Abstract
AIM: To investigate the clinical course of untreatable hepatocellular carcinoma (HCC) identified at any stage and to identify factors associated with mortality.
METHODS: From January 1999 to December 2010, 320 out of 825 consecutive patients with a diagnosis of HCC and not appropriate for curative or palliative treatments were followed and managed with supportive therapy. Cirrhosis was diagnosed by histological or clinical features and liver function was evaluated according to Child-Pugh score. The diagnosis of HCC was performed by Ultra-Sound guided biopsy or by multiphasic contrast-enhanced computed tomography or gadolinium-enhanced magnetic resonance imaging. Data were collected for each patient including all clinical, laboratory and imaging variables necessary for the outcome prediction staging systems considered. HCC staging was performed according Barcelona Clinic Liver Cancer (BCLC) and Cancer of the Liver Italian Program scores. Follow-up time was defined as the number of months from the diagnosis of HCC to death. Prognostic baseline variables were analyzed by multivariate Cox analysis to identify the independent predictors of survival.
RESULTS: Seventy-five per cent of patients had hepatitis C. Ascites was present in 169 patients (53%), while hepatic encephalopathy was present in 49 patients (15%). The Child-Pugh score was class A in 105 patients (33%), class B in 142 patients (44%), and class C in 73 patients (23%). One hundred patients (31%) had macroscopic vascular invasion and/or extra-hepatic spread of the tumor. A single lesion > 10 cm was observed in 34 patients (11%), while multinodular HCC was present in 189 patients (59%). Thirty nine patients (12%) were BCLC early (A) stage, 55 (17%) were BCLC intermediate (B) stage, 124 (39%) were BCLC advanced (C) stage, and 102 (32%) were end-stage BCLC (D). At the time of this analysis (July 2011), 28 (9%) patients were still alive. Six (2%) patients who were lost during follow-up were censored at the last visit. The overall median survival was 6.8 mo, and the 1-year survival was 32%. The 1-year survival according to BCLC classes was 100%, 79%, 12% and 0%, for BCLC A, B, C and D, respectively. There was a significant difference in survival between each BCLC class. The median survival of patients of BCLC stages A, B, C and D was 33, 17.4, 6.9, and 1.8 mo, respectively (P < 0.05 for comparison between stages). The median survival of Child-Pugh A, B and C classes were 9.8 mo (range 6.4-13), 6.1 (range 4.9-7.3), and 3.7 (range 1.5-6), respectively (P < 0.05 for comparison between stages). By univariate analysis, the variables significantly associated to an increased liklihood of mortality were Eastern Cooperative Oncology Group performance status (PS), presence of ascites, low level of albumin, elevated level of bilirubin, international normalized ratio (INR) and Log-[(α fetoprotein (AFP)]. At multivariate analysis, mortality was independently predicted by bad PS (P < 0.0001), high INR values (P = 0.0001) and elevated Log-(AFP) levels (P = 0.009).
CONCLUSION: This study confirms the heterogeneous behavior of untreated HCC. BCLC staging remains an important prognostic guide and may be important in decision-making for palliative treatment.
Keywords: Hepatocellular carcinoma, Liver, Cancer, Survival, Prognosis, Natural history
INTRODUCTION
Hepatocellular carcinoma (HCC) is associated with a high rate of mortality[1] and, despite extensive application of intensive surveillance programs, considerable therapeutic progress, and technological improvement observed over the past few years, prognosis of this tumor is poor even when treatments have been considered potentially curative[2].
Curative treatments for early-stage tumors include liver transplantation, resection and percutaneous ablation. Transarterial chemoembolization (TACE) and sorafenib can improve survival for patients with intermediate and advanced tumors, respectively[3].
Although in two large-scale studies[4,5], sorafenib has been shown to improve survival in unresectable HCC patients with well-preserved liver function, response rates remain poor. Moreover, recent studies showed that tolerability was moderate and that most patients need reduction or interruption of treatment[6-8]. So, there is a need for further properly designed randomized controlled trials (RCTs) to assess the survival benefits of second-line systemic therapies. The design of such trials would require an accurate estimation of the survival of patients with untreated disease, preferably with stratification according to known prognostic factor. In addition, as soon as the results of such trials are available, patients and their physicians will become aware of the natural history of the untreated disease will be better able to decide whether or not to accept other palliative treatments.
