Abstract
Cumulative damage to cellular macromolecules via oxidative stress is a hallmark of aging and neurodegenerative disease. Whether such damage is a cause or a subsequent effect of neurodegeneration is still unknown. This paper describes the development of an age-associated mild parkinsonian model in mice that lack the DNA repair enzyme 8-oxoguanine glycosylase 1 (Ogg1). Aged OGG1 knock-out (OGG1 KO) mice show a decreased spontaneous locomotor behavior and evidence a decrease in striatal dopamine levels, a loss of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra (SN), and an increase in ubiquitin-positive inclusions in their remaining SN neurons. In addition, young OGG1 KO mice are more susceptible to the dopaminergic toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) than their wild-type (WT) counterparts. Age-associated increases in 7,8-dihydro-2′-deoxyguanine (oxo8dG) have been reported in brain regions and neuronal populations affected in Parkinson’s disease (PD), toxin-induced parkinsonian models, and mice harboring genetic abnormalities associated with PD. Because of these increased oxo8dG levels, the OGG1 KO mouse strain could shed light on molecular events leading to neuronal loss as a consequence of cumulative oxidative damage to DNA during aging and after toxicological challenge.
Keywords: DNA Damage; DNA Repair; 7,8-dihydroxy-2′-deoxyguanine; Ogg1; Aging; Parkinson’s disease; MPTP
1 Introduction
Parkinson’s disease (PD) is an age-associated movement disorder, characterized by a loss in the number of dopaminergic neurons in the substantia nigra (SN) and the formation of Lewy bodies, ubiquitin and synuclein positive eosinophillic aggregates (Alves-Rodrigues et al., 1998; Wakabayashi et al., 1998). There is growing evidence that reactive oxygen species (ROS) play a major role in the neurodegenerative process and targeting their formation or deleterious effects can serve as a therapeutic approach (Fukae et al., 2007; Mancuso et al., 2007).
All major macromolecules are targets for ROS-induced damage (Halliwell and Gutteridge, 1986). Among the four DNA bases, guanine has the lowest oxidation potential (Devasagayam et al., 1991; Milligan et al., 2001; Steenken and Jovanovic, 1997); thus, 7,8-dihydro-2′-deoxyguanine (oxo8dG) is the most prevalent form of oxidative base modifications produced (Dizdaroglu et al., 2002). Oxo8dG levels have become biomarkers of oxidative stress associated with aging and diseases ranging from cancer to neurological deficits (Chiou et al., 2003; Proteggente et al., 2002; Wang et al., 2005). Oxo8dG formation and accumulation are associated with deleterious cellular effects such as altering of telomere ends stability and maintenance (Opresko et al., 2005), activation of the cell cycle and apoptosis in neurons (Kruman et al., 2004).
Oxo8dG has been shown to be significantly higher in PD postmortem brain samples than in age-matched controls (Alam et al., 1997; Sanchez-Ramos, 1994; Zhang et al., 1999). Oxidative damage to DNA in PD is not restricted to the nigrostriatal pathway as oxo8dG has been found to be elevated in lymphocytes (Migliore et al., 2002; Petrozzi et al., 2001), cerebrospinal fluid, and serum (Abe et al., 2003). Urinary oxo8dG levels have also been found to be elevated in PD patients correlating with the progression of the disease (Sato et al., 2005). Also, the expression of enzymes that repair or remove oxidized guanine bases are found to be upregulated in PD brains (Nakabeppu et al., 2007). Recently, it has been shown that alterations in 8-oxoguanine glycosylase 1 (Ogg1) are also a component in AD brains (Shao et al., 2008).
High oxo8dG levels are not restricted to clinical cases of PD. Increased oxidative damage to DNA has been found after exposure to the dopaminergic toxins 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone (Beretta et al., 2006; Betarbet et al., 2000; Chen et al., 2005; de Lima et al., 2005; Mandavilli et al., 2000). Despite its association with both neuronal loss in the nigrostriatal system and the effect of dopaminergic neurotoxins, oxo8dG accumulation has been regarded as a marker for but not as an initiator of neurodegenerative processes.
Mice lacking Ogg1, the enzyme responsible for the removal of oxo8dG from DNA, accumulate oxo8dG at a faster rate than wild-type (WT) mice (Klungland et al., 1999; Osterod et al., 2001). The mice age normally without a significant phenotype (Friedberg and Meira, 2006). Oxo8dG accumulation in OGG1 deficient mice is accentuated in organs with reduced cell proliferation as well as in mitochondria (Minowa et al., 2000; Stuart et al., 2005). Recent studies indicate that the OGG1 deficient mice are more susceptible to a challenge with methamphetamine during development (Wong et al., 2008), and primary cortical neurons from these mice are more susceptible to ischemic episodes (Liu et al., 2011). Thus, supporting a role of accumulation of oxo8dG in neurodegenerative cascades. Studies looking at biological systems involved in reducing oxidative burden in the nucleotide triphosphate pools indicate that mice lacking the enzyme MTH1, responsible for removing free radical-damaged nucleotides, exhibit increase damage to RNA and DNA and are more susceptible to a neurotoxic challenge with MPTP. These results lend support to the role of DNA damage in neurodegeneration of the nigrostriatal system (Yamaguchi et al., 2006). However, despite the overwhelming evidence of oxo8dG as a major component of the pathology in PD no study has directly determined the role of increased DNA damage in the nigrostriatal system in relationship to aging and neurotoxicological susceptibility.
We present data demonstrating that mice lacking Ogg1 evidence age-associated spontaneous motor behavior deficiencies paralleled by neurochemical and histological changes in the nigrostriatal system, loss of striatal DA, loss of TH-positive neurons in the SN, and the development of ubiquitin-positive inclusions in surviving SN neurons. In addition similar to published results of increased toxicological susceptibility in these mice, mice lacking Ogg1 are more susceptible to a challenge with MPTP. The age-dependent changes in striatum are neurochemically specific, striatal glutamate levels are unaffected and no or less drastic histopathological changes are seen in non-SN neuronal populations. This study evidences the role of oxidative stress and the susceptibility of the nigrostriatal pathway. Specifically, the role increased oxidative damage to DNA plays in neuronal vulnerability and how deficiencies in repair capacity can alter neuronal fate.
