Abstract
Problem
Human uterine macrophages must maintain an environment hospitable to implantation and pregnancy and simultaneously provide protection against pathogens. Although macrophages comprise a significant portion of leukocytes within the uterine endometrium, the activation profile and functional response of these cells to endotoxin is unknown.
Method of Study
Flow cytometric analysis of surface receptors and intracellular markers expressed by macrophages isolated from human endometria was performed. Uterine macrophages were stimulated with LPS. Cytokines, chemokines, and growth factors expressed by these cells were analyzed using Bio-Plex analysis.
Results
CD163high human endometrial macrophages constitutively secrete both pro- and anti-inflammatory cytokines as well as pro-angiogenic factors and secretion of these factors is LPS-inducible.
Conclusions
A major population of human uterine macrophages is alternatively activated. These cells secrete factors in response to LPS that are involved in the activation of immune responses and tissue homeostasis.
Keywords: Macrophage, endometrium, CD163, M2
Introduction
The uterine endometrium is an immunologically unique mucosal site, as it must simultaneously protect against pathogens and tolerate allogeneic sperm and a semi-allogeneic fetus. In this regard, uterine macrophages play a key role in ensuring immune defense within the endometrium, as they recognize invading microbes and elaborate a broad range of chemokines and cytokines in response to pathogenic challenge.
Macrophages function as key effectors of both innate and humoral immunity, as they actively phagocytose foreign molecules and display antigens on their surface for recognition by T lymphocytes. The phenotype and function of tissue macrophages are affected by and uniquely dependent on the cellular milieu to which they are exposed, including local cytokines, chemokines and other biological effector molecules, as well as extracellular matrix and cellular components. Thus, the local microenvironment plays a significant role in macrophage activation and polarization [1]. In this regard, previous studies have demonstrated that macrophages derived from unique anatomical sites, including mucosal tissue (intestines), adipose tissue and alveolar tissue, possess properties and activation states that are distinct (reviewed in [2]). Despite the importance of uterine macrophages in the regulation of immunity within the uterine endometrium, relatively little is known about the influence of the uterine microenvironment on human uterine macrophage polarization and activation.
Polarized macrophages can be broadly classified as either “classically activated” (M1) or “alternatively activated” (M2), which represent the extremes of a continuum of functional states [3]. M1 macrophages are elicited through stimulation with IFN-γ, select cytokines such as GM-CSF and TNF-α, and microbial products such as LPS. These cells are considered microbicidal and pro-inflammatory and have a high capacity for antigen presentation and elevated production of reactive oxygen intermediates (ROI). In contrast, M2 macrophages are cells that have been activated by any means other than those used to generate M1 macrophages. Recent studies have expanded the definition of M2 macrophages to many subsets, including M2a, M2b and M2c. M2 cells are diverse—they are activated by different types of stimuli and elaborate distinct cytokines and chemokines following stimulation. Despite these differences, M2 macrophages share some common features. In general, M2 macrophages are poorly microbicidal and exhibit decreased expression of ROI, MHC Class II and IL-12, but have enhanced expression of anti-inflammatory mediators such as IL-10 (reviewed in [4]). Significantly, M1 macrophages are thought to initiate humoral immunity, while M2 macrophages are considered key players in angiogenesis, the resolution of inflammation and the coordination of tissue repair following an acute inflammatory reaction (reviewed in [3, 5, 6]. M2 polarized macrophages can be further characterized in terms of their activation status. M2a and M2c produce low levels of pro-inflammatory cytokines and high levels of IL-10. In contrast, M2b macrophages, which are activated by TLR agonists, produce high levels of pro-inflammatory IL-1, TNFα and IL-6 in addition to anti-inflammatory IL-10 [3].
In addition to their role in host immune defense, macrophages also have an active part in maintaining endometrial tissue homeostasis. Macrophage density in the human endometrium increases during the secretory stage of the menstrual cycle in preparation for menstruation, where macrophages help mediate tissue breakdown through expression of degrading enzymes like matrix metalloproteinases (MMPs) [7, 8]. As phagocytes, macrophages participate in clearance of the shed endometrial lining [7]. Since macrophages have a well-defined role in wound healing and angiogenesis [9], they may also contribute to regeneration of the endometrial lining and angiogenesis through secretion of growth and angiogenic factors.
Although macrophages comprise approximately 10 percent of the total leukocyte population within the human endometrium [10, 11], the activation profile of these cells is largely unknown. To date, most studies involving endometrial macrophages rely on identification of these cells by expression of CD68 [8, 12, 13] or CD14 [11, 14-16]. Although expression of these molecules is enriched in macrophages, recent studies have shown that CD68 and CD14 are also expressed by other cell types. Indeed, CD68 immunoreactivity has been detected in both myeloid and non-myeloid cell types, including dendritic cells, NK cell, basophils and endothelial cells and fibroblasts [17-19]. Isolation of CD68+ cells from tissue is difficult because this marker is expressed intracellularly. Moreover, although CD14 is expressed largely on monocytes and macrophages, granulocytes also express low levels of CD14 [20].
In contrast, the scavenger receptor CD163 is a surface molecule expressed exclusively on monocytes and macrophages [21-23] and is a marker of M2 macrophages [5, 24-27]. CD163 is an endocytic receptor for hemoglobin-haptoglobin complexes that mediates the clearance of free hemoglobin and minimizes oxidative tissue damage [28]. CD163 is expressed by mature tissue macrophages [29, 30] and CD163+ cells are present during the healing phase of acute inflammation [30, 31].
Because of the role that macrophages play in immune defense and the importance of macrophages in angiogenesis and tissue remodeling, we hypothesized that human uterine macrophages would be M2 or alternatively activated. In the present study, we demonstrate the human endometrial macrophages are predominantly CD163+, a marker of M2 macrophages. Flow cytometric analysis of this previously uncharacterized uterine macrophage population demonstrated that these cells also express CD14 and CD68, as well as the co-stimulatory molecules CD40, CD80 and CD86. Because infection within the endometrium has serious negative consequences on reproductive success, we determined the responsiveness of CD163+ human endometrial macrophages to TLR stimulation. We now report that human uterine endometrial macrophages produce both pro- and anti-inflammatory mediators as well as high levels of pro-angiogenic factors, indicating that these cells are characteristic of the alternatively activated M2b phenotype.
Methods
Human uterine endometrial tissues and blood
Endometrial tissue was obtained immediately following hysterectomy for non-endometrial pathology from female patients who had given informed consent. A summary of the patient population is provided in Table 1. Menstrual cycle staging of endometrial tissues was determined in accordance with accepted histologic practice using hematoxylin/eosin-stained paraffin sections. Approval to use human tissues was obtained from the Dartmouth Institutional Review Board in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services. Uterine endometrial-donor-matched PBMCs were isolated from heparinized whole blood with Ficoll-Hypaque (d=1.077). Monocytes were purified from mononuclear cell fractions as described by Mentzer et al.[32]. Monocyte purity was >95% as determined by CD14 expression using flow cytometry (data not shown). To generate M1-or M2-polarized macrophages, monocytes were incubated with either 10 ng/ml GM-CSF or 100 ng/ml M-CSF for 7 days [33, 34].