The natural course of unresectable HCC has recently been evaluated in a meta-analysis[9] which analyzed the survival rates of the placebo and untreated arms of several RCTs on HCC patients, showing that the 1- and 2-year survival is extremely heterogeneous.
For ethical reasons it is not possible to evaluate the natural history of early HCC in RCTs. However a milestone paper[10] published in 1989 showed that 1- and 2-year overall survival (OS) of asymptomatic patients with HCC and cirrhosis was 96% and 50%, respectively.
To provide updated survival data on untreated HCC in Italy, we analyzed the clinical data of a cohort of HCC patients followed in our Liver Unit.
MATERIALS AND METHODS
Patients
From January 1999 to December 2010, 825 consecutive patients with cirrhosis and a new diagnosis of HCC were observed at our Liver Unit. Cirrhosis was diagnosed by histological or clinical features and the liver function was evaluated according to Child-Pugh score. The diagnosis of HCC was performed by ultrasound guided biopsy or by multiphasic contrast-enhanced computed tomography or gadolinium-enhanced magnetic resonance imaging. Performance status (PS) was scored according to the Eastern Cooperative Oncology Group (ECOG)[11].
All patients were evaluated according to European Association for the Study of the Liver criteria[12] up to 2005, and to American Association for the Study of Liver Diseases criteria[13] from January 2006. HCC staging and the choice of treatment were performed according to the Barcelona Clinic Liver Cancer (BCLC) schedule[14].
Patients with early tumors (BCLC A) were considered for curative therapies [resection, orthotopic liver transplantation (OLT), or radiofrequency thermal ablation (RFTA)]. TACE was performed in patients at intermediate stage (BCLC B) according to BCLC and in early-stage (BCLC A) patients for whom percutaneous RFTA was not feasible because of tumor location (proximity to gall-bladder, biliary tree, or blood vessel) or in whom surgery could not be performed because of comorbidities[6,15-19]. Combined treatments were used when indicated to achieve a better radical cure[20]. Starting July 2008, patients with advanced HCC and patients with an intermediate HCC who were not eligible for or failed loco-ablative therapies were treated with sorafenib.
The current study analyzed the natural course of patients with HCC at any stage who were untreated for any cause. Follow-up was censored on July 31, 2011. The main causes for non-treatment were the presence of severe co-morbidities or impaired PS[3,4], advanced age, refusal of treatment, diffuse or massive tumor with or without macro-vascular invasion or extra-hepatic spread before the advent of sorafenib, poor residual liver function (Child-Pugh > B8) precluding OLT.
Outcome
The primary outcome measure in this analysis was survival. Follow-up time was defined as the number of months from the diagnosis of HCC to death. All subjects were followed as outpatients or inpatients at our Liver Unit and clinical data were collected by telephone follow-up when clinical worsening did not allow the patient to present for medical controls.
Statistical analysis
Data collected for each patient included all clinical, laboratory and imaging variables necessary for the outcome prediction staging systems considered. Patients were also stratified according to Child-Pugh, BCLC and Italian (Cancer of the Liver Italian Program) classifications. Continuous variables were expressed as mean ± SD. The Kaplan-Meier estimator was applied to survival. Differences in the survival rate were assessed by log-rank testing. Variables listed in Table 1 were analyzed using univariate analysis. All variables with a P-value less than 0.05 by univariate analysis were subjected to multivariate analysis. The multivariate analysis was performed by the Cox proportional hazard model. All statistical analyses were performed with the Statistical Analysis System (SAS) version 8.1 (SAS Institute, Inc., Cary, NC, United States).
Table 1.
