Abstract
Objective
The safety and efficacy of laparoscopic adrenalectomy has been well documented and it has become the standard of care for benign adrenal pathology, and small (<5cm) pheochromocytomas. Its applicability for pheochromocytomas larger than 6 cm has been questioned, due to concerns including cardiovascular complications, malignancy, and recurrence. The aim of this study is to determine if laparoscopic adrenalectomy in patients without radiologic evidence of cancer, compromises the peri-operative and long-term outcomes of large ≥6 cm pheochromocytomas.
Methods
We analyzed a prospective adrenal database of 163 consecutive patients who underwent adrenalectomy at our institution between September 2000 and September 2010, under an Institutional Review Board approved protocol. Patients diagnosed with a pheochromocytoma, who underwent laparoscopic adrenalectomy (LA) were investigated. Patients with tumors <6 cm (SmPheo) were compared to those presenting with tumors ≥6 cm (LgPheo).
Results
Twenty-five patients presented with 26 pheochromocytomas. There were 52% females with a mean age of 53±3. The mean tumor size was 5.2±0.5cm and 11 (42%) pheochromocytomas were ≥6 cm. The tumor size was significantly different between the LgPheo and the SmPheo groups (7.6±0.4 vs. 3.6±0.4 cm, p<0.001) but there was no significant difference in intra-operative complications, estimated blood loss, cancer diagnosis, or recurrence. The length of stay was comparative amongst the two cohorts and there were no incidents of capsular invasion or adverse cardiovascular events.
Conclusion
Laparoscopic adrenalectomy of pheochromocytomas larger than 6 cm is feasible and safe with comparable results to smaller ones.
Keywords: pheochromocytomas, laparoscopic adrenalectomy, outcomes, recurrence, malignant
INTRODUCTION
Laparoscopic adrenalectomy was first described by Gagner et al, in 1992, and is now considered the gold standard for the treatment of benign adrenal pathologies (1–3). In contrast to other adrenal tumors, pheochromocytomas present as a management challenge, due to their size, vascularity, and demanding pre-, intra-, and post-operative care, secondary to their hormonal secretion. A cancer diagnosis is also of concern, as 5–6% of pheochromocytomas are malignant (4–6). As a result, a diagnosis of pheochromocytoma was once considered a contraindication for laparoscopy. Improved peri-operative management, understanding of the pathophysiology of the disease, and advances made in laparoscopic surgery, to maximize visualization and minimize gland manipulation, have resulted in laparoscopic adrenalectomy becoming the standard of care for small pheochromocytomas. The progress made in minimally invasive surgery has allowed it to be expanded to larger tumors, metastatic malignancies, and the presentation of bilateral pathology (7–12). Lastly, the intra-abdominal insufflation and the hypercapnia and acidosis resulting from the CO2 pneumoperitoneum required for laparoscopic surgery, may increase serum catecholamine levels and hypertension, but not necessarily result in a higher incidence of adverse peri-operative cardiovascular events, over time (13, 14).
Large pheochromocytomas present a two-fold challenge. First, large adrenal tumors, regardless of their pathology, have traditionally been considered a relative contraindication for minimally invasive surgery (15–17). Concerns regarding distorted anatomy, and increased gland manipulation play a role in advocating open surgery for larger adrenal lesions. Second, size has traditionally played a key role in the diagnosis of malignancy in pheochromocytomas, and it has been purported that all malignant adrenal tumors are best treated with open surgery (18). Both of these concerns have been challenged over the years. As surgeons have gained experience with the resection of larger adrenal tumors, it seems that size is no longer a contraindication for laparoscopic surgery. The concern for increased incidence of local recurrence following laparoscopic resection of malignant adrenal tumors has also been a matter of debate, and currently small, localized malignant tumors with no evidence of local invasion, may be treated via the minimally invasive approach with similar outcome. Recent studies with long term follow up suggest that minimally invasive surgery for small pheochromocytomas results in similar outcomes as open surgery, with respect to intra-operative hypertension, blood loss, post-operative biochemical cure and malignant recurrence rates (2, 12, 15, 19–22). The aim of this study is to investigate the short and long term outcomes of laparoscopic resection of large (≥ 6 cm) pheochromocytomas, without obvious pre-operative radiographic evidence of extra-adrenal extension, resected in a single tertiary referral institution.