2 Materials and methods
2.1 Animals and Behavioral Tests
All animal use was conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with protocols approved by the Institutional Animal Care and Use Committee.
All chemicals were purchased from Sigma (Sigma, St. Louis, MO), unless otherwise indicated. Male and female WT, heterozygous (HZ), and OGG1 knock-out (KO) mice all on 129/SvJ background were used for these studies. Experimental groups consisted of littermates with ages at either 3 (young) to 26 (aged) months old. Original colony founders were kindly donated by Dr. Tomas Lindahl.
Motor behavior was evaluated by a 10 minutes single-session open field test. The tests were performed in a 30 cm. square box, under a 20 LUX light intensity and during the light hours of the day. All the animals were evaluated at the same time, after a 1-hour conditioning period.
2.2 MPTP treatment
MPTP hydrochloride was dissolved in PBS at a concentration of 10 mg/mL and injected at a volume of 1.25 μL/g for a total dose of 25 mg/Kg (2 i.p. injections of 12.5 mg/Kg 8 hours apart). MPTP treated mice were compared to saline treated control littermates of each genotype. All mice were sacrificed by cervical dislocation followed by rapid decapitation 7 days post-injection. Safety precautions for the handling of MPTP and MPTP-treated animals were carefully followed (Przedborski et al., 2001) http://www.nih.gov/od/ors/ds/pubs/mptp/.
2.3 Measurement of Ogg1 enzymatic activity
The extraction and activity of Ogg1 was performed as described elsewhere (Bolin et al., 2004; Cardozo-Pelaez et al., 2000).
2.4 Neurotransmitter Analysis by RP-HPLC
Levels of DA, dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in caudate/putamen (CP) were measured using reverse-phase high performance liquid chromatography (RP-HPLC) with electrochemical detection (HPLC-ECD) as previously described (Cardozo-Pelaez et al., 2005; Cardozo-Pelaez et al., 1999). Briefly, CP were weighed and homogenized by sonication in 500 L of perchloric acid (0.05M) containing 3,4-dihydroxybenzylamine (DBA, 31 ng/mL). Homogenates were centrifuged for 20 minutes at 14000 g, 4°C and filtered through a 0.45 μm filter. Sample filtrates were injected into the HPLC using an ESA Model 542 autosampler (ESA Chelmsford, MA). The mobile phase consisted of water:acetonitrile (9:1, vol/vol) containing 0.15 M monochloroacetic acid, 0.12 M sodium hydroxide, 0.60 mM EDTA, and 1.30 mM sodium octyl sulfate. An ultrasphere ODS column with a length of 25 cm and internal diameter of 4.6 mm was used for separation (Beckman Instruments, San Ramon, CA). The flow rate was kept at 1 mL/min (ESA Model 582 Solvent Delivery Module, Chelmsford, MA) and the column eluent was analyzed with an electrochemical detector (ESA Model 5600A CoulArray Detector, 3 ESA Model 6210 four channel electrochemical cells, Chelmsford, MA). All RP-HPLC data was recorded, stored and analyzed on a PC Pentium computer using CoulArray for Windows 32Software (ESA Chelmsford, MA). DA, DOPAC, and DBA were monitored at 100 mV; HVA was monitored at 300 mV. The ratio of the peak heights produced by DA and its metabolites (DOPAC and HVA) to the peak height produced by DBA (internal standard) in the samples were used to obtain the CP analyte levels from a calibration curve. Data was expressed as micrograms of analyte per gram of wet tissue weight (μg/g wt).
Glutamate levels were measured in CP homogenates using a precolumn o-phthaldialdehyde/β-mercaptoethanol (OPA/βME) derivatization method as previously described (Donzanti and Yamamoto, 1988). Derivatization was performed by mixing 20 μl of sample with 20 μl of the OPA/βME working derivatizing reagent. After 1 minute, 20 μl of the reaction mixture was injected into the HPLC. This process was automated by the ESA Model 542 autosampler. The mobile phase consisted of 100mM disodium hydrogen phosphate, 20% methanol, and 3.5% acetonitrile; the pH was adjusted to 6.7 with phosphoric acid. A Waters Xterra™ MS C18 (3.0 × 50 mm, 2.5 μL; Milford, MA) column was used for separation, the flow rate was kept at 0.3 mL/min. The OPA/βME glutamate derivative was monitored at 380 mV. Data was expressed as micrograms of analyte per gram of wet tissue weight (μg/g wt).
2.5 Isolation and analysis of Oxo8dG and 2-deoxyguanosine in caudate putamen
The procedures for extraction, purification, and enzymatic hydrolysis of DNA were based on previously published methods with minor modifications (Bolin et al., 2004; Cardozo-Pelaez et al., 1999). Analysis of the levels of oxo8dG, expressed as the ratio of oxo8dG/ 2-deoxyguanosine (2-dG) was performed as follows. Oxo8dG and 2-dG were resolved by HPLC with a reverse phase YMCbasic column (4.6 × 150 mm; particle size 3-micron) (YMC Inc., Wilmington, NC) and quantified using a CoulArray electrochemical detection system (ESA, Inc., Chelmsford, MA). An isocratic mobile phase consisting of 100 mM sodium acetate, pH 5.2, 4% Methanol (HPLC Grade) diluted in water polished with C18 Sep-Pak cartridges (Waters Corp., Milford, MA) was utilized to elute the nucleosides from the column. The mobile phase was filtered using 0.2 m Nylon filters and degassed by sonication before use with the HPLC. Potentials of the four coulometric analytical cells of the CoulArray system, placed in series, were as follows: 50, 125, 175, 200, 250, 380, 500, 700, 785, 850, 890, 900 mV. Calibration curves were generated from standards of 2-dG ranging from 100 ng to 2 μg (0.350 nmol to 7.01 nmol) and oxo8dG (Cayman Chemical Company, Ann Arbor, MI) ranging from 5 pg to 100 pg (17.6 fmol to 353 fmol). The calibration curve for oxo8dG was created based on the peak area of the oxo8dG standard, eluting at 9.0 min, in the 250 mV channel. The calibration curve for 2-dG was created based on the sum of peak areas of the 2-dG standard, eluting at 7.8 min, in the 850, 890, and 900 mV channels. The electrochemical cells were allowed to equilibrate and wake up standards (three times the highest concentration of calibration standards) were injected before calibration standards. The amount of oxo8dG and 2-dG was calculated by comparing the peak area and sum of peak areas respectively from the 10μL injection of reconstituted enzymatic hydrolysate of the DNA sample with the calibration curves for oxo8dG and 2-dG. Levels of oxo8dG in the samples were expressed relative to the content of 2-dG (e.g. the molar ratio of oxo8dG to 2-dG [fmol of oxo8dG/ nmol of 2-dG]). Data were recorded, analyzed, and stored using CoulArray for Windows data analysis software (ESA Inc., Chelmsford, MA).