Baseline demographic, laboratory, clinical and tumour staging characteristics of 320 untreated hepatocellular carcinoma patients (mean ± SD) n (%)
| Variable | Patients (n = 320) |
| Age (yr) | 68 ± 9.8 |
| Sex | |
| Male | 226 (71) |
| Female | 94 (29) |
| Etiology of cirrhosis | |
| Hepatitis C only | 241 (75) |
| Hepatitis B only | 27 (8) |
| Alcohol abuse only | 12 (4) |
| Multiple | 9 (3) |
| Other | 31 (10) |
| Biopsy-proven HCC diagnosis | 60 (19) |
| Non-invasive HCC diagnosis | 260 (81) |
| ECOG performance status1 | |
| 0 | 94 (30) |
| 1-2 | 123 (38) |
| 3-4 | 103 (32) |
| Hepatic encephalopathy | |
| None | 271 (85) |
| Grades I-II | 39 (12) |
| Grades III-IV | 10 (3) |
| Ascites | |
| Absent | 151 (48) |
| Slight | 122 (38) |
| Moderate-severe | 47 (14) |
| Child-pugh score | 8 ± 2 |
| Child-pugh classes | |
| A | 105 (33) |
| B | 142 (44) |
| C | 73 (23) |
| Albumin (g/dL) | 3.1 ± 0.6 |
| International normalized ratio | 1.4 ± 0.4 |
| Total bilirubin (mg/dL) | 2.8 ± 4.2 |
| Platelet × 103/mmc | 112 ± 80 |
| Uninodular HCC | 131 (41) |
| Multinodular HCC | 189 (59) |
| Single lesion > 10 cm | 34 (11) |
| Macroscopic vascular invasion and/or extrahepatic spread | 100 (31) |
| AFP (ng/mL) | |
| Median | 35 |
| Range | 1-115.000 |
| AFP ≥ 200 ng/dL | 79 (24) |
| BCLC stage | |
| A (early) | 39 (12) |
| B (intermediate) | 55 (17) |
| C (advanced) | 124 (39) |
| D (end-stage) | 102 (32) |
| CLIP score | |
| 0 | 23 (7) |
| 1 | 49 (15) |
| 2 | 127 (40) |
| 3 | 67 (21) |
| 4 | 34 (11) |
| 5 | 17(5) |
| 6 | 3 (1) |
Eastern Cooperative Oncology Group-Performance Status. AFP: α fetoprotein; BCLC: Barcelona Clinic Liver Cancer; CLIP: Cancer of LiverItalian Program; HCC: Hepatocellular carcinoma; ECOG: Eastern Cooperative Oncology Group.
RESULTS
Patient features at baseline
The study population consisted of 320 patients with HCC secondary to cirrhosis of various etiologies. The demographical, clinical and tumor staging features of the 320 patients are given in Table 1. Chronic hepatitis C virus infection was the dominant etiology (75%).
At presentation, the Child-Pugh score was class A in 105 patients (33%), class B in 142 patients (44%), and class C in 73 patients (23%). Ascites was present in 169 patients (53%), while hepatic encephalopathy was present in 49 patients (15%).
Regarding the features of HCC, 100 patients (31%) had macroscopic vascular invasion and/or extra-hepatic spread of the tumour. A single lesion > 10 cm was observed in 34 patients (11%), while multinodular HCC was present in patients 189 (59%). Thirty nine patients (12%) were BCLC early (A) stage, 55 (17%) were BCLC intermediate (B) stage, 124 (39%) were BCLC advanced (C) stage, and 102 (32%) were end-stage BCLC (D).
At the time of this analysis (July 2011), 28 (9%) patients were still alive. Six (2%) patients who were lost during follow-up were censored at their last visit.
Survival
The median OS was 6.8 mo (95%CI: 5.8-7.7), corresponding to 33% of the patients being alive at 1 year. The 1-year survival according to BCLC class was 100%, 79%, 12% and 0%, for BCLC A, B, C and D, respectively (Table 2 and Figure 1).
Table 2.