Table 1.
| Patient # | Age (years) | Diagnosis | Staging |
|---|---|---|---|
| 4960 | 39 | Fibroids | Inactive |
| 4970 | 44 | Pelvic Pain | Unknown |
| 4975 | 42 | Prolapse | Proliferative |
| 4983 | 39 | Prolapse | Secretory |
| 5034 | 40 | Colonic Inertia | Secretory |
| 5045 | 52 | Prolapse | Unknown |
| 5815 | 40 | Prolapse | Proliferative |
| 5845 | 47 | Prolapse | Secretory |
| 6048 | 37 | Menorrhagia | Proliferative |
| 6078 | 41 | Fibroids | Proliferative |
| 6084 | 44 | Pelvic Pain | Secretory |
| 6098 | 44 | Fibroids | Secretory |
Two-color immunophenotyping and confocal scanning laser microscopy
Six tissue sections were dissected and stained as previously described [35]. Sections were maintained in ice-cold PBS throughout processing to prevent internalization of surface markers and immunofluorescent staining was performed immediately after cutting. Two mg/100 ml each of CD68-FITC (Thermo Scientific, clone KiM7) and CD14-PE (Caltag, clone TUK4) or CD163-PE (Trillium Diagnostics, clone MAC2-158) were used to stain sections. C ytoplasmic staining with antibody specific for CD68 was achieved by fixing cells with paraformaldehyde, followed by treatment with saponin. Fixed and permeabilized tissue was incubated with specific or control antibody. Stained sections were wet-mounted in anti-fade (Molecular Probes, Eugene, OR), sealed with nail varnish, and stored at 4°C in the dark for up to 10 days before confocal imaging.
Immunofluorescently labeled sections were optically sectioned using a Zeiss LSM5-10 Confocal Scanning Laser Microscope System. Two-color fluorescent sections were evaluated for the presence of CD68 and/or CD14 or CD163. Unstained and fluorochrome isotype controls were used to control for auto-fluorescence and non-specific antibody binding, respectively.
Uterine endometrial tissue digestion and macrophage isolation
Endometrial tissue sections were processed as described by White et al. [36]. Briefly, tissue sections were minced and incubated in an enzyme cocktail containing final concentrations of 3.4 mg/ml pancreatin (Gibco), 0.1 mg/ml hyaluronidase (Worthington) and 1.6 mg/ml collagenase I (Worthington) in Hank’s Buffered Saline Solution (HBSS) containing 2 mg/ml D-glucose (Sigma) at 37°C for 2 hours. Following digestion, cells were dispersed by straining through a 250 μm mesh screen and washed with HBSS. Tissue cells were stained and fixed for flow cytometric analysis. Prior to macrophage isolation, dead cells were removed from the culture using the dead cell removal kit (Miltenyi). Cell viability was assessed by trypan blue exclusion, which ranged between 80% and 95%. Cells were incubated with biotinylated anti-CD163 mAb (Maine Biotechnology) for one hour at room temperature. Following thorough washing, cells were incubated with strepavadin-conjugated magnetic microbeads (Miltenyi) and passed over a magnetic column. The CD163+ cells retained on the column were eluted and used in LPS stimulation assays. In control studies, peripheral blood monocyte-matured macrophages were incubated with the enzyme cocktail used to digest uterine endometrial tissue. No effect was observed on surface expression of MHC Class II, CD80, CD86, CD40, TLR4, CD14, CD16, and CD163, consistent with results reported by White et al [36].
Flow cytometry
To assess surface expression of macrophage markers, endometrial tissue cells were stained for two-color flow cytometry with anti-CD163 and antibodies to CD14, MHC-II (HLA-DR), CD16, TLR4, CD40, CD80 or CD86. Surface marker expression of control blood-derived macrophages was analyzed using single color flow cytometry. To assess CD68 expression in macrophages, cells were initially stained for surface expression of CD14 or CD163, fixed in 1% MFF and permeabilized in saponin, and then stained intracellularly for CD68. Table 2 lists the fluorochrome-conjugated antibodies utilized in flow cytometric analysis of intracellular and surface marker expression. Cells were analyzed using CellQuest software and a FACScan flow cytometer (BD Biosciences).
Table 2.
| Antigen | Fluorochrome | Source | Antibody Clone |
|---|---|---|---|
| CD14 | FITC | Medarex | AML223 |
| CD16 | FITC | Medarex | 3G8 |
| CD40 | PE | R and D Systems | 82111 |
| CD68 | FITC | Thermo Scientific | Kim7 |
| CD80 | FITC | R and D Systems | 37711 |
| CD86 | PE | Beckman Coulter | HA5.2B7 |
| CD163 | PE | Maine Biotechnology | Mac 2-158 |
| CD163 | FITC | Maine Biotechnology | Mac 2-158 |
| MHC-II | FITC | R and D Systems | L203 |
| TLR4 | PE | R and D Systems | 267518 |
LPS stimulation, Bio-Plex assay, and ELISA
CD163+ isolated endometrial macrophages or blood-derived monocyte-differentiated macrophages were plated at 2×105 cells per well in 24-well tissue culture dishes in complete RPMI. Cells were either unstimulated or treated with 10 ng/ml ultrapure E. coli LPS (Invitrogen), for 24 hours. Cell-free culture supernatants were aliquoted and stored at −80°C until further use. The Bio-Plex suspension array system using fluorescently dyed Luminex microspheres (beads) was used (Bio-Rad, Hercules, CA) to determine the responsiveness of endometrial macrophages to LPS stimulation. This assay system is ideally suited to measure multiple cytokines from one sample. Standards were prepared in the same fresh medium that was used for culturing experimental samples and were assayed in triplicate. Spiked controls accurately reflected the added cytokine, chemokine or growth factor concentration. Assays were performed in a 96-well filtration plate (Millipore, Billerica, MA). The fluorescence intensity for each bead was measured using the Bio-Plex array reader, and Bio-Plex Manager software with five-parametric-curve fitting was used for data analysis. The level of detection of each analyte was 7.8 pg/ml. Analysis of IL-10, IL-6, and IL-12p40 in control blood-derived monocyte-matured macrophages was performed by ELISA (R and D Systems, Minneapolis, MN).