2.6 Histology, tissue preparation, and immunofluorescence labeling
Midbrains and forebrains were fixed by immersion in fresh 4% paraformaldehyde/100 mM phosphate buffer (pH 7.4) for 4 days at 4°C. Tissues were then paraffin embedded and sectioned coronally at 10 μm. Every third section through the SN was immunohistochemically stained for tyrosine hydroxylase (TH). Adjacent sections were later used for ubiquitin and 8-hydroxydeoxyguanosine (8-OHdG) immunoreactivity, as well as hematoxylin and eosin (H&E) staining.
Single chromogenic immunolabeling studies for TH (1:600, rabbit polyclonal, Chemicon, Temecula, CA) and ubiquitin (1:100, rabbit polyclonal, Abcam, Cambridge, MA) were performed according to the biotin-avidin peroxidase method. All sections were incubated in 3% H2O2 for 10 minutes to eliminate endogenous peroxidase activity. All rinses were done with 0.1 M Tris buffer (pH 7.4). Non-specific binding was reduced by using 0.5% bovine serum albumin in 0.1 M Tris buffer. Blocking was performed by incubating sections in 4% normal goat serum for 20 minutes. Slices were then exposed to a primary antibody, anti-TH 1:600 or anti-ubiquitin 1:100 for 18 hours at 21°C. Sections were rinsed and incubated with a biotinylated goat anti-rabbit IgG, 1:400 (Vector Laboratories, Burlingame, CA) for 1 hour at 21°C. The antibody signal was amplified using the Vectastain Elite ABC Kit (Vector Laboratories), 1 hour at 21°C. Color was developed by a 3,3′-diaminobenzidine (DAB) hydrogen peroxide reaction for 10 minutes. A sodium-citrate buffer antigen retrieval method was used prior to ubiquitin immunolabeling. After deparaffinization and rehydration, sections were immersed in a solution of 10 mM sodium citrate and 0.05% Tween 20 (pH 6.0) for 25 minutes at 95-100°C.
Single immunofluorescence labeling studies for oxo8dG and ubiquitin were performed as follows: Midbrains were fixed by immersion in fresh 4% paraformaldehyde/100 mM phosphate buffer (pH 7.4) for 4 days at 4°C. Tissues were then paraffin embedded and sectioned coronally at 10 μm. All rinses were done with 0.1 M Tris buffer (pH 7.4). Non-specific binding was reduced by using 0.5% bovine serum albumin in 0.1 M Tris buffer. Sections through the SN were incubated with an anti oxo 8dG (1:200, goat polyclonal, Chemicon) antibody for 18 hours at 21°C. Blocking was performed with 4% normal donkey serum. Sections were then incubated with an AlexaFluor®-488 (1:400, donkey anti-goat IgG, Molecular Probes, Eugene, OR) secondary antibody for 1 hour at 21°C. Adjacent SN sections were incubated with an anti-ubiquitin (1:100, rabbit polyclonal, Abcam) antibody for 18 hours at 21°C. Blocking was performed with 4% normal goat serum. Sections were then exposed to a Texas Red® (1:400, goat anti-rabbit IgG, Molecular Probes) secondary antibody for 1 hour at 21°C.
A green NeuroTrace™ (Molecular Probes) fluorescent Nissl stain, 1:300 and 20 minutes at 21°C, was used as a counterstain to reveal the location of intraneruonal ubiquitin-positive inclusions. A sodium-citrate buffer antigen retrieval method, as mentioned above, was used prior to ubiquitin immunolabeling. Fluorescence was visualized and imaged with a Nikon TE300 (Melville, NY) inverted microscope equipped with a Bio-Rad Radiance 2000 confocal laser scanning system (Hercules, CA) and Lasersharp 2000 imaging software. A Plan Apochromat 60× 1.4 oil immersion objective was used for collecting images. Laser parameters and pinhole sizes were kept constant for each respective study.
H&E staining was performed with a Varistain™ 24-4 Automatic Slide Stainer (Shandon Scientific Limited, Cheshire, England).
2.7 Neuronal quantitation
Tissue sections were viewed with a Nikon E-800 microscope attached to a Cohu videocamera connected to a Pentium PC computer. The following anatomical features were used to define the borders of the SN. Rostral: the first coronal section moving from rostral to caudal in the midbrain region that contains TH-positive neurons in the A9 (SN) cell group; Caudal: the last coronal section that includes TH-positive in the A9 cell group; Medial: The appearance of TH-positive neurons that belong to A10 cell group (ventral tegmental area, VTA); Lateral: the disappearance of TH-positive neurons of the A9 cell group. Based on these defined boundaries, numbers of TH-positive neurons in the substantia nigra were determined. Neuron counts were obtained from one focal plane of non-overlapping images. Within each section counted, the field of view was focused on the dorsal region of the SN using a 40X objective. Sequential images were captured and saved as the field of view moved towards the VTA. Counting was performed by assistants blind to genotype using the public domain NIH Image J program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). Mean cell number per plane and SEM were then calculated for each group.
2.8 Statistical analysis
For the aging study group, spontaneous motor activity was recorded by using ANYMAZE software. Means and SEMs were determined for DA, DOPAC and HVA. Quantitative data from histological analysis (number of TH-positive neurons) are expressed as number of TH-positive neurons per section and SEMs. Two-way ANOVA tests were used to determine OGG1 genotype and age effect in the status of the nigrostriatal system (DA levels or TH-positive neurons). One-way ANOVA tests were used to compare measured parameters in CP followed by the student Newman Keuls test for significant differences for DA levels, OGG1 genotype, and age. Student t-test was used to evaluate parameters that include a single condition (age or treatment).