METHODS
A review of the University of Wisconsin Prospective Adrenal Surgery Database was performed including all patients who had undergone adrenal resection between September 2000 and September 2010. Institutional approval was obtained from the local institutional review board.
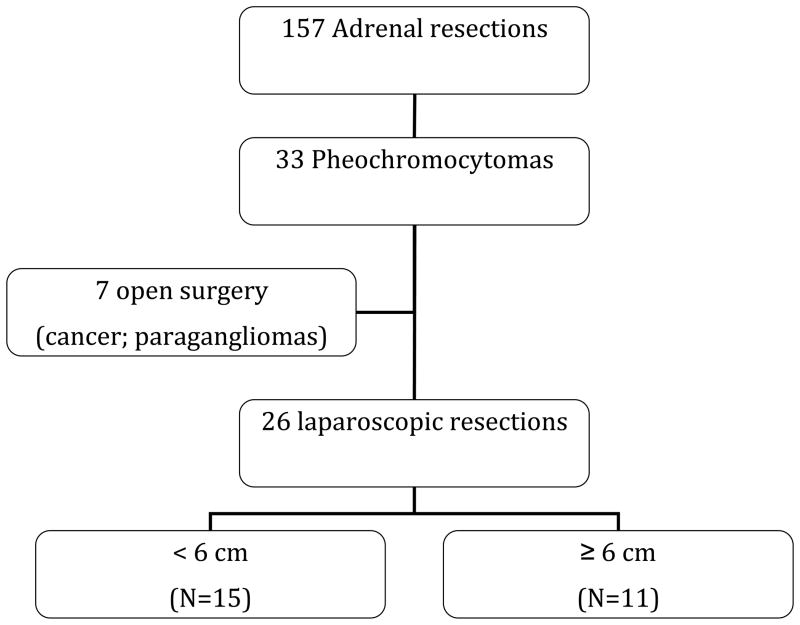
This study included patients with the diagnosis of pheochromocytoma based on biochemical laboratory values and radiographic images, and confirmed by final pathology. Patients with other adrenal pathologies were excluded. Seven patients who underwent open operations for pheochromocytoma were also excluded. The reasons for open surgery in these seven patients were suspicion for malignancy (n=1) and extra-adrenal paraganglioma (n=6). Figure 1 is a summary of the adrenal resections performed for pathologies, with a focus on pheochromocytomas. Data were collected on patient demographics, pre-operative evaluation (including biochemical hormonal status and imaging studies), operative narrative, and postoperative course. Specific attention was paid to intra-operative course, including hemodynamic status, complications, estimated blood loss and conversion from laparoscopic to open surgery. All operations were performed at a tertiary care hospital by experienced laparoscopic endocrine surgeons. Patient charts were investigated to correctly identify the final pathology, including tumor size and malignancy status, as well as post-operative short and long term complications. Patients were grouped according to the tumor size. Patients with tumors <6 cm (SmPheo) were compared to those with tumors ≥6 cm (LgPheo).
Figure 1.
Outline of adrenalectomies performed for pheochromocytomas
To identify differences between the two size groups in the laparoscopic cohort, univariate analysis with t-tests were used. Data are expressed as mean ± SEM, unless otherwise specified. Statistical calculations were completed using statistical software SPSS version 17 (SPSS, Inc., Chicago, IL) and a p value <0.05 was considered to represent statistical significance for all comparisons.
PRE-OPERATIVE CARE
All patients who presented with pre-operative signs and symptoms of catecholamine excess received alpha-adrenergic blockade for at least two weeks prior to surgery. Concomitant additional oral fluid and salt intake were encouraged with the institution of alpha blockade therapy. Patients with persistent tachycardia, despite these measures, were supplemented with either beta- or calcium channel blockade. Patients were also treated with intravenous volume loading in the pre-operative holding area in order to increase intra-vascular volume. Intra-operatively, all patients had an arterial line and 2 large-bore peripheral intravenous lines or a central venous catheter placed, prior to the induction of anesthesia.
SURGICAL TECHNIQUE
The adrenalectomies were performed laparoscopically through a lateral decubitus transperitoneal approach. Briefly, the patient is placed in the lateral decubitus position and a pneumoperitoneum is established to a pressure of 15 mmHg. A 30-degree laparoscope is inserted into the peritoneal cavity, and two or three additional trocars are introduced in the right subcostal space, from the mid-clavicular line to the posterior axillary line.