Results
CD163 is widely expressed on uterine endometrial macrophages
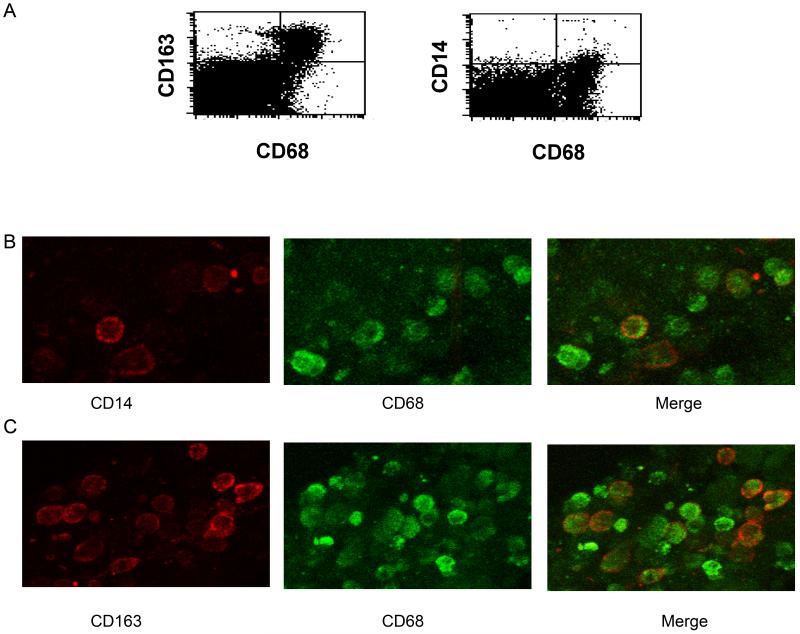
Although macrophages have been identified immunohistochemically in the human uterine endometrium on the basis of CD14 and CD68 expression [37], no studies to date have determined the polarization of primary human non-pregnant uterine macrophages. Flow cytometric analysis was used to discern the activation profile of macrophages within the human non-pregnant uterine endometrium. Single cell suspensions of whole uterine endometrial tissue digests were stained for the pan-macrophage marker CD68 and for CD14, which is enriched in M1 macrophages or CD163, which is highly expressed by M2 macrophages. CD163 was expressed on 71.9% of CD68+ cells, indicating that CD163 is widely expressed on human endometrial tissue macrophages (Figure 1A). As demonstrated in Figure 1A, CD14+ cells represented a minor population of CD68+ cells (10.3%). Low expression of CD14 was not attributable to proteolytic cleavage of this molecule from the cell surface during cell isolation, as previous work indicates that the methodology used for tissue dispersal in this study does not cleave surface expression of CD14 from macrophages [36]. Confocal imaging confirmed that uterine endometrial macrophages express CD163 and imaging analysis corroborates flow cytometric findings that indicate more uterine macrophages are CD163high compared with CD14high (Figures 1B and 1C). The high level of CD163 expression on CD68+ macrophages suggests that these cells are polarized toward an alternatively activated or M2 phenotype.
Figure 1. CD163 is highly expressed by uterine macrophages.
Freshly obtained uterine endometrial tissue sections were enzymatically digested into single cell suspensions, stained for CD68 and CD163 or CD68 and CD14 (A) and analyzed by flow cytometry. The numbers inset in the histograms are the percentage of CD163+/CD68+ or CD14+/CD68+ cells. (B and C) Confocal imaging of uterine endometrial macrophages. Endometrial tissues were sectioned and stained for either (B) CD14 and CD68 or (C) CD163 and CD68. Images shown are representative of tissue sections from six individuals.
To validate the characterization of M1 and M2 polarization in these cells, we performed control studies in parallel using immunophenotypic analysis of donor-matched peripheral blood monocytes that were differentiated with either GM-CSF or M-CSF. Differentiation of monocytes with these cytokines permits good discrimination of M1 vs. M2 activation [5]. Results of flow cytometric analysis of surface marker expression on blood-derived macrophages are summarized in Table 3. Cells were also stimulated with LPS and expression of IL-6, IL-10, and IL-12p40 was evaluated by ELISA to verify macrophage polarization. As expected, GM-CSF-differentiated cells produced high levels of IL-6 and IL-12p40, while M-CSF-differentiated macrophages secreted enhanced levels of anti-inflammatory IL-10 (data not shown).
Table 3.
| Measurement | M1 Mφ | M2 Mφ |
|---|---|---|
| CD163 | + | ++++ |
| CD14 | +++ | + |
| CD16 | +/- | +++ |
| CD40 | ++ | +++ |
| CD80 | +++ | ++ |
| CD86 | +++ | + |
| MHC-II | +++ | + |
| TLR4 | +++ | ++ |
Uterine macrophage surface expression
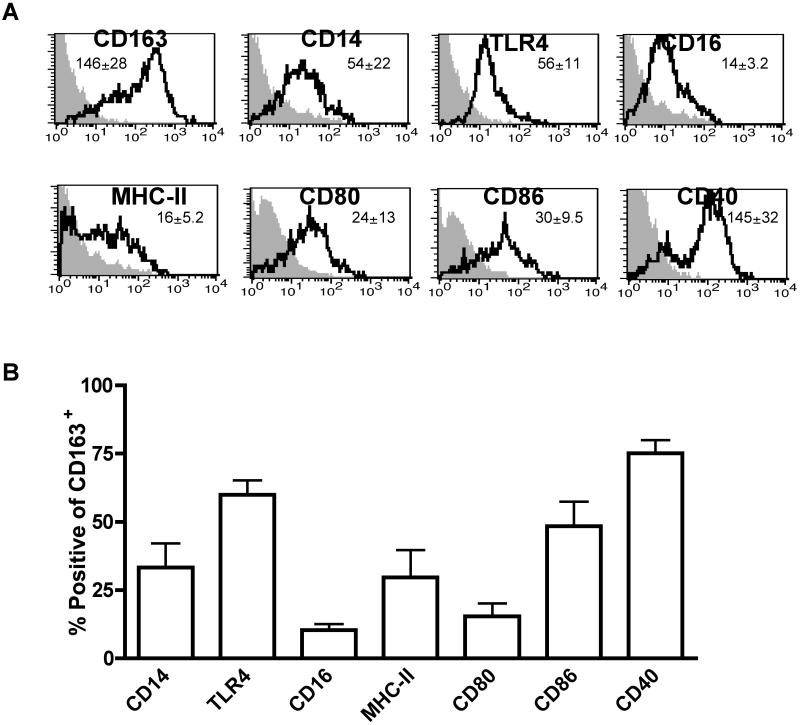
Because human uterine CD163+ macrophages have not been previously characterized, we examined the expression pattern of innate immune receptors present on these cells. Whole tissue human uterine cell suspensions were analyzed by flow cytometry and macrophages were gated based on CD163 expression (Figure 2A). Because of their importance in the recognition of Gram-negative microbes, our initial studies focused on analysis of CD14 and TLR4. CD14 is a co-receptor for LPS with TLR4 and MD-2 and is necessary for macrophage activation in response to bacterial infection [38]. As indicated in Figure 2A, levels of CD14 and TLR4 surface expression are similar on CD163+ macrophages. However, although CD14 is present on a subset of these cells (30%), TLR4 expression is more widely distributed (62%) (Figure 2B). We next examined this cell population for expression of CD16 (FCγRIII), which recognizes the Fc portion of IgG antibodies and stimulates pro-inflammatory cytokine production [39]. Enhanced surface expression of CD16 has also been reported on a subset of alternatively activated CD163+ macrophages [5]. As demonstrated in Figures 2A and 2B, CD16 is expressed at low levels on approximately 12% of CD163+ uterine macrophages. These data imply that human CD163+ uterine macrophages differ from gut-derived mucosal macrophages in terms of TLR4, CD14 and CD16 expression. In contrast to uterine endometrial macrophages, intestinal macrophages express uniformly low levels of CD14, CD16, and TLR4, which may enable them to remain hypo-responsive to environmental antigens and commensals in the gut [40].