The toxicological effects of MPTP in the 3 month old mice (young) were analyzed as follows: All group values were expressed as mean ± SEM. Between group differences for striatal concentrations of DA evaluated for treatment group by one-way ANOVA tests followed by the student Newman Keuls test for significant differences for DA levels. The significance level of all evaluations was set at p<0.05. Statistical tests were performed with Graphpad Prism® statistical software (Sorrento, CA).
3 Results
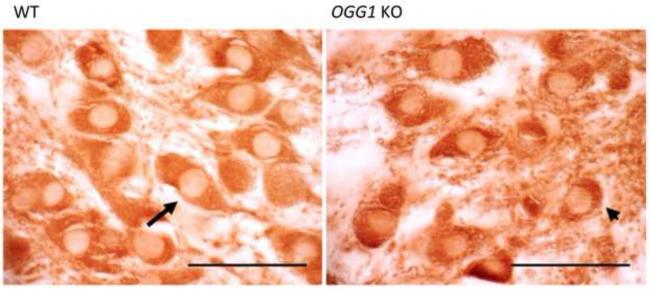
It has previously been established that as OGG1 KO mice age they accumulate oxo8dG at a faster rate than age matched WT mice (Klungland et al., 1999). Although the age-dependent accumulation of oxo8dG has been demonstrated in whole brain, no reports have aimed to demonstrate the same phenomena in discrete brain regions. Using HPLC-ECD and oxo8dG histological assessment, we identified higher levels of the damaged base in CP (Figure 1A) and in SN (Figure 1B) of aged OGG1 KO mice when compared with age-matched WT. Both approaches indicate that oxo8dG levels are increased in the OGG1 KO mice.
Fig. 1.
(A) oxo8dG levels in DNA from caudate putamen (CP) of aged (26 month old) WT (white bar) and OGG1 KO (black bar) mice. Data expressed as mean ± SEM. *Significant difference as compared to age-matched control values (p<0.05; n=8-9). (B) Microphotograph of oxo8dG immunostaining in the substantia nigra (SN) of aged (26 months old) WT and OGG1 KO mice.
3.1 Spontaneous motor behavior
Aged OGG1 KO mice exhibited decreased line crossings (Figure 2A), speed (Figure 2B), and number of rearings (Figure 2C), while time immobile was longer compared to aged-matched WT mice (Figure 2D). These differences found in the aging group were not found in young mice (Corresponding inset for each Figures 2A, 2B, 2C, and 2D).
Fig. 2.
Open field test in aged WT (white bars) and OGG1 KO (black bars) mice. A) Line crossings; B) Mean speed; C) Number of rearings; D) Time immobile. * denotes significant difference from WT mice (p<0.05; n=10). Insets are data for same analysis in young WT (white bars) and OGG1 KO (black bars) mice.
3.2 Effects of aging in the nigrostriatal system of the OGG1 KO mice
Given these findings, along with reports of increased oxo8dG levels in brains of PD patients, we opted to evaluate the status of the nigrostriatal system in aged WT and OGG1 KO mice. Our data indicates that at 3 months of age there are not significant differences between WT and OGG1 KO mice (Figure 3A). Also, no significant differences were detected in the DA index of turnover between young WT and OGG1 KO mice (not shown). However, aged OGG1 KO mice have a striatal DA reduction of 35% as compared to age-matched WT mice (Figure 3B). This loss of striatal DA is paralleled by a reduction in the number of TH-positive neurons in the SN of the OGG1 KO (Figures 3C and 3D). It is worth noting that the difference in striatal DA between the WT and the OGG1 KO is only significant in the aged mice, indicating an age-dependent phenomenon that is related to the deficiency in repair of oxo8dG.
Fig. 3.
A) Striatal DA levels in young WT (white bar) and OGG1 KO (black bar); B) Striatal DA levels in aged WT (white bar) and OGG1 KO (black bar); C) number of TH-positive neurons in SN aged WT (white bar) and OGG1 KO (black bar) mice. D) Low magnification of TH-positive neurons in SN of WT (left) and OGG1 KO (right) aged mice. Data expressed as mean ± SEM. *Significant difference as compared to age-matched control values (p<0.05; n=6).
Neuronal degeneration is typically accompanied by many histological changes. High magnification of TH-positive neurons in the SN from Figure 3D reveals that the neurons in the OGG1 KO mice exhibit an increased level of atrophy, vacuolization, cytosolic condensation, and nuclear displacement (Figure 4).
Fig. 4.
High magnification of TH-positive neurons in the substantia nigra (SN) of WT (left) and OGG1 KO (right) aged mice. Neurons in the aged OGG1 KO mice exhibit increased levels of atrophy, vacuolization, and cytosolic condensation (arrow head). Long arrow shows a typical healthy TH-positive neuron in the SN of the aged WT mice. Scale Bar 50 μm.
H&E staining was employed to identify histopathological differences between aged WT and OGG1 KO mice. Gross pathological changes between aged WT and aged OGG1 KO mice were assessed in the SN (Figure 5A and 5B), CP (Figure 5C and 5D), and HP (Figure 5E and 5F). OGG1 KO SN neurons exhibit signs of nuclear condensation and displacement, shrinkage of the cytoplasm (arrow head in Figure 5B) not seen in the WT SN. No evidence of chromatolysis or nuclear displacement is evident in the CP (Figures 5C and D). However, HP neurons in the aged OGG1 KO (Figure 5F) mice exhibit a reduced cell volume but retain their cytoarchitectural organization. Lower magnification picture indicates areas used for histological assessments in HP and SN (supplemental Figure 1).
Fig. 5.
Representative pictures of hematoxylin and eosin staining in the substantia nigra (A, B), caudate putamen (C, D) and hippocampus (E, F) of aged WT (A, C, E) and OGG1 KO (B, D, F) mice. Long arrows show nuclear location, indicating nuclear condensation in the SN of the OGG1 KO. Open arrowhead shows a chromatolytic neuron with a displaced nucleus. Scale Bar 75 μm.
Histological changes in the SN, the decreased number of TH-positive neurons and signs of nuclear condensation, are paralleled by a reduction in striatal levels of the neurotransmitter DA (Figure 3A). To test the specificity of this neurochemical deficiency, levels of striatal glutamate were determined in both WT and OGG1 KO mice at 3 and 26 months of age. No significant difference was detected between the genotypes and no age related changes were evident (Figure 6).
Fig. 6.
