar]
- Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Med Sci Sports Exerc. 1988;20(5 Suppl):S132–S134. doi: 10.1249/00005768-198810001-00008. [DOI] [PubMed] [Google Scholar]
- Thompson HS, Maynard EB, Morales ER, Scordilis SP. Exercise-induced HSP27, HSP70 and MAPK responses in human skeletal muscle. Acta Physiol Scand. 2003;178(1):61–72. doi: 10.1046/j.1365-201X.2003.01112.x. [DOI] [PubMed] [Google Scholar]
- Thompson HS, Scordilis SP, Clarkson PM, Lohrer WA. A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiol Scand. 2001;171(2):187–193. doi: 10.1046/j.1365-201x.2001.00795.x. [DOI] [PubMed] [Google Scholar]
- Trevors JT, Pollack GH. Hypothesis: the origin of life in a hydrogel environment. Prog Biophys Mol Biol. 2005;89(1):1–8. doi: 10.1016/j.pbiomolbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Tsivitse SK, McLoughlin TJ, Peterson JM, Mylona E, McGregor SJ, Pizza FX. Downhill running in rats: influence on neutrophils, macrophages, and MyoD + cells in skeletal muscle. Eur J Appl Physiol. 2003;90(5–6):633–638. doi: 10.1007/s00421-003-0909-0. [DOI] [PubMed] [Google Scholar]
- Uehara K, Goto K, Kobayashi T, Kojima A, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T, Aoki H. Heat-stress enhances proliferative potential in rat soleus muscle. Jpn J Physiol. 2004;54(3):263–271. doi: 10.2170/jjphysiol.54.263. [DOI] [PubMed] [Google Scholar]
- Ginneken MM, Graaf-Roelfsema E, Keizer HA, Dam KG, Wijnberg ID, Kolk JH, Breda E. Effect of exercise on activation of the p38 mitogen-activated protein kinase pathway, c-Jun NH2 terminal kinase, and heat shock protein 27 in equine skeletal muscle. Am J Vet Res. 2006;67(5):837–844. doi: 10.2460/ajvr.67.5.837. [DOI] [PubMed] [Google Scholar]
- Vissing K, Bayer ML, Overgaard K, Schjerling P, Raastad T. Heat shock protein translocation and expression response is attenuated in response to repeated eccentric exercise. Acta Physiol (Oxf) 2009;196(3):283–293. doi: 10.1111/j.1748-1716.2008.01940.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Grammatikakis N, Siganou A, Stevenson MA, Calderwood SK. Interactions between extracellular signal-regulated protein kinase 1, 14-3-3epsilon, and heat shock factor 1 during stress. J Biol Chem. 2004;279(47):49460–49469. doi: 10.1074/jbc.M406059200. [DOI] [PubMed] [Google Scholar]
- Wei H, Vander Heide RS. Heat stress activates AKT via focal adhesion kinase-mediated pathway in neonatal rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;295(2):H561–H568. doi: 10.1152/ajpheart.00401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547(Pt 3):977–987. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara K, Ohno Y, Kojima A, Uehara K, Beppu M, Sugiura T, Fujimoto M, Nakai A, Ohira Y, Yoshioka T, Goto K (2011) Absence of heat shock transcription factor 1 retards the regrowth of atrophied soleus muscle in mice. J Appl Physiol. doi:10.1152/japplphysiol.00471.2011 [DOI] [PubMed]
- Zhang BT, Yeung SS, Allen DG, Qin L, Yeung EW. Role of the calcium–calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol. 2008;105(1):352–357. doi: 10.1152/japplphysiol.90320.2008. [DOI] [PubMed] [Google Scholar]
- Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD (2011) The {alpha}7{beta}1 integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol. doi:10.1152/japplphysiol.00081.2011 [DOI] [PubMed]