Figure 2. Uterine macrophage surface expression phenotype.
Freshly obtained uterine endometrial tissue sections were enzymatically digested into single cell suspensions. Cells were stained for surface antigens in addition to CD163 and analyzed by flow cytometry. (A) Representative histograms of mean fluorescence intensity of macrophages gated on CD163 expression. Inset numbers indicate mean MFI ± SEM for N = 4-7 patients. (B) Percentage of positive cells gated on CD163 expression.
Macrophages present antigen to T cells through expression of MHC on the cell surface, and co-stimulatory molecule signaling is necessary for the generation of adaptive immune responses. As shown in Figures 2A and 2B, approximately 30% of all CD163+ uterine macrophages express low levels of MHC-II. Notably, these cells express similar levels of the co-stimulatory molecules CD80 and CD86 (Figure 2A). CD86 is expressed on almost 50% and CD80 is expressed by roughly 15% of CD163+ uterine macrophages. (Figure 2B). This pattern is similar to that of alveolar and intestinal macrophages, which also express low levels of MHC-II, CD80 and CD86 [40, 41].
CD40 is a co-stimulatory receptor expressed by macrophages and binding of its ligand, CD40L (CD154), results in potent activation. CD40L is expressed mainly by activated T cells and allows for back talk from T cells to antigen presenting cells [42]. In contrast to macrophages derived from other mucosal sites [43, 44], CD40 is highly expressed on most CD163+ uterine macrophages (Figures 2A and 2B). This suggests that uterine macrophages are particularly sensitive to activation by CD40L.
Uterine macrophage cytokine expression
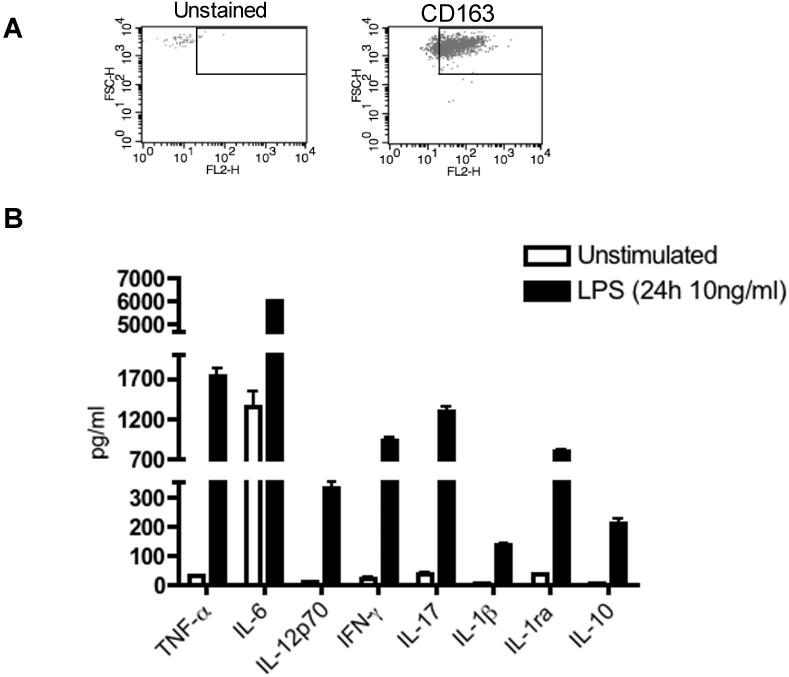
Microbial infection is a major cause of pre-term birth, infertility and ectopic pregnancy; therefore, protection from uterine infection is crucial to ensuring reproductive success [45]. Given the important role of the endometrium in the maintenance of fetal implantation and development, it is advantageous to mount a rapid immune response to microbial challenge. To determine the responsiveness of uterine macrophages to endotoxin challenge, CD163+ macrophages were isolated from uterine tissue by positive selection. Cell purity ranged between 89-95%, as determined by CD163 staining. Flow cytometric data in Figure 3A are representative of cell isolations from three individual donors. Following isolation, cells were stimulated with 10 ng/ml of ultra pure E. Coli LPS for 24 hours and cytokine secretion was measured by Bio-Plex assay. As demonstrated in Figure 3B, uterine macrophages secrete a wide range of pro-inflammatory cytokines in response to LPS including TNFα, IL-12, IL-17 and IL-1β. These data indicate that TLR4 signaling is functional in these cells.
Figure 3. Isolation of CD163+ macrophages and cytokine expression.
(A) Uterine macrophages were isolated by CD163 positive selection. The purity of the isolated cells typically ranged between 89-95 percent, as determined by staining for CD163 compared to unstained controls. (B) CD163+ selected macrophages from 3 individual donors were stimulated with 10 ng/ml LPS for 24 hours and analyzed for cytokine secretion in culture supernatants by Bio-Plex analysis.
IL-1β and its receptor antagonist, IL-1ra, co-ordinate a wide range of biological activities within the human uterine endometrium, both facilitating embryonic implantation as well as conferring protection from pathogenic challenge [45]. In previous studies, we have demonstrated that human uterine macrophages produce bioactive IL-1β in response to LPS [15]. We now show that in addition to IL-1β, uterine macrophages also express high levels of IL-1ra (Figure 3B). Because the secretion of IL-1ra exceeds that of IL-1β by 6-fold, IL-1β signaling within the human uterine endometrium may be attenuated. Similarly, CD163+ uterine macrophages also secrete IL-10 in response to LPS, which may also dampen the effects of pro-inflammatory cytokines (Figure 3B). These data suggest that CD163+ endometrial macrophages are most likely M2b polarized because they produce both pro- and anti-inflammatory cytokines in response to LPS stimulation.