For tumors located on the right side, the liver is mobilized, lifted, and gently retracted for safe access to the vena cava and adrenal vein. The edge of the adrenal gland is identified and small tributaries to the adrenal gland are divided with ultrasonic shears. The adrenal vein is identified and divided between clips. The remaining retroperitoneal attachments are dissected with ultrasonic shears. The adrenal gland with the tumor is then removed in a bag via the 10-mm port and the tumor is examined and cut on the back table, to ensure inclusion of the entire tumor prior to submission to pathology.
For left sided tumors, the left colon is taken down to gain access to the spleen. With the patient slightly rotated to the right, the spleen is medially retracted along with the distal pancreas. Similarly, ultrasonic shears are used to divide small adrenal vessels and the adrenal vein is divided between clips. The remaining dissection follows the description above.
POST-OPERATIVE CARE
Our routine post-operative monitoring includes two hours in post-anesthesia care unit (PACU) with arterial line in place and continuous monitoring of the heart rate and blood pressure. From PACU patients are transferred to the surgical floor and complete 23 hour admission (short stay). Surgical intensive care unit is available at our institution for patients with labile blood pressure or for those who cannot be extubated; however, none of the patients in this cohort required this safety measure. Post-operative hypotension may result from decreased sympathetic tone, intraopertaive bleeding, and inadequate intraopertaive intravenous fluids. Such hypotension episodes are initially treated with intravenous fluid bolus and only rarely sympathomimetic medications are required. When intra-operative bleeding is encountered patients may also require blood transfusion. Patients are discharged after they are ambulating, tolerating a regular diet, and have established adequate pain control. Follow-up consists of an office visit 7 to 10 days post-operatively, and subsequently, as needed. For those patients from remote areas of the state, they maintain follow up appointments with their primary care physician. Long-term follow up includes frequent blood pressure monitoring for the first year, then yearly thereafter. Plasma metanephrines are measured annually. Urinary metanephrine levels and abdominal imaging were obtained only in those patients with recurrent or poorly managed hypertension, elevated plasma metanephrines, malignant lesions, or paragangliomas.
RESULTS
One hundred and sixty three adrenalectomies were performed between September 2000 and September 2010 at the University of Wisconsin. Of these, 25 patients underwent laparoscopic adrenalectomy for resection of 26 adrenal pheochromocytomas – one patient presented with bilateral tumors. The mean age for the entire cohort was 54±3 years and there were 13 (52%) females. The localization of the adrenal mass was documented in all patients on CT or MRI scans and elevated urinary or plasma metanephrines confirmed the diagnosis, and all tumors were functional. Of the entire cohort 4 (16%) patients had the diagnosis of multiple endocrine neoplasia (MEN) 2A and one (4%) patient had neurofibromatosis (NF1). All four patients with MEN 2A presented with their pheochromocytoma years after being diagnosed with medullary thyroid cancer, and having their thyroid gland removed.
Twenty-three (88%) patients presented with hypertension and 7 (28%) patients had documented coronary artery disease. All patients were placed on alpha adrenergic blocker systemic therapy prior to surgery and 10 (40%) were also treated with beta adrenergic or calcium channel blockers. The average size of the adrenal masses was 5.2±0.5 cm (range 1.5–9 cm) and they were evenly distributed with 15 (57.7%) presenting on the left side. Intra-operative complications were documented in 3 (12%) patients and included a splenic capsular tear, a liver laceration, and entrance into normal adrenal tissue. There were no conversions to open operations. The diagnosis of benign pheochromocytoma was confirmed in all patients. The mean hospital length of stay was 1.3±0.2 days, and no post-operative complications were documented. None of the patients had recurrence on a mean follow of 53±7 months.
Laparoscopic adrenalectomy (<6 vs. ≥6 cm)
Of the 26 pheochromocytomas that were laparoscopically resected, 15 (57.7%) tumors were <6cm and 11 (42.3%) tumors were ≥ 6cm. Table 1 summarizes the differences between the groups. There were no differences in age, gender, or tumor location. There was one complication in the SmPheo group (entrance into normal adrenal gland) and two in the LgPheo group (liver laceration and splenic capsular tear), and median intra-operative blood loss was higher in the LgPheo group (150 versus 100 ml, p=0.04). Both groups had no post-operative complication and there was no evidence of recurrence or malignancy. The mean length of stay was also similar in both groups.