Glutamate levels in the caudate putamen of WT (white bars) and OGG1 KO (black bars) mice at 3 and 26 months of age. Data expressed as mean ± SEM (n=5-7).
In idiopathic PD, the striatal DA loss is accompanied by the formation of proteinaceous inclusions in the SN. These eosinophillic inclusions stain positive for ubiquitin and -synuclein (Alves-Rodrigues et al., 1998; Gibb et al., 1991; Wakabayashi et al., 1998). Given the histological changes seen in the SN of the aged OGG1 KO mice, we opted to evaluate brain sections for the presence of protein aggregations. In addition to a reduced number of TH-positive neurons in the SN of the aged OGG1 KO mice and to morphological changes in the surviving neurons, there is a development of ubiquitin-positive inclusions (Figure 7B, arrowheads). These inclusions are not present in the SN of the age-matched WT mice (Figure 7A). Also, the ubiquitin-positive inclusions appear to be restricted to the SN as indicated by a lack of staining in the VTA (Figure 7C WT, Figure 7D OGG1 KO). Despite the positive staining for ubiquitin we found little -synuclein reactivity in the SN of adjacent sections in the aged WT and OGG1 KO mice (Data not shown).
Fig. 7.
Representative microphotographs of the substantia nigra (A, B) and ventral tegmental area (C, D) of aged WT (A,C) and OGG1 KO (B,D) stained for ubiquitin. Long arrows denote neuronal localization; arrowheads identify intraneuronal ubiquitin aggregates. Scale Bar 50 μm.
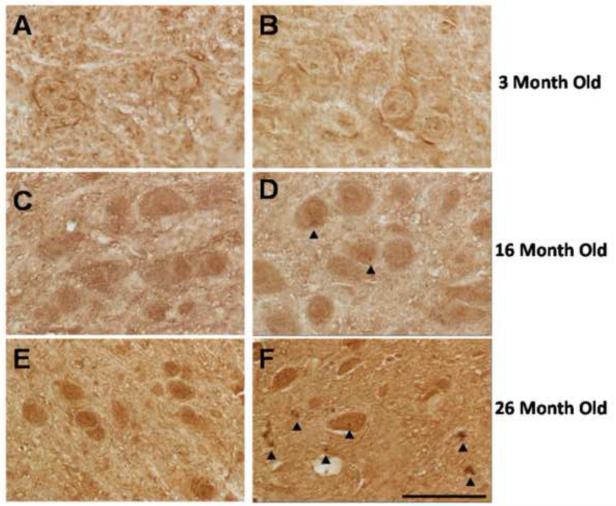
In order to establish the time associated events with ubiquitin aggregate formation, we stained the SN of WT and OGG1 KO mice at 3, 16, and 26 months of age. We found ubiquitin-positive staining to be age dependent in the SN of the OGG1 KO mice. No ubiquitin-positive inclusions were visible in the SN of WT mice at any age (Figure 8 A, C, and E); nor positive ubiquitin accumulation was found in the OGG1 KO at 3-months of age (Figure 8B). Inclusions were few and diffuse in the 16-month-old OGG1 KO SN (Figure 8D), and they became more prominent in the SN of the 26-month-old OGG1 KO mice (Figure 8E).
Fig. 8.
Microscopic assessment of age-associated accumulation of ubiquitin-positive intraneuronal inclusions in the WT and OGG1 KO mice. Representative photographs of the substantia nigra of WT (A,C,E) and OGG1 KO (B,D,F) mice. Arrow heads identify intraneuronal ubiquitin aggregates. Scale Bar 50 m.
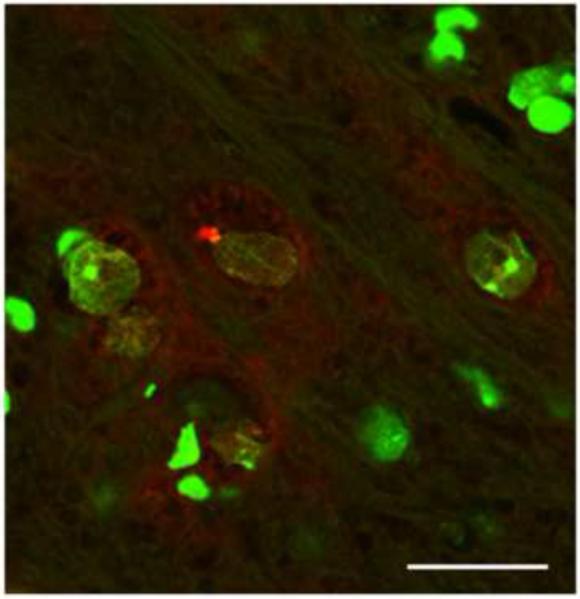
High magnification of NeuroTrace fluorescent Nissl stain (green) followed by immunoflorescense ubiquitin staining in the SN of a 26 month old mice indicate that the ubiquitin-positive inclusions are located inside of SN neurons (Figure 9).
Fig. 9.
Representative confocal micrograph of green Nissl NeurTrace staining (green) and ubiquitin positive immunostaining (red) in the SN of aged OGG1 KO mouse.
3.3 Neurotoxic response in the OGG1 KO mice
The objective of this study is to establish a relationship between increased oxidative burden to DNA due to deficient DNA repair and the susceptibility of the nigrostriatal system to an acute challenge with MPTP. Two-way ANOVA analysis identified an MPTP effect (F=10.72; df=1,48 ; p<0.05) and an interaction with age effect (F=8.50; df=1,48; p<0.05) in DA and DA index of turnover. One-way ANOVA analysis followed by student Newman’s Keul post-hoc test indicate a significant reduction (P<0.05) in DA levels and a significant elevation in DA index of turnover in the MPTP treated cohort, as compared to saline treated mice. The dose used in these studies is known to produce small changes in the nigrostriatal system of the 129/SvJ mice (Dauer et al., 2002). Young WT mice did not present DA reduction in striatum one week after a challenge with 25 mg/Kg of MPTP (two 12.5 mg/Kg i.p. injections 8 hours apart) (Figure 10A). However, we found that young OGG1 KO mice had an increased vulnerability with a 35% reduction in striatal DA levels one week after MPTP exposure (Figure 10 B). The reduction in DA is paralleled by a reduction in TH-positive staining in SN of MPTP-treated OGG1 KO mice (Figure 10 D) as compared to TH-positive staining in SN of MPTP-treated WT mice (Figure 10 C)
Fig. 10.