Uterine macrophage chemokine expression
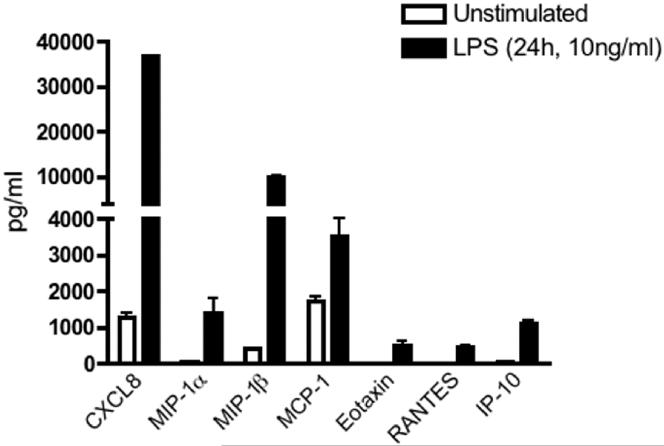
Leukocytes are recruited to the uterine endometrium throughout the menstrual cycle and are an important component of tissue turnover and repair [7]. The influx of migratory cells is orchestrated through local chemokine expression within the cycling endometrium [46]. In Figure 4, we demonstrate that CD163+ uterine macrophages constitutively express low levels of MIP-1β and MCP-1, implicating these cells in the active recruitment of neutrophils and monocytes to the endometrium. In addition, recent studies implicate a role for MCP-1 in M2 macrophage polarization [47]. Constitutive expression of MCP-1 may be important in the maintenance of this phenotype in uterine macrophages.
Figure 4. Uterine macrophage chemokine expression.
Culture supernatants were analyzed for chemokine expression from uterine macrophages as described in Figure 3.
Because tissue resident macrophages produce chemokines in response to microbial challenge as an early step in the recruitment of additional immune effector cells, we next investigated whether LPS activation elicits chemokine secretion from uterine macrophages. As demonstrated in Figure 4, LPS stimulation markedly induces MIP-1α and MIP-1β secretion by uterine macrophages. Similarly, MCP-1, eotaxin, RANTES and IP-10 are LPS-inducible in uterine macrophages. As these chemokines are involved in the recruitment of monocytes, dendritic cells, T cells and eosinophils, these results suggest that macrophages mediate localization of these immune cell subsets to the uterine endometrium in response to microbial challenge.
Uterine macrophage growth factor expression
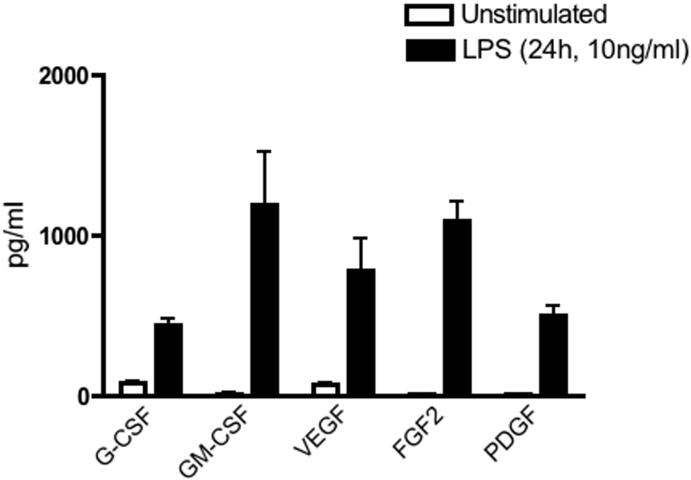
Macrophages have an active role in tissue turnover and remodeling in the human endometrium [48]. Following shedding of the endometrial lining during menstruation, expression of growth factors and angiogenic molecules promotes tissue growth and vascular repair. As demonstrated in Figure 5, uterine macrophages secrete G-CSF and GM-CSF in response to LPS. In addition to regulating the survival and differentiation of granulocytes and macrophages, GM-CSF is also a chemo-attractant for neutrophils [49].
Figure 5. Uterine macrophage growth factor expression.
Growth factor expression in response to LPS stimulation was analyzed by Bio-Plex analysis as described in Figure 3.
Angiogenesis occurs during endometrial repair and vascular integrity is imperative for successful embryo implantation (reviewed in [50]). In this regard, uterine macrophages secrete low constitutive levels of the pro-angiogenic factors VEGF, FGF2, and PDGF, which are enhanced by LPS stimulation (Figure 5). Activated platelets are a major source of PDGF within the uterine endometrium [51], and as demonstrated in Figure 5, macrophages provide an additional source of endometrial PDGF. These data demonstrate that CD163+ uterine macrophages produce important factors involved in the maintenance of endometrial tissue homeostasis and angiogenesis.
Discussion
The uterine endometrium is an immunologically unique site, as it must simultaneously protect against microbial infection and tolerate allogeneic sperm and a semi-allogeneic fetus. Macrophages within the uterine endometrium have a significant role in mediating host defense in addition to maintaining tissue homeostasis. Although macrophages comprise a significant number of leukocytes within the non-pregnant uterine endometrium, no studies to our knowledge have addressed the functional polarization of these cells. To address this question, we characterized the repertoire of immunoreceptors expressed by human uterine macrophages and the profile of cytokines, chemokines and growth factors produced by these cells in response to LPS.
CD163 expression is restricted to cells of monocytic lineage and is widely expressed by mature tissue macrophages [29, 30], making it an excellent marker for identification and isolation of tissue macrophages. Moreover, high CD163 expression is a bona fide marker of the M2 macrophage subtype [5]. Figure 1 shows that CD163 is expressed at high levels on 71.9% of CD68+ endometrial macrophages, whereas CD14 expression is limited to a smaller sub-population of macrophages. These findings demonstrate that the majority of macrophages within the human endometrium express high levels of CD163, consistent with an M2 phenotype. Moreover, our data corroborate results of a recent study in which CD14highCD68+ M1 polarized macrophages were shown to constitute a relatively small population of the total immune cell population in the human non-pregnant myometrium [37]. In our current study, we have identified and characterized for the first time a distinct CD163highCD68+ M2 polarized uterine macrophage population.
To further characterize these cells, CD163+ macrophages were analyzed for surface expression of other macrophage markers. In Figure 2, we show that a subset (approximately 30%) of CD163+ human uterine macrophages also express CD14, a marker of classically activated macrophages. Intriguingly, expression of CD16, which is characteristic of M2 macrophages, is low and limited to only 10% of total CD163+ cells. This may be attributable to the diverse nature of alternatively activated macrophages. Down-regulation of CD14 and CD16 is also observed in macrophages derived from other mucosal sites, including the lamina propria of the gut [52, 53] and the vaginal mucosa [54].