Summary of follow-up in 320 untreated hepatocellular carcinoma patients (%)
| Outcome | Patients (n = 320) |
| Death | 292 (91) |
| Overall survival | |
| Median (95%CI) (mo) | 6.8 (5.8-7.7) |
| 1-yr survival rate | 32 |
| 2-yr survival rate | 13 |
| BCLC A (early stage) survival | |
| Median (95%CI) (mo) | 33 (20-46) |
| 1-yr survival rate | 100 |
| 2-yr survival rate | 57 |
| 3-yr survival rate | 41 |
| BCLC B (intermediate stage) survival | |
| Median (95%CI) (mo) | 17.4 (14.8-20) |
| 1-yr survival rate | 79 |
| 2-yr survival rate | 22 |
| 3-yr survival rate | 5 |
| BCLC C (advanced stage) survival | |
| Median (95%CI) (mo) | 6.9 (6.3-7.3) |
| 1-yr survival rate | 12 |
| BCLC D (end-stage) survival | |
| Median (95%CI) (mo) | 1.8 (1.2-2.4) |
| 1-yr survival rate | 0 |
BCLC: Barcelona Clinic Liver Cancer classification.
Figure 1.
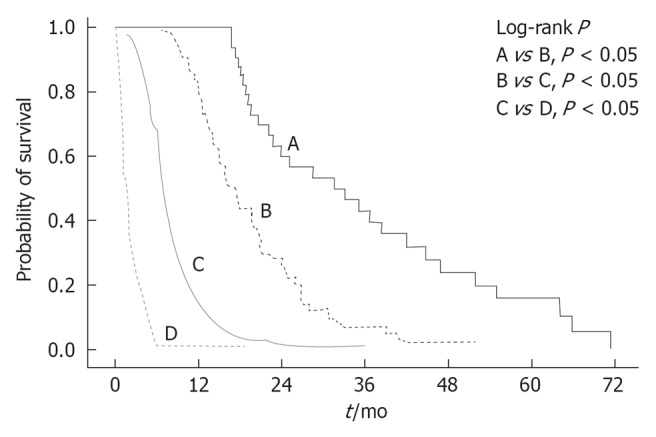
Kaplan-Meier analysis of 320 untreated hepatocellular carcinoma patients. Survival according to Barcelona Clinic Liver Cancer classification.
There was a significant progressive difference in survival between each BCLC class. The median survival of BCLC A (33 mo; range 20-46) was significantly longer than that of BCLC B (17.4 mo; range 14.8-20), BCLC C (6.9 mo; range 6.3-7.3), and BCLC D (1.8 mo; range 1.2-2.4).
The median survival of Child-Pugh A, B and C classes were 9.8 mo (range 6.4-13), 6.1 mo (range 4.9-7.3), and 3.7 mo (range 1.5-6), respectively (P < 0.05 for comparison between stages).
By univariate analysis, the variables significantly associated to an increased likelihood of mortality were ECOG PS, presence of ascites, low level of albumin, elevated level of bilirubin, international normalized ratio (INR) and Log-[α fetoprotein (AFP)]. Cox regression analysis showed that PS [hazard ratio (HR) 2.875, 95%CI: 2.547-3.245, P < 0.0001], INR (HR 1.811, 95%CI: 1.328-2469, P = 0.0001), and Log-(AFP) (HR 1.078, 95%CI: 1.018-1.142, P = 0.009) were independent risk factors for mortality (Table 3).
Table 3.
Multivariate Cox-regression models for predicting over all survival in 320 patients with hepatocellular carcinoma patients in cirrhosis
| Variable | HR | 95%CI | P value |
| Performance status1 | 2.875 | 2.547-3.245 | < 0.0001 |
| INR | 1.811 | 1.328-2469 | 0.0001 |
| Log-(AFP) | 1.078 | 1.018–1.142 | 0.009 |
| Albumin | - | - | - |
| Total Bilirubin | - | - | - |
| Presence of ascites | - | - | - |
Eastern Cooperative Oncology Group-Performance Status. HR: Hazard ratio; INR: International Normalized Ratio; AFP: α fetoprotein.
DISCUSSION
HCC secondary to cirrhosis is a complex and heterogeneous disease with wide variations during its clinical course. In this context, the management of cirrhotic patients with neoplasm is a major clinical issue[21,22].
A better knowledge of the natural history of the tumor as well as the development of clinically based staging systems, such the BCLC classification which stratifies patients according to the stage of the tumor and liver disease, has meant that life expectancies can be confidently predicted, and the appropriate treatment can be chosen according to stage.