Table 1.
Comparison of peri-operative factors for laparoscopic adrenalectomy for pheochromocytomas <6 cm versus ≥6 cm:
| Variable | SmPheo (<6 cm) (N=15) | LgPheo (>6 cm) (N=11) | p value |
|---|---|---|---|
| Age (yrs) | 51±3 | 57±4 | NS |
| Gender (% female) | 53% | 45% | NS |
| Tumor location (% left) | 53% | 64% | NS |
| Mean tumor size (cm) | 3.4±0.3 | 7.6±0.4 | <0.01 |
| Intraoperative complications | 1 | 2 | NS |
| Median EBL (ml, range) | 100 (50–150) | 150 (50–1200) | 0.04 |
| pRBC transfusion | 0 | 2 | NS |
| Postoperative complications | 0 | 0 | NS |
| Mean length of stay (days) | 1±0 | 2±1 | NS |
| Malignancy | 0 | 0 | NS |
| Recurrence | 0 | 0 | NS |
| Mean follow-up (months) | 50±8 | 55±11 | NS |
| Cardiovascular data | |||
| Mean SBP (mmHg) | 142±9 | 154±8 | NS |
| Mean DBP (mmHg) | 87±5 | 82±5 | NS |
| Mean pulse (beats/minute) | 82±4 | 86±5 | NS |
| Intraoperative hypotension | 2 (13.3%) | 4 (36.4%) | 0.03 |
| Intraoperative hypertension | 0 | 2 (18.2%) | NS |
EBL – estimated blood loss, pRBC – packed red blood cells, SBP – systolic blood pressure, DBP – diastolic blood pressure
Persistent intra-operative hypo- and hypertension were defined as systolic blood pressure <90 and ≥180 mmHg, respectively and lasting for more than 5 minutes. Hypertension occurred in one (9%) patient with 6 cm pheochromocytoma in the LgPheo group. The overall incidence of intra-operative hypotension was significantly higher in the LgPheo cohort (36.4% versus 13.3%, p=0.03) and the majority of these patients were successfully managed with additional intravenous fluids only. One patient in each cohort required the anesthesiologists to administer medication (i.e. intravenous adrenergic agonists), in addition to volume resuscitation, to elevate and maintain the systolic blood pressure ≥ 90mmHg (9% vs. 7%, p=0.45). The higher intra-operative blood loss and hypotension in the LgPheo group was the result of one patient requiring a wedge resection of his kidney with the pheochromocytoma (1200ml) and another patient with coagulopathy secondary to advanced liver cirrhosis (1000ml). Overall, there were no major operative complications, three minor operative complications (one splenic capsular tear, one hepatic tear and entrance into normal adrenal tissue), and no post-operative complications. None of the patients in either group had any adverse peri-operative cardiovascular events.
DISCUSSION
The results of our study reveal no disadvantages in employing the laparoscopic approach to large (≥6 cm) pheochromocytomas. In our series, there was no difference in intra- operative hypertension, length of stay, or post-operative complications. Patients who presented with larger tumors, had a similar immediate post-operative recovery as the SmPheo group. There were no incidents of capsular invasion, irrespective of size, and to date, there have been no recurrences or cancer diagnoses. Our patients also did not experience any adverse cardiovascular events (myocardial infarction or cerebrovascular accident). Minor liver and spleen tears were encountered in two patients.
The benefits of laparoscopic surgery are many including decreased length of stay, faster functional recovery, early ambulation, safety, decreased peri-operative morbidity, and similar rate of conversion and morbidity to other adrenal tumors, despite their average larger size (1, 2, 4, 23). These benefits refer to laparoscopic adrenalectomy for all adrenal pathologies, including pheochromocytomas. Some studies have cited an upper size limit of 12 cm to 14 cm for adrenal tumors that are resected laparoscopically (24, 25). However, due to the catecholamine surge and possible adverse peri-operative cardiovascular events, the upper limit for pheochromocytomas has traditionally been much smaller (5, 9). Using the 6 cm cutoff for performing laparoscopic adrenalectomy we would have deprived 42.3% of our patients the benefits of a minimally invasive approach. Similarly, a cutoff of 8 cm would deny 23% of our patients the same benefits.