However, in contrast to macrophages of the gut mucosa where TLR4 expression is low or undetectable [52, 55-57], a large percentage of uterine macrophages (~60%) is positive for TLR4 expression. Since commensal bacteria colonize the gut, limiting TLR expression may be advantageous for minimizing inappropriate immune activation. Commensal organisms also colonize the lower regions of the female reproductive tract; however, they are absent from the upper tract, including the uterine endometrium and Fallopian tubes [58]. Our previous work has shown that TLR4 expression progressively declines in tissues from the upper to lower reproductive tract, with the highest levels expressed in the Fallopian tube and uterine endometrium [59]. High expression of TLR4 in the uterine endometrium may be critical to ensuring reproductive success, since this tissue is most likely to be challenged by Gram-negative N. gonorrhoeae and C. trachomatis [58]. Increased innate surveillance at this site (manifested by enhanced TLR4 expression) may provide a means of ensuring sterile conditions while conferring protection from microbial challenge. In this regard, it has recently been reported that in addition to recognizing hemoglobin-haptoglobin complexes, CD163 also functions as an immune receptor for both Gram-negative and Gram-positive bacteria [60]. Therefore, it is notable that uterine macrophages express elevated levels of CD163 in addition to TLR4. High expression of these receptors suggests that these cells are poised to recognize bacterial infection within the uterine endometrium.
As key effector cells of the innate immune system, macrophages interact with CD4+ T cells through MHC II and co-stimulatory molecule expression. As demonstrated in Figure 2, MHC II, CD80 and CD86 expression on endometrial macrophages is low, indicating that these cells may have reduced ability to mediate CD4+ T cell activation. CD80 and CD86 have overlapping expression patterns and identical function. Both molecules serve as ligands for CD28, the activating receptor expressed on the surface of T cells, as well as CTLA-4, an inhibitory receptor expressed by T cells [61]. Whether CD80 and CD86 provide activating or inhibitory signals depends on the relative expression of CD28 and CTLA-4 on uterine CD4+ T cells and is an area of ongoing investigation in our laboratory.
CD40 is a member of the tumor necrosis factor-α family and is expressed on antigen presenting cells including macrophages and B cells (reviewed in [42]). CD40L, the endogenous ligand for CD40, is expressed mainly on activated T cells and is also present in soluble form in the human endometrium [62]. In contrast to monocytes and in vitro derived macrophages, which express low levels of CD40 [63], uterine macrophages express high levels of CD40. Macrophage activation through CD40 stimulation leads to the production of both pro- and anti-inflammatory cytokines as well as the up-regulation of MHC II, CD80, CD86 and CD40 itself [64]. Activated platelets serve as a reservoir of sCD40L [65]. Since platelet numbers in the endometrium increase during menstruation [11], sCD40L levels may be an important signal for macrophage involvement in uterine endometrial tissue turnover and repair. Therefore, high CD40 expression on uterine macrophages is likely important in both the context of infection and in tissue homeostasis.
We also investigated whether CD163+ uterine macrophages were responsive to endotoxin challenge. In response to LPS, isolated uterine endometrial macrophages secrete the pro-inflammatory cytokines TNFα, IL-12, IL-17 and IL-1β as well as anti-inflammatory IL-1ra and IL-10. As previously reported, endometrial macrophages express bioactive IL-1β in response to endotoxin challenge, and expression of this cytokine elicits the secretion of HBD2 by the endometrial epithelium [15]. Interestingly, IL-1ra is expressed in excess of IL-1β, a characteristic of alternatively activated macrophages [66]. It is notable that a similar level of recombinant IL-1β induces higher levels of HBD2 than does conditioned media from LPS-stimulated endometrial macrophages [15]. Although IL-1ra levels were not measured in that study, our results suggest that high levels of IL-1ra expression may explain this observation. Therefore, in addition to secreting pro-inflammatory cytokines to combat microbial infection, uterine macrophages also produce anti-inflammatory factors that aid in the resolution of inflammation. These characteristics are consistent with M2b macrophage alternative activation.
Intriguingly, uterine macrophages produce high levels of IL-17 in response to LPS. IL-17 is a pro-inflammatory cytokine that also induces neovascularization and can promote the expression of other angiogenic factors [67]. In humans, T cells are the major source of IL-17; however, monocytes and macrophages have now also been identified as significant producers of IL-17 [68-70]. IL-17 also up-regulates chemokine and MMP expression, which enables recruitment of inflammatory cells to sites of infection (reviewed in [71]). Given that MMPs contribute to the breakdown of tissue during menstruation, this highlights a potential role for IL-17 in regulation of endometrial tissue turnover, and suggests that this cytokine is an important mediator of macrophage maintenance of uterine endometrial tissue homeostasis.
In addition to cytokines, uterine macrophages also produce significant levels of chemokines. As demonstrated in Figure 4, endometrial macrophages express MIP-1α, MIP-1β and MCP-1. Levels of these chemokines in the human endometrium vary throughout the menstrual cycle with highest levels reported during the late secretory phase [72, 73], consistent with the role these factors play in the recruitment of leukocytes for endometrial shedding. Because rapid trafficking of immune effector cells to sites of infection is essential, it is fitting that uterine macrophage production of chemokines is LPS-inducible. In addition to recruiting leukocytes to the endometrium, macrophages also produce factors important in leukocyte maturation and differentiation. In this work, we have shown that endometrial macrophages produce G-CSF and GM-CSF, which are important in the differentiation of granulocytes and macrophages.
Most mature human tissues do not exhibit significant angiogenesis except in response to injury. However, the human uterine endometrium regularly undergoes extensive vascular remodeling in preparation for embryo implantation. The process of endometrial angiogenesis is mediated by local production of growth factors, most prominently VEGF [74]. Our study suggests that macrophages in the uterine endometrium may contribute to the process of angiogenesis through both constitutive and induced expression of angiogenic factors such as VEGF, FGF2 and PDGF. Notably, secretion of pro-angiogenic factors is also characteristic of M2 macrophages.
Notably, uterine macrophages constitutively secrete many chemokines and cytokines, including MCP-1 and IL-6. Intriguingly, recent work demonstrates that MCP-1 skews macrophages towards an alternatively activated phenotype (M2b or M2d) [47]. Furthermore, IL-6 secretion may also play a role in maintaining M2 polarization, as IL-6 mediates up-regulation of CD163, a validated marker of alternatively activated macrophages [27, 75].
In this regard, the functional plasticity of macrophages has been well-documented. Because macrophages are exquisitely sensitive to micro-environmental signals, they readily adapt functional patterns to respond to changes in the local cellular milieu [76]. In recent studies, we have shown that culture of M2-polarized uterine macrophages with LPS enhances expression of CD14 and MHC-II and inhibits expression of CD163 (data not shown). These results suggest that microbial encounter induces phenotypic and functional changes in M2 uterine macrophages that result in an activation program aimed at inhibition of infection rather than implantation. Further studies are needed to elucidate the mechanism and functional consequences of macrophage repolarization.
Because the local uterine micro-environment plays a role in the modulation of macrophage activation and function, it is possible that differences in hormone levels (such as those observed during the proliferative and secretory phases of the menstrual cycle) influence macrophage polarization. All tissues were staged prior to macrophage isolation and flow analysis. Review of our data failed to establish a statistically significant link between menstrual cycle status and macrophage activation. However, this may be attributable to the relatively limited sample size assessed in our study. Current work in our laboratory may provide greater insight as to the influence of cycle-dependence on macrophage polarization, as this work is focused on determining how estradiol and/or progesterone modulate macrophage activation.