Assessment of MPTP susceptibility in 3 month old wild-type (A) Striatal DA levels in young WT treated with saline (white bar) or with MPTP (black bar) (B) Striatal DA levels in young OGG1 KO treated with saline (white bar) or with MPTP (black bar). Data expressed as mean ± SEM, *Significant differences from saline treated control. (p<0.05; n=6-8) (C) TH-positive staining in young WT after MPTP treatment (D) TH-positive staining in young OGG1 KO after MPTP treatment
4 Discussion
Our results suggest that elevated levels of oxo8dG due to deficient DNA repair capacity are a major player in the vulnerability of the nigrostriatal pathway in relationship to aging and to a challenge with MPTP. The notion that accumulation of oxidative damage to DNA is deleterious to specific neuronal populations, particularly those found in the nigrostriatal pathway, is supported by the age-dependent loss of TH-positive neurons, reduction in striatal dopamine, histopathological evidence, and the evidence of intraneuronal ubiquitin-positive inclusions only found in the aged OGG1 KO mice. The decreased spontaneous motor behavior found in aged OGG1 KO mice suggests that the modulatory effect of DA neurotransmission is affected by nigrostriatal pathway damage. Further support comes from the OGG1 expression-dependent susceptibility to MPTP.
Relevant clinical findings in PD patients are not restricted to the nigrostriatal pathway. For instance, systemic deficiencies such as decreased mitochondrial complex I activity have been found in patients with PD (Mizuno et al., 1989; Schapira et al., 1989). The mitochondrial complex I deficiency seen in PD was exploited to generate an animal model that reproduces deficits seen in PD. Rats chronically treated with the complex I inhibitor rotenone show neurochemical and histological changes similar to those seen in PD (Betarbet et al., 2000). Our results, attained by using a mouse deficient in the ability to remove the oxidatively modified base oxo8dG, fall into this category of whole system impact coupled with specific vulnerability of the nigral neurons, suggesting a role for oxo8dG accumulation prior to neuronal loss. Clinical findings of systemic increased oxo8dG levels in cases of PD (Abe et al., 2003; Alam et al., 1997; Sanchez-Ramos, 1994; Sato et al., 2005) are in agreement with our findings and lend support to the hypothesis that increased oxo8dG levels are precursors in neuronal loss. Reproduction of some of the deficiencies seen in PD using the DNA repair deficient mouse, OGG1 KO, suggests that accumulation of oxo8dG is a primary event in dopaminergic neuronal loss. Relevant to the results we have presented, in vitro studies show that chronic treatment with the mitochondrial complex I inhibitor rotenone leads to free radical production and oxidative damage to DNA (Beretta et al., 2006; de Lima et al., 2005).
Although Ogg1 has been extensively studied in the context of cancer, little is known of its activity in the central nervous system as a function of aging, neuropathological changes, or as a result of exposure to neurotoxicants. Recent reports demonstrate that Ogg1 is neuroprotective in neurotoxicological paradigms that impact the nigrostriatal pathway (Cardozo-Pelaez et al., 2005; Wong et al., 2008) similar to the results presented in our studies. Also, mice lacking the nucleotide-cleansing enzyme MTH1 exhibit increased levels of oxidative damage to RNA and DNA and are more susceptible to a challenge with MPTP (Yamaguchi et al., 2006). However, no assessment of the impact of aging has been done in the context of increased oxidative burden to DNA. Other investigators point that under pathological conditions that mimic ischemia in cellular and animal models Ogg1 activity ameliorates the neurodegenerative process and acts as neuroprotective mechanism (Liu et al., 2011). In conjunction with our results, all these studies support a major role for DNA damage in neurodegenerative processes.
The histological assessment and the lack of a gene- or age-dependent loss of striatal glutamate in the aged mice points to a specific impact to the dopaminergic neurons of the SN. It has been established that the metabolic pathway involved in DA removal leads to increased oxidative stress (Chiueh et al., 1993; Fahn and Cohen, 1992). In addition, we have previously shown that during aging the nigrostriatal pathway accumulates more oxo8dG than other brain regions (Cardozo-Pelaez et al., 1999). Consequently, this normal oxidative stress endogenous to the nigrostriatal pathway, coupled to impaired DNA repair in OGG1 KO, could render a population of dopaminergic neurons more susceptible to aging and toxicological challenge.
Results from genetic manipulation studies attempting to reproduce genetic anomalies found in familial cases of PD lend additional support to the role of oxidative stress in the neurological deficit. In particular, there is evidence of increased levels of oxo8dG in some of these models. Two -synuclein mutations, A30P and A53T, have been associated with the development of PD (Kruger et al., 1998; Polymeropoulos et al., 1997). Mice and cell cultures overexpressing the most toxic A53T form show evidence of oxidative stress, and in cell cultures its toxicity correlates with increased levels of oxo8dG (Giasson et al., 2002; Lee et al., 2001; Lee et al., 2002). The lack of overt -synuclein-positive aggregates in the aged OGG1 KO mice and the increased oxo8dG levels found in models harboring -synuclein mutations suggest that -synuclein aggregates are upstream of oxo8dG accumulation in the neurodegenerative cascade. Future studies of mice with Ogg1 deficiencies and -synuclein mutations could help to evaluate this possibility.
An understanding of the mechanisms associated with neuronal loss after an MPTP challenge has aided the development of PD therapeutics and PD animal models (Greenamyre et al., 2003; MacMahon and Bland, 1996; Olanow et al., 1996). We have shown that OGG1 KO mice are more susceptible to the toxic effects of MPTP than WT mice. This suggests a direct relationship between burden of damage to DNA and neuronal vulnerability. These findings could help to understand neurodegenerative mechanisms not previously addressed by other models and facilitate novel therapeutic advancements.
The susceptibility to toxic agents in the OGG1 KO mice is not restricted to MPTP. We have previously reported that Ogg1 plays an important role in protecting dopaminergic neurons against a neurotoxicological challenge with manganese (Cardozo-Pelaez et al., 2005). Exposure to manganese has been associated with the development of Parkinson-like deficiencies; however, most exposure protocols have failed to reproduce the effects of manganese in rodents (Newland, 1999; Pappas et al., 1997). Our previous manganese experiments contrast such findings and point to oxidative stress, specifically oxo8dG levels, as an indicator of neuronal health and susceptibility to toxins.