Cardiovascular instability due to excessive catecholamine release caused by CO2 pneumoperitoneum and/or dissection, has always been a concern, irrespective of the surgical approach. In Gagner’s initial report, he cited 58% intra-operative hypertension (SBP > 200mmHg) and 53% hypotension (SBP <90mmHg) in 1996 (1). Despite this being a new frontier in surgery, his results were favorable to those of the traditional open approach (26). Our results show continued improvement compared with the literature. With the use of more stringent criteria for hypertension (SBP ≥180mmHg) only 8% experienced intra-operative hypertension and only 23% had hypotension. One patient in the LgPheo group did require intra-operative intravenous anti-hypertensive medication for his hypertension, and two patients (one in each group) required pharmacologic pressure support, in addition to volume resuscitation, for their hypotension. Only one patient in the LgPheo group required transfusion of blood products.
Cardiovascular instability is at the core of intra-operative complications in patients who present with pheochromocytomas, requiring continuous invasive monitoring by both anesthesia and surgical teams, with pharmacologic intervention when warranted, and minimal tumor manipulation. Several studies have shown that tumor manipulation is the most significant intra-operative stimulus for catecholamine release during both open and laparoscopic approaches (13, 27–29). This is where operative experience with larger pheochromocytomas can make a difference in outcome. Rocha et al. reported that even with early adrenal vein ligation, it is most likely the vascularity that results in the large amount of hormone release (13). Even though we did not measure intra-operative catecholamine levels, our strategy of minimal direct manipulation of both the tumor and the adrenal gland and early venous control suggests that this surgical technique employed by experienced surgeons results in low incidence of catecholamine-induced cardiovascular instability.
It has been suggested that an increased pheochromocytoma size is associated with increased risk of malignancy. We believe that any sign of suspected malignancy on pre-operative imaging should result in open surgery. None of the laparoscopic adrenalectomies performed was for cancer, as the only one malignant pheochromocytoma was suspected pre-operatively and that patient underwent an open operation. In this case, the capsule was preserved, and there has been no evidence of recurrence. Despite undergoing the open procedure, this patient did not experience any significant morbidity or mortality. The benefits of performing an open adrenalectomy in the face of malignant pheochromocytoma include safer removal of the cancer, without risking malignant pheochromocytomatosis, and the ability to evaluate metastases.
Our study has a few limitations. First, it is a retrospective study on a prospective database. Due to the rare incidence of large pheochromocytomas it is unlikely that a prospective randomized trial will ever be conducted. The small number of patients in our study is comparable to other studies, some of which are multi-institutional and/or include other adrenal pathologies (1, 21, 30). Despite the length of the study, where some patients have at least a five year follow-up, the rare occurrence of these tumors may require an even longer follow up. Our exclusion of patients with paragangliomas or open surgical resection resulted in selection bias and no known malignancy in our cohort. The aim of this study was to assess the role of laparoscopic surgery in large adrenal pheochromocytomas and the inclusion of paragangliomas and patients with initial open surgery was not appropriate to the study design. Lastly, the small number of patients in this study does not allow more than two subgroups and therefore our conclusions are limited to the 6 cm cutoff.
The extremely small incidence of pheochromocytomas also limited our ability to evaluate malignant pheochromocytomas. One patient presented with locally invasive tumor, which was identified on pre-operative imaging, and another presented with a recurrence of her initial pheochromocytoma from five years earlier. Both of these patients underwent open resection, due to the invasiveness of the tumors.
CONCLUSION
Minimal peri-operative morbidity, mortality, and long-term eradication of endocrinopathy support the minimally invasive approach for patients with pheochromocytomas ≥ 6 cm without pre-operative suspicion of malignancy. This can be achieved by experienced endocrine surgeons and correct pre and intra-operative patient management.
Synopsis.
The aim of this study is to investigate the long-term oncologic outcomes, morbidity and mortality following laparoscopic resection of 6 cm and larger pheochromocytomas. Cancer diagnosis, recurrence and morbidity incidence were similar, despite the size difference. The data suggest that tumor size should not be the sole deterrent to laparoscopic resection of larger pheochromocytomas.