In summary, we have now shown that the major population of human uterine macrophages exhibits characteristics of alternatively activated or M2 macrophages. These CD163+ cells express a repertoire of immunoreceptors similar to that of other mucosal macrophages, but with higher levels of TLR4 and CD40. Elevated expression of TLR4 is likely important in mounting rapid responses to invading pathogens to ensure reproductive success in the face of infection. As endometrial macrophages play a significant role in tissue remodeling, high CD40 expression may permit these cells to respond to sCD40L produced by activated platelets during menstruation. In this study, we have shown that endometrial macrophages are sensitive to endotoxin challenge and respond by producing a profile of cytokines, chemokines, growth and pro-angiogenic factors similar to that of M2b activated macrophages. Collectively, these data suggest that CD163+ endometrial macrophages play an important role in host defense and the regulation of tissue homeostatic functions including tissue breakdown, clearance and angiogenic remodeling.
Acknowledgements
This study was supported by the Centers of Biomedical Research Excellence (COBRE) P20 RR 016437 grant and NIH grant RO1AI051547. AJM received support from an NIH Autoimmunity and Connective Tissue Training Grant (T32AR007576).
References
- 1.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 2.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181(6):3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 5.Verreck FA, et al. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79(2):285–93. doi: 10.1189/jlb.0105015. [DOI] [PubMed] [Google Scholar]
- 6.Timmer AM, Nizet V. IKKbeta/NF-kappaB and the miscreant macrophage. J Exp Med. 2008;205(6):1255–9. doi: 10.1084/jem.20081056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salamonsen LA, et al. Regulation of matrix metalloproteinases in human endometrium. Hum Reprod. 2000;15(Suppl 3):112–9. doi: 10.1093/humrep/15.suppl_3.112. [DOI] [PubMed] [Google Scholar]
- 8.Bonatz G, et al. Macrophage- and lymphocyte-subtypes in the endometrium during different phases of the ovarian cycle. Int J Gynaecol Obstet. 1992;37(1):29–36. doi: 10.1016/0020-7292(92)90974-n. [DOI] [PubMed] [Google Scholar]
- 9.Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18(8):349–51. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 10.Givan AL, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38(5):350–9. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 11.Eidukaite A, Tamosiunas V. Endometrial and peritoneal macrophages: expression of activation and adhesion molecules. Am J Reprod Immunol. 2004;52(2):113–7. doi: 10.1111/j.1600-0897.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 12.Dechaud H, et al. Evaluation of endometrial inflammation by quantification of macrophages, T lymphocytes, and interleukin-1 and -6 in human endometrium. J Assist Reprod Genet. 1998;15(10):612–8. doi: 10.1023/A:1020337528607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berbic M, et al. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. 2009;24(2):325–32. doi: 10.1093/humrep/den393. [DOI] [PubMed] [Google Scholar]
- 14.Stewart JA, Bulmer JN, Murdoch AP. Endometrial leucocytes: expression of steroid hormone receptors. J Clin Pathol. 1998;51(2):121–6. doi: 10.1136/jcp.51.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pioli PA, et al. Lipopolysaccharide-induced IL-1 beta production by human uterine macrophages up-regulates uterine epithelial cell expression of human beta-defensin 2. J Immunol. 2006;176(11):6647–55. doi: 10.4049/jimmunol.176.11.6647. [DOI] [PubMed] [Google Scholar]
- 16.Basu S, et al. Human uterine NK cells interact with uterine macrophages via NKG2D upon stimulation with PAMPs. Am J Reprod Immunol. 2009;61(1):52–61. doi: 10.1111/j.1600-0897.2008.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travaglione S, et al. Epithelial cells and expression of the phagocytic marker CD68: scavenging of apoptotic bodies following Rho activation. Toxicol In Vitro. 2002;16(4):405–11. doi: 10.1016/s0887-2333(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 18.Strobl H, et al. Flow cytometric analysis of intracellular CD68 molecule expression in normal and malignant haemopoiesis. Br J Haematol. 1995;90(4):774–82. doi: 10.1111/j.1365-2141.1995.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 19.Heinemann DE, et al. Human osteoblast-like cells phagocytose metal particles and express the macrophage marker CD68 in vitro. J Bone Joint Surg Br. 2000;82(2):283–9. [PubMed] [Google Scholar]
- 20.Schutt C. Cd14. Int J Biochem Cell Biol. 1999;31(5):545–9. doi: 10.1016/s1357-2725(98)00153-8. [DOI] [PubMed] [Google Scholar]
- 21.Law SK, et al. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol. 1993;23(9):2320–5. doi: 10.1002/eji.1830230940. [DOI] [PubMed] [Google Scholar]
- 22.Hogger P, et al. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161(4):1883–90. [PubMed] [Google Scholar]
- 23.Gronlund J, et al. Cloning of a novel scavenger receptor cysteine-rich type I transmembrane molecule (M160) expressed by human macrophages. J Immunol. 2000;165(11):6406–15. doi: 10.4049/jimmunol.165.11.6406. [DOI] [PubMed] [Google Scholar]
- 24.Moestrup SK, Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med. 2004;36(5):347–54. doi: 10.1080/07853890410033171. [DOI] [PubMed] [Google Scholar]
- 25.Komohara Y, et al. AM-3K, an anti-macrophage antibody, recognizes CD163, a molecule associated with an anti-inflammatory macrophage phenotype. J Histochem Cytochem. 2006;54(7):763–71. doi: 10.1369/jhc.5A6871.2006. [DOI] [PubMed] [Google Scholar]
- 26.Jensen TO, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27(20):3330–7. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 27.Buechler C, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67(1):97–103. [PubMed] [Google Scholar]
- 28.Graversen JH, Madsen M, Moestrup SK. CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol. 2002;34(4):309–14. doi: 10.1016/s1357-2725(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 29.Pulford K, et al. A monocyte/macrophage antigen recognized by the four antibodies GHI/61, Ber-MAC3, Ki-M8 and SM4. Immunology. 1992;75(4):588–95. [PMC free article] [PubMed] [Google Scholar]
- 30.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210(2-4):153–60. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Porcheray F, et al. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142(3):481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentzer SJ, et al. Spontaneous aggregation as a mechanism for human monocyte purification. Cell Immunol. 1986;101(2):312–9. doi: 10.1016/0008-8749(86)90144-9. [DOI] [PubMed] [Google Scholar]
- 33.Lacey DC, et al. Defining GM-CSF- and Macrophage-CSF-Dependent Macrophage Responses by In Vitro Models. J Immunol. 2012;188(11):5752–65. doi: 10.4049/jimmunol.1103426. [DOI] [PubMed] [Google Scholar]
- 34.Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184(9):5029–37. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeaman GR, et al. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J Immunol. 1998;160(10):5145–53. [PubMed] [Google Scholar]