This study shows that in patients with untreated HCC, survival can be predicted from information collected by the physician as part of the initial assessment. In fact, the identified prognostic factors (PS, INR and AFP) are easily measured. Decreased PS has been previously found to have prognostic value in patients with HCC[9,14]. In our cohort, advanced liver diseases, assessed by high INR was associated with improvement in survival, while elevated serum AFP reflects the degree of cellular differentiation and thus the spread of the tumor. Moreover, our study confirms that the BCLC staging classification sensitively identifies HCC patients with a good or unfavorable prognosis.
Although, the median survival for early stage observed in our study was good (33 mo), data on survival rates of early HCC patients confirm, even in absence of data from RCT, the effectiveness of any current treatment for this cancer.
Clearly, the impact of sorafenib for patients with advanced HCC and with intermediate HCC who were unfit or failed to respond to ablative therapies, is a landmark in the treatment of liver cancer. However, given the high rate of therapy discontinuation for adverse events or radiological progression, data on survival of untreated intermediate/advanced stage patients could give useful information in the design of RCTs on second-line treatment with new agents after failure of sorafenib therapy[23,24].
In our cohort, HCC patients in BCLC D stage at baseline have a 1-year survival of less than 5%. Given this poor life expectancy, BCLC suggests only symptomatic treatment.
The study had some bias. Firstly, it was conducted retrospectivelyalthough, because the only defined endpoint was survival, it is unlikely that the results were affected. Another weakness is the lack of data on molecular factors, such as gene expression profiling, which can have some impact on patient outcome[25,26].
The treatment of HCC has changed dramatically. Years ago, there were no safe or reliable therapies for patients diagnosed with this cancer and their prognosis was uniformly grim. Now, all stages of the disease may receive effective therapy and current research will expand the existing benefits. However, the current therapeutic approach still needs significant improvement. Firthermore, the therapeutic options for patients with advanced HCC have limited impact and thus, development of new agents and strategies for this group of patients is of majorimportance.
In untreated HCC patients, the available evidence is sufficient to conclude that poor PS, high INR values and high AFP levels are associated with worse prognosis. BCLC staging classification sensitively identifies HCC untreated patients with a good or unfavorable prognosis. Patients at BCLC end-stage should only receive symptomatic treatment.
COMMENTS
Background
Hepatocellular carcinoma (HCC) is an insidious disease, with no particular or specific signs and symptoms of manifestation and whose behavior is usually unpredictable. Its natural history is also dependent on functional impairment of the underlying liver disease which often limits the application of therapeutic modalities and influences survival.
Research frontiers
The spontaneous course of the unresectable disease has recently been evaluated in a meta-analysis which analyzed the survival rates of the placebo and untreated arms of several randomized controlled trials (RCTs) on HCC patients, showing that the 1- and 2-year survival is extremely heterogeneous.
Innovations and breakthroughs
For ethical reasons it is not possible to evaluate the natural history of early HCC in RCTs. In patients with untreated HCC, survival can be predicted from information collected by the physician as a part of the initial assessment. In fact, the prognostic factors identified [performance status (PS), international normalized ratio (INR) and α-fetoprotein (AFP)] are easily measured. Moreover, the Barcelona Clinic Liver Cancer (BCLC) staging classification sensitively identifies HCC untreated patients with a good or unfavorable prognosis, and patients at BCLC end-stage should only receive symptomatic treatment.
Applications
Knowing the spontaneous outcome of HCC is important for designing RCTs of new therapeutic approaches, for assessing the validity of biological and radiological surrogate markers, and in controlling for confounding factors in observational studies.
Peer review
The study entitled “Natural history of untreatable HCC: A retrospective cohort study” by Giuseppe Cabibbo et al describes the natural history of untreated patients with HCC. Poor prognosis (survival) is related to bad PS, high INR and AFP values. The study is interesting for the readers even though it does not include any molecular analysis which could give additional information.