5 Conclusion
Mice lacking the enzyme Ogg1 show pathologic changes in the nigrostriatal system. This deficiency is age-dependent and appears to be localized in dopaminergic neurons of the SN. In addition, these mice show an age-dependent brakykinetic phenotype and an increased susceptibility to a challenge with MPTP. These results support the hypothesis that free radical mechanisms are heavily involved in the process of neurodegeneration and that oxidative damage to DNA affects neuronal populations, specifically the dopaminergic neurons of the SN. This mouse model of mild PD could be used to propose novel mechanisms of neurodegeneration and for the development of antioxidant therapies affording neuronal protection. Thus, our findings of increased vulnerability of the nigrostriatal system to neurotoxins such as manganese and MPTP, along with the age-dependent loss of SN neurons and intraneuronal ubiquitin accumulation in the OGG1 KO, lead us to infer that the OGG1 KO mice are a unique model for the neuronal loss seen in PD. Alternatively, it is possible to speculate that genetic or environmentally induced changes in OGG1 expression or activity can predispose an individual to the effects of other environmental or genetic factors, eventually leading to neuronal loss.
Supplementary Material
Highlights.
Mice Lacking the DNA repair enzyme Ogg1 present an age-dependent Parkinson Disease phenotype
Dopaminergic neurons in OGG1 KO mice have a higher susceptibility to the neurotoxin MPTP
Loss of dopamine neurons during aging is accompanied by the increased formation of ubiquitin positive inclusions
This reports supports the role of oxidative damage to DNA and the risk of Parkinson’s disease
Acknowledgements
This work was supported by NIA grant RO1 AG031184 to F.C.P. and by NIH P20 RR015583 and NIH P20RR017670. The investigator thanks Lou Herritt for her help at the Histology Core facility at U of M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors do not have a conflict of interest.
References
- Abe T, Isobe C, Murata T, Sato C, Tohgi H. Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci Lett. 2003;336:105–108. doi: 10.1016/s0304-3940(02)01259-4. [DOI] [PubMed] [Google Scholar]
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 1998;21:516–520. doi: 10.1016/s0166-2236(98)01276-4. [DOI] [PubMed] [Google Scholar]
- Beretta S, Wood JP, Derham B, Sala G, Tremolizzo L, Ferrarese C, Osborne NN. Partial mitochondrial complex I inhibition induces oxidative damage and perturbs glutamate transport in primary retinal cultures. Relevance to Leber Hereditary Optic Neuropathy (LHON) Neurobiol Dis. 2006;24:308–317. doi: 10.1016/j.nbd.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bolin C, Stedeford T, Cardozo-Pelaez F. Single extraction protocol for the analysis of 8-hydroxy-2′-deoxyguanosine (oxo8dG) and the associated activity of 8-oxoguanine DNA glycosylase. Journal of Neuroscience Methods. 2004;136f:69–76. doi: 10.1016/j.jneumeth.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Brooks PJ, Stedeford T, Song S, Sanchez-Ramos J. DNA damage, repair, and antioxidant systems in brain regions: a correlative study. Free Radic Biol Med. 2000;28:779–785. doi: 10.1016/s0891-5849(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Cox DP, Bolin C. Lack of the DNA repair enzyme OGG1 sensitizes dopamine neurons to manganese toxicity during development. Gene Expr. 2005;12:315–323. doi: 10.3727/000000005783992007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Song S, Parthasarathy A, Hazzi C, Naidu K, Sanchez-Ramos J. Oxidative DNA damage in the aging mouse brain. Mov Disord. 1999;14:972–980. doi: 10.1002/1531-8257(199911)14:6<972::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Chen LJ, Gao YQ, Li XJ, Shen DH, Sun FY. Melatonin protects against MPTP/MPP+ -induced mitochondrial DNA oxidative damage in vivo and in vitro. J Pineal Res. 2005;39:34–42. doi: 10.1111/j.1600-079X.2005.00209.x. [DOI] [PubMed] [Google Scholar]
- Chiou CC, Chang PY, Chan EC, Wu TL, Tsao KC, Wu JT. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin Chim Acta. 2003;334:87–94. doi: 10.1016/s0009-8981(03)00191-8. [DOI] [PubMed] [Google Scholar]
- Chiueh CC, Miyake H, Peng MT. Role of dopamine autoxidation, hydroxyl radical generation, and calcium overload in underlying mechanisms involved in MPTP-induced parkinsonism. Adv Neurol. 1993;60:251–258. [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima PD, Yamada ES, da Costa ET, Cdo O. Pessoa, Rabenhorst SH, Mde O. Bahia, Cardoso PC, Santos RA, Mde A. Smith, Burbano RR. Genotoxic effects of rotenone on cultured lymphocytes. Genet Mol Res. 2005;4:822–831. [PubMed] [Google Scholar]
- Devasagayam TP, Steenken S, Obendorf MS, Schulz WA, Sies H. Formation of 8-hydroxy(deoxy)guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry. 1991;30:6283–6289. doi: 10.1021/bi00239a029. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sci. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol. 1992;32:804–812. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Meira LB. Database of mouse strains carrying targeted mutations in genes affecting biological responses to DNA damage Version 7. DNA Repair (Amst) 2006;5:189–209. doi: 10.1016/j.dnarep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Fukae J, Mizuno Y, Hattori N. Mitochondrial dysfunction in Parkinson’s disease. Mitochondrion. 2007;7:58–62. doi: 10.1016/j.mito.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Scott T, Lees AJ. Neuronal inclusions of Parkinson’s disease. Mov Disord. 1991;6:2–11. doi: 10.1002/mds.870060103. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism Relat Disord. 2003;9(Suppl 2):S59–64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986;246:501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE. Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proc Natl Acad Sci U S A. 1999;96:13300–13305. doi: 10.1073/pnas.96.23.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Kruman II, Wersto RP, Cardozo-Pelaez F, Smilenov L, Chan SL, Chrest FJ, Emokpae R, Jr., Gorospe M, Mattson MP. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41:549–561. doi: 10.1016/s0896-6273(04)00017-0. [DOI] [PubMed] [Google Scholar]