- 36.White HD, et al. A method for the dispersal and characterization of leukocytes from the human female reproductive tract. Am J Reprod Immunol. 2000;44(2):96–103. doi: 10.1111/j.8755-8920.2000.440205.x. [DOI] [PubMed] [Google Scholar]
- 37.Ivanisevic M, et al. Antigen-presenting cells in pregnant and non-pregnant human myometrium. Am J Reprod Immunol. 2010;64(3):188–96. doi: 10.1111/j.1600-0897.2010.00858.x. [DOI] [PubMed] [Google Scholar]
- 38.Kitchens RL. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem Immunol. 2000;74:61–82. doi: 10.1159/000058750. [DOI] [PubMed] [Google Scholar]
- 39.Fossati G, Bucknall RC, Edwards SW. Fcgamma receptors in autoimmune diseases. Eur J Clin Invest. 2001;31(9):821–31. doi: 10.1046/j.1365-2362.2001.00881.x. [DOI] [PubMed] [Google Scholar]
- 40.Platt AM, Mowat AM. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett. 2008;119(1-2):22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Nicod LP, Isler P. Alveolar macrophages in sarcoidosis coexpress high levels of CD86 (B7.2), CD40, and CD30L. Am J Respir Cell Mol Biol. 1997;17(1):91–6. doi: 10.1165/ajrcmb.17.1.2781. [DOI] [PubMed] [Google Scholar]
- 42.Chatzigeorgiou A, et al. CD40/CD40L signaling and its implication in health and disease. Biofactors. 2009;35(6):474–83. doi: 10.1002/biof.62. [DOI] [PubMed] [Google Scholar]
- 43.Rugtveit J, et al. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112(5):1493–505. doi: 10.1016/s0016-5085(97)70030-1. [DOI] [PubMed] [Google Scholar]
- 44.Rugtveit J, Bakka A, Brandtzaeg P. Differential distribution of B7.1 (CD80) and B7.2 (CD86) costimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD) Clin Exp Immunol. 1997;110(1):104–13. doi: 10.1046/j.1365-2249.1997.5071404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horne AW, Stock SJ, King AE. Innate immunity and disorders of the female reproductive tract. Reproduction. 2008;135(6):739–49. doi: 10.1530/REP-07-0564. [DOI] [PubMed] [Google Scholar]
- 46.Jones RL, et al. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89(12):6155–67. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, et al. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene. 2011 doi: 10.1038/onc.2011.112. [DOI] [PubMed] [Google Scholar]
- 48.Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update. 2000;6(1):16–27. doi: 10.1093/humupd/6.1.16. [DOI] [PubMed] [Google Scholar]
- 49.Yong KL, et al. Granulocyte-macrophage colony-stimulating factor induces neutrophil adhesion to pulmonary vascular endothelium in vivo: role of beta 2 integrins. Blood. 1992;80(6):1565–75. [PubMed] [Google Scholar]
- 50.Torry DS, et al. Angiogenesis in implantation. J Assist Reprod Genet. 2007;24(7):303–15. doi: 10.1007/s10815-007-9152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chegini N, Rossi MJ, Masterson BJ. Platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and EGF and PDGF beta-receptors in human endometrial tissue: localization and in vitro action. Endocrinology. 1992;130(4):2373–85. doi: 10.1210/endo.130.4.1312455. [DOI] [PubMed] [Google Scholar]
- 52.Rogler G, et al. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112(2):205–15. doi: 10.1046/j.1365-2249.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamada N, et al. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009;183(3):1724–31. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 54.Shen R, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83(7):3258–67. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith PD, et al. Intestinal macrophages lack CD14 and CD89 and consequently are down-regulated for LPS- and IgA-mediated activities. J Immunol. 2001;167(5):2651–6. doi: 10.4049/jimmunol.167.5.2651. [DOI] [PubMed] [Google Scholar]
- 56.Grimm MC, et al. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995;100(2):291–7. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hausmann M, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122(7):1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 58.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57(1-2):61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 59.Pioli PA, et al. Differential expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun. 2004;72(10):5799–806. doi: 10.1128/IAI.72.10.5799-5806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fabriek BO, et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113(4):887–92. doi: 10.1182/blood-2008-07-167064. [DOI] [PubMed] [Google Scholar]
- 61.Sharpe AH. Mechanisms of costimulation. Immunol Rev. 2009;229(1):5–11. doi: 10.1111/j.1600-065X.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King AE, et al. Cd40 expression in uterine tissues: a key regulator of cytokine expression by fibroblasts. J Clin Endocrinol Metab. 2001;86(1):405–12. doi: 10.1210/jcem.86.1.7133. [DOI] [PubMed] [Google Scholar]
- 63.Alderson MR, et al. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178(2):669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suttles J, Stout RD. Macrophage CD40 signaling: a pivotal regulator of disease protection and pathogenesis. Semin Immunol. 2009;21(5):257–64. doi: 10.1016/j.smim.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 65.Henn V, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391(6667):591–4. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 66.Vannier E, Miller LC, Dinarello CA. Coordinated antiinflammatory effects of interleukin 4: interleukin 4 suppresses interleukin 1 production but up-regulates gene expression and synthesis of interleukin 1 receptor antagonist. Proc Natl Acad Sci U S A. 1992;89(9):4076–80. doi: 10.1073/pnas.89.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Numasaki M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 68.Song C, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181(9):6117–24. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 69.Fujino S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52(1):65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chabaud M, et al. IL-17 derived from juxta-articular bone and synovium contributes to joint degradation in rheumatoid arthritis. Arthritis Res. 2001;3(3):168–77. doi: 10.1186/ar294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okamoto H, et al. Molecular targets of rheumatoid arthritis. Inflamm Allergy Drug Targets. 2008;7(1):53–66. doi: 10.2174/187152808784165199. [DOI] [PubMed] [Google Scholar]
- 72.Simon C, et al. Cytokines and embryo implantation. J Reprod Immunol. 1998;39(1-2):117–31. doi: 10.1016/s0165-0378(98)00017-5. [DOI] [PubMed] [Google Scholar]
- 73.Kitaya K, et al. Expression of macrophage inflammatory protein-1beta in human endometrium: its role in endometrial recruitment of natural killer cells. J Clin Endocrinol Metab. 2003;88(4):1809–14. doi: 10.1210/jc.2002-020980. [DOI] [PubMed] [Google Scholar]
- 74.Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121(2):181–6. doi: 10.1530/rep.0.1210181. [DOI] [PubMed] [Google Scholar]
- 75.Weaver LK, et al. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol. 2007;81(3):663–71. doi: 10.1189/jlb.0706428. [DOI] [PubMed] [Google Scholar]
- 76.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76(3):509–13. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]