Acknowledgments
We thank Nicholas Yeutter for his contribution to the data collection and editing of this paper.
Supported by NIH/NCI Supplemental Grant RO1CA12115-S1
References
- 1.Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327:1033. doi: 10.1056/NEJM199210013271417. [DOI] [PubMed] [Google Scholar]
- 2.Smith CD, Weber CJ, Amerson JR. Laparoscopic adrenalectomy: new gold standard. World J Surg. 1999;23:389–396. doi: 10.1007/pl00012314. [DOI] [PubMed] [Google Scholar]
- 3.Vargas HI, Kavoussi LR, Bartlett DL, et al. Laparoscopic adrenalectomy: a new standard of care. Urology. 1997;49:673–678. doi: 10.1016/s0090-4295(97)00083-6. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelm SM, Prinz RA, Barbu AM, Onders RP, Solorzano CC. Analysis of large versus small pheochromocytomas: operative approaches and patient outcomes. Surgery. 2006;140:553–559. doi: 10.1016/j.surg.2006.07.008. discussion 9–60. [DOI] [PubMed] [Google Scholar]
- 5.Toniato A, Boschin IM, Opocher G, Guolo A, Pelizzo M, Mantero F. Is the laparoscopic adrenalectomy for pheochromocytoma the best treatment? Surgery. 2007;141:723–727. doi: 10.1016/j.surg.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Kalady MF, McKinlay R, Olson JA, Jr, et al. Laparoscopic adrenalectomy for pheochromocytoma. A comparison to aldosteronoma and incidentaloma. Surg Endosc. 2004;18:621–625. doi: 10.1007/s00464-003-8827-0. [DOI] [PubMed] [Google Scholar]
- 7.Heniford BT, Arca MJ, Walsh RM, Gill IS. Laparoscopic adrenalectomy for cancer. Semin Surg Oncol. 1999;16:293–306. doi: 10.1002/(sici)1098-2388(199906)16:4<293::aid-ssu4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Kebebew E, Siperstein AE, Clark OH, Duh QY. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasms. Arch Surg. 2002;137:948–951. doi: 10.1001/archsurg.137.8.948. discussion 52–3. [DOI] [PubMed] [Google Scholar]
- 9.Novitsky YW, Czerniach DR, Kercher KW, Perugini RA, Kelly JJ, Litwin DE. Feasibility of laparoscopic adrenalectomy for large adrenal masses. Surg Laparosc Endosc Percutan Tech. 2003:13106–110. doi: 10.1097/00129689-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Imai T, Kikumori T, Shibata A, Fujiwara M, Hibi Y, Nakao A. Laparoscopic adrenalectomy for incidentaloma and bilateral adrenal disease. Asian J Surg. 2003;26:64–70. doi: 10.1016/S1015-9584(09)60223-2. [DOI] [PubMed] [Google Scholar]
- 11.Hasan R, Harold KL, Matthews BD, Kercher KW, Sing RF, Heniford BT. Outcomes for laparoscopic bilateral adrenalectomy. J Laparoendosc Adv Surg Tech A. 2002;12:233–236. doi: 10.1089/109264202760267989. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez R, Smith CD, McClusky DA, 3rd, et al. Laparoscopic approach reduces likelihood of perioperative complications in patients undergoing adrenalectomy. Am Surg. 2004;70:668–674. [PubMed] [Google Scholar]
- 13.Flavio Rocha M, Faramarzi-Roques R, Tauzin-Fin P, Vallee V, Leitao de Vasconcelos PR, Ballanger P. Laparoscopic surgery for pheochromocytoma. Eur Urol. 2004;45:226–232. doi: 10.1016/j.eururo.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 14.de La Chapelle A, Deghmani M, Dureuil B. Peritoneal insufflation can be a critical moment in the laparoscopic surgery of pheochromocytoma. Ann Fr Anesth Reanim. 1998;17:1184–1185. doi: 10.1016/s0750-7658(00)80020-9. [DOI] [PubMed] [Google Scholar]
- 15.Cheah WK, Clark OH, Horn JK, Siperstein AE, Duh QY. Laparoscopic adrenalectomy for pheochromocytoma. World J Surg. 2002;26:1048–1051. doi: 10.1007/s00268-002-6669-x. [DOI] [PubMed] [Google Scholar]