- Lee M, Hyun D, Halliwell B, Jenner P. Effect of the overexpression of wild-type or mutant alpha-synuclein on cell susceptibility to insult. J Neurochem. 2001;76:998–1009. doi: 10.1046/j.1471-4159.2001.00149.x. [DOI] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Croteau DL, Souza-Pinto N, Pitta M, Tian J, Wu C, Jiang H, Mustafa K, Keijzers G, Bohr VA, Mattson MP. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. Journal of cerebral blood flow and metabolism. 2011 doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMahon DG, Bland R. Effect of adding selegeline to levodopa in early, mild Parkinson’s disease. Selegeline is effective and safe in early stages. Bmj. 1996;312:703. author reply 704-705. [PMC free article] [PubMed] [Google Scholar]
- Mancuso C, Scapagini G, Curro D, Stella A.M. Giuffrida, De Marco C, Butterfield DA, Calabrese V. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- Mandavilli BS, Ali SF, Van Houten B. DNA damage in brain mitochondria caused by aging and MPTP treatment. Brain Res. 2000;885:45–52. doi: 10.1016/s0006-8993(00)02926-7. [DOI] [PubMed] [Google Scholar]
- Migliore L, Petrozzi L, Lucetti C, Gambaccini G, Bernardini S, Scarpato R, Trippi F, Barale R, Frenzilli G, Rodilla V, Bonuccelli U. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology. 2002;58:1809–1815. doi: 10.1212/wnl.58.12.1809. [DOI] [PubMed] [Google Scholar]
- Milligan JR, Aguilera JA, Ward JF. Redox equilibrium between guanyl radicals and thiocyanate influences base damage yields in gamma irradiated plasmid DNA. Estimation of the reduction potential of guanyl radicals in plasmid DNA in aqueous solution at physiological ionic strength. Int J Radiat Biol. 2001;77:1195–1205. doi: 10.1080/09553000110083988. [DOI] [PubMed] [Google Scholar]
- Minowa O, Arai T, Hirano M, Monden Y, Nakai S, Fukuda M, Itoh M, Takano H, Hippou Y, Aburatani H, Masumura K, Nohmi T, Nishimura S, Noda T. Mmh/Ogg1 gene inactivation results in accumulation of 8-hydroxyguanine in mice. Proc Natl Acad Sci U S A. 2000;97:4156–4161. doi: 10.1073/pnas.050404497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun. 1989;163:1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Tsuchimoto D, Yamaguchi H, Sakumi K. Oxidative damage in nucleic acids and Parkinson’s disease. J Neurosci Res. 2007;85:919–934. doi: 10.1002/jnr.21191. [DOI] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese’s neurotoxicity. Neurotoxicology. 1999;20:415–432. [PubMed] [Google Scholar]
- Olanow CW, Godbold JH, Koller W. Effect of adding selegeline to levodopa in early, mild Parkinson’s disease. Patients taking selegeline may have received more levodopa than necessary. Bmj. 1996;312:702–703. author reply 704-705. [PMC free article] [PubMed] [Google Scholar]
- Opresko PL, Fan J, Danzy S, Wilson DM, 3rd, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterod M, Hollenbach S, Hengstler JG, Barnes DE, Lindahl T, Epe B. Age-related and tissue-specific accumulation of oxidative DNA base damage in 7,8-dihydro-8-oxoguanine-DNA glycosylase (Ogg1) deficient mice. Carcinogenesis. 2001;22:1459–1463. doi: 10.1093/carcin/22.9.1459. [DOI] [PubMed] [Google Scholar]
- Pappas BA, Zhang D, Davidson CM, Crowder T, Park GA, Fortin T. Perinatal manganese exposure: behavioral, neurochemical, and histopathological effects in the rat. Neurotoxicol Teratol. 1997;19:17–25. doi: 10.1016/s0892-0362(96)00185-7. [DOI] [PubMed] [Google Scholar]
- Petrozzi L, Lucetti C, Gambaccini G, Bernardini S, Del Dotto P, Migliore L, Scarpato R, Bonuccelli U. Cytogenetic analysis oxidative damage in lymphocytes of Parkinson’s disease patients. Neurol Sci. 2001;22:83–84. doi: 10.1007/s100720170058. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Proteggente AR, England TG, Rehman A, Rice-Evans CA, Halliwell B. Gender differences in steady-state levels of oxidative damage to DNA in healthy individuals. Free Radic Res. 2002;36:157–162. doi: 10.1080/10715760290006475. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Ramos J, Ames B. A Marker of Oxyradical-Mediated DNA Damage (8-Hydroxy-2′-Deoxyguanosine) is Increased in Nigro-Striatum of Parkinson’s Disease Brain. Neurodegeneration. 1994;3:197–204. [Google Scholar]
- Sato S, Mizuno Y, Hattori N. Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology. 2005;64:1081–1083. doi: 10.1212/01.WNL.0000154597.24838.6B. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Shao C, Xiong S, Li GM, Gu L, Mao G, Markesbery WR, Lovell MA. Altered 8-oxoguanine glycosylase in mild cognitive impairment and late-stage Alzheimer’s disease brain. Free Radic Biol Med. 2008;45:813–819. doi: 10.1016/j.freeradbiomed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenken S, Jovanovic S. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997;119:617–618. [Google Scholar]
- Stuart JA, Bourque BM, de Souza-Pinto NC, Bohr VA. No evidence of mitochondrial respiratory dysfunction in OGG1-null mice deficient in removal of 8-oxodeoxyguanine from mitochondrial DNA. Free Radic Biol Med. 2005;38:737–745. doi: 10.1016/j.freeradbiomed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, Yamada M, Toyoshima Y, Yoshimoto M, Takahashi H. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 1998;96:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J Neurochem. 2005;93:953–962. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]
- Wong AW, McCallum GP, Jeng W, Wells PG. Oxoguanine glycosylase 1 protects against methamphetamine-enhanced fetal brain oxidative DNA damage and neurodevelopmental deficits. J Neurosci. 2008;28:9047–9054. doi: 10.1523/JNEUROSCI.2557-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Kajitani K, Dan Y, Furuichi M, Ohno M, Sakumi K, Kang D, Nakabeppu Y. MTH1, an oxidized purine nucleoside triphosphatase, protects the dopamine neurons from oxidative damage in nucleic acids caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Cell death and differentiation. 2006;13:551–563. doi: 10.1038/sj.cdd.4401788. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.