Footnotes
Peer reviewers: Lang Zhuo, PhD, Team Leader and Principal Research Scientist, Institute of Bioengineering and Nanotechnology, 31 Biopolis Way, The Nanos #04-16, Singapore 138669, Singapore; Jordi Muntané, PhD, Unidad de Investigacion, Hospital Universitario Reina Sofía, Av. Menéndez Pidal s/n, Cordoba 14004, Spain
S- Editor Jia F L- Editor Hughes D E- Editor Zheng XM
References
- 1.Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M, Talwalkar J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 7.Pinter M, Sieghart W, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Matejka J, et al. Prognostic factors in patients with advanced hepatocellular carcinoma treated with sorafenib. Aliment Pharmacol Ther. 2011;34:949–959. doi: 10.1111/j.1365-2036.2011.04823.x. [DOI] [PubMed] [Google Scholar]
- 8.Schütte K, Zimmermann L, Bornschein J, Csepregi A, Rühl R, Ricke J, Malfertheiner P. Sorafenib therapy in patients with advanced hepatocellular carcinoma in advanced liver cirrhosis. Digestion. 2011;83:275–282. doi: 10.1159/000320377. [DOI] [PubMed] [Google Scholar]
- 9.Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. doi: 10.1002/hep.23485. [DOI] [PubMed] [Google Scholar]
- 10.Cottone M, Virdone R, Fusco G, Orlando A, Turri M, Caltagirone M, Maringhini A, Sciarrino E, Demma I, Nicoli N. Asymptomatic hepatocellular carcinoma in Child’s A cirrhosis. A comparison of natural history and surgical treatment. Gastroenterology. 1989;96:1566–1571. doi: 10.1016/0016-5085(89)90528-3. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 14.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–33. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 15.Cabibbo G, Genco C, Di Marco V, Barbara M, Enea M, Parisi P, Brancatelli G, Romano P, Craxì A, Cammà C. Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Aliment Pharmacol Ther. 2011;34:196–204. doi: 10.1111/j.1365-2036.2011.04694.x. [DOI] [PubMed] [Google Scholar]
- 16.Sandonato L, Cipolla C, Fulfaro F, Lo Re G, Latteri F, Terranova A, Mastrosimone A, Bova V, Cabibbo G, Latteri MA. Minor hepatic resection using heat coagulative necrosis. Am Surg. 2009;75:1213–1219. [PubMed] [Google Scholar]
- 17.Cammà C, Di Marco V, Cabibbo G, Latteri F, Sandonato L, Parisi P, Enea M, Attanasio M, Galia M, Alessi N, et al. Survival of patients with hepatocellular carcinoma in cirrhosis: a comparison of BCLC, CLIP and GRETCH staging systems. Aliment Pharmacol Ther. 2008;28:62–75. doi: 10.1111/j.1365-2036.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- 18.Latteri F, Sandonato L, Di Marco V, Parisi P, Cabibbo G, Lombardo G, Galia M, Midiri M, Latteri MA, Craxì A. Seeding after radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis: a prospective study. Dig Liver Dis. 2008;40:684–689. doi: 10.1016/j.dld.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Cammà C, Di Marco V, Orlando A, Sandonato L, Casaril A, Parisi P, Alizzi S, Sciarrino E, Virdone R, Pardo S, et al. Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol. 2005;42:535–540. doi: 10.1016/j.jhep.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 20.Cabibbo G, Latteri F, Antonucci M, Craxì A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. 2009;6:159–169. doi: 10.1038/ncpgasthep1357. [DOI] [PubMed] [Google Scholar]
- 21.Cabibbo G, Palmeri L, Palmeri S, Craxì A. Should cirrhosis change our attitude towards treating non-hepatic cancer. Liver Int. 2012;32:21–27. doi: 10.1111/j.1478-3231.2011.02629.x. [DOI] [PubMed] [Google Scholar]
- 22.Cabibbo G, Rolle E, De Giorgio M, Genco C, Pressiani T, Spada F, Sacco R. Management of cirrhotic patients with hepatocellular carcinoma treated with sorafenib. Expert Rev Anticancer Ther. 2011;11:1807–1816. doi: 10.1586/era.11.139. [DOI] [PubMed] [Google Scholar]
- 23.Villanueva A, Llovet JM. Second-line therapies in hepatocellular carcinoma: emergence of resistance tzfo sorafenib. Clin Cancer Res. 2012;18:1824–1826. doi: 10.1158/1078-0432.CCR-12-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 26.Villanueva A, Hoshida Y, Toffanin S, Lachenmayer A, Alsinet C, Savic R, Cornella H, Llovet JM. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]