- 16.Inabnet WB, Pitre J, Bernard D, Chapuis Y. Comparison of the hemodynamic parameters of open and laparoscopic adrenalectomy for pheochromocytoma. World J Surg. 2000;24:574–578. doi: 10.1007/s002689910094. [DOI] [PubMed] [Google Scholar]
- 17.Staren ED, Prinz RA. Adrenalectomy in the era of laparoscopy. Surgery. 1996;120:706–709. doi: 10.1016/s0039-6060(96)80020-1. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 18.Winfield HN, Hamilton BD, Bravo EL, Novick AC. Laparoscopic adrenalectomy: the preferred choice? A comparison to open adrenalectomy. J Urol. 1998;160:325–329. doi: 10.1016/s0022-5347(01)62884-2. [DOI] [PubMed] [Google Scholar]
- 19.Brunt LM. The positive impact of laparoscopic adrenalectomy on complications of adrenal surgery. Surg Endosc. 2002;16:252–257. doi: 10.1007/s00464-001-8302-8. [DOI] [PubMed] [Google Scholar]
- 20.Jaroszewski DE, Tessier DJ, Schlinkert RT, et al. Laparoscopic adrenalectomy for pheochromocytoma. Mayo Clin Proc. 2003;78:1501–1504. doi: 10.4065/78.12.1501. [DOI] [PubMed] [Google Scholar]
- 21.Kim AW, Quiros RM, Maxhimer JB, El-Ganzouri AR, Prinz RA. Outcome of laparoscopic adrenalectomy for pheochromocytomas vs aldosteronomas. Arch Surg. 2004;139:526–529. doi: 10.1001/archsurg.139.5.526. discussion 9–31. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Sippel RS, O'Dorisio MS, Vinik AI, Lloyd RV, Pacak K. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39:775–783. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyer-Rochow GY, Soon PS, Delbridge LW, et al. Outcomes of minimally invasive surgery for phaeochromocytoma. ANZ J Surg. 2009;79:367–370. doi: 10.1111/j.1445-2197.2009.04891.x. [DOI] [PubMed] [Google Scholar]
- 24.Liao CH, Chueh SC, Lai MK, Hsiao PJ, Chen J. Laparoscopic adrenalectomy for potentially malignant adrenal tumors greater than 5 centimeters. J Clin Endocrinol Metab. 2006;91:3080–3083. doi: 10.1210/jc.2005-2420. [DOI] [PubMed] [Google Scholar]
- 25.Shen WT, Sturgeon C, Clark OH, Duh QY, Kebebew E. Should pheochromocytoma size influence surgical approach? A comparison of 90 malignant and 60 benign pheochromocytomas. Surgery. 2004;136:1129–1137. doi: 10.1016/j.surg.2004.05.058. [DOI] [PubMed] [Google Scholar]
- 26.van Heerden JA, Sheps SG, Hamberger B, Sheedy PF, 2nd, Poston JG, ReMine WH. Pheochromocytoma: current status and changing trends. Surgery. 1982;91:367–373. [PubMed] [Google Scholar]
- 27.Fernandez-Cruz L, Saenz A, Taura P, Sabater L, Astudillo E, Fontanals J. Helium and carbon dioxide pneumoperitoneum in patients with pheochromocytoma undergoing laparoscopic adrenalectomy. World J Surg. 1998;22:1250–1255. doi: 10.1007/s002689900554. [DOI] [PubMed] [Google Scholar]
- 28.Feldman JM, Blalock JA, Fagraeus L, Miller JN, Farrell RE, Wells SA., Jr Alterations in plasma norepinephrine concentration during surgical resection of pheochromocytoma. Ann Surg. 1978;188:758–768. doi: 10.1097/00000658-197812000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marty J, Desmonts JM, Chalaux G, et al. Hypertensive responses during operation for phaeochromocytoma: a study of plasma catecholamine and haemodynamic changes. Eur J Anaesthesiol. 1985;2:257–264. [PubMed] [Google Scholar]
- 30.Kercher KW, Novitsky YW, Park A, Matthews BD, Litwin DE, Heniford BT. Laparoscopic curative resection of pheochromocytomas. Ann Surg. 2005;241:919–926. doi: 10.1097/01.sla.0000164175.26785.06. discussion 26–8. [DOI] [PMC free article] [PubMed] [Google Scholar]