Abstract
AIM: To compare clinicopathological features of acute presentation of type 1 autoimmune hepatitis (AIH) with or without centrilobular necrosis (CN).
METHODS: Our study comprised 41 patients with biopsy-proven acute presentation (acute exacerbation phase 36, acute hepatitis phase 5) of type 1 AIH at our hospital from 1975 to 2009. Elevated serum alanine aminotransferase (ALT) (> 5x upper limit of normal) identified acute presentation of the disease. We compared clinicopathological features of these AIH patients with or without CN. The data used for analysis included patient background (age, sex, type of disease, presence of complications with other autoimmune diseases, human leukocyte antigen, and International Autoimmune Hepatitis Group score), clinical parameters at presentation (ALT, alkaline phosphatase, IgG, anti-nuclear antibodies, and anti-smooth muscle antibodies), histology and therapy.
RESULTS: CN was found in 13 (31.7%) patients with acute presentation (acute exacerbation phase 10, acute hepatitis phase 3) of AIH. Serum IgG levels of patients with CN were significantly lower than those of patients without CN (mean: 2307 mg/dL vs 3126 mg/dL, P < 0.05), while antinuclear antibody-negative rates were significantly higher (30.7% vs 3.5%, P < 0.05). However, other clinical features were similar between the two groups. The frequency of advanced fibrosis in patients with CN was significantly lower than in patients without CN (F0-2: 84.6% vs 35.7%, F3-4: 15.4% vs 64.3%, P < 0.05). Other histological features were similar between the two groups. Although there was no significant difference between groups when evaluated using the revised original score (12 vs 14), the simplified AIH score of patients with CN was significantly lower (6 vs 7, P < 0.05). Frequency of DR4 was similar between patients with and without CN.
CONCLUSION: CN is observed in both Japanese patients with acute hepatitis phase and acute exacerbation phase of type 1 AIH, although AIH with CN often shows clinical features of the genuine acute form.
Keywords: Autoimmune hepatitis, Centrilobular necrosis, Acute presentation, Acute exacerbation phase, Acute hepatitis phase
INTRODUCTION
Autoimmune hepatitis (AIH) manifests as chronic liver inflammation of unknown cause. AIH generally affects young to middle-aged women, and is associated with the presence of autoantibodies and hypergammaglobulinemia[1]. The histological hallmark of AIH is interface hepatitis, although other histological findings are compatible with the disease. Human leukocyte antigen DR status is considered to affect the clinical features of patients with type 1 AIH. In Japanese patients, DR4 is dominantly associated with the disease. Patients with DR4 are typically older and respond better to corticosteroid treatment than those with DR3[2]. Centrilobular necrosis (CN) is a non-specific histological finding caused by hepatotoxins, such as acetaminophen, paracetamol, thioacetamide, tetrachloride, congestive hepatic injury and cardiac hepatopathy due to acute right side cardiac failure[3-5]. CN probably reflects early stage AIH detected primarily in patients with an acute onset[6-8]. Cases of acute presentation of AIH have frequently been reported. The first report showed two AIH cases presenting histologically acute hepatitis[9]. Other reports revealed cases of the clinically acute phase of AIH not associated with typical AIH[7,10]. Pathological examination of acute presentations revealed that the cases with CN demonstrate different characteristics from those of typical AIH[11]. Moreover, a recent report indicated that it is possible to distinguish the acute phase of AIH from the acute exacerbation phase of AIH by checking the existence of both CN and portal inflammation[12]. Recently, it has been reported from the AIH study group in Japan[13] that there are two types of acute presentation in patients with AIH, acute exacerbation phase and acute hepatitis phase. Here, we compared the clinicopathological features of acute presentation of AIH with and without CN.
MATERIALS AND METHODS
Patients
Our study comprised 41 patients with biopsy-proven acute presentation of AIH at our hospital from 1975 to 2009 (38 women and 3 men, mean age: 52.2 years). These patients were reviewed according to the revised original scoring system or the simplified scoring system of the International Autoimmune Hepatitis Group (IAIHG)[14-16]. We only applied pre-treatment criteria for AIH diagnosis. In the revised original system, scores of 10 to 15 points support a probable diagnosis while scores greater than 15 points support a definitive diagnosis. With the simplified system, a score of 6 points constitutes a probable diagnosis as a definitive diagnosis requires a score of 7 or more points. None of the patients showed evidence of other liver diseases such as viral hepatitis, haemochromatosis, Wilson’s disease, primary biliary cirrhosis or non-alcoholic steatohepatitis. We excluded patients with overlapping syndromes and patients with a daily alcohol intake of > 50 g (men) or > 25 g (women).
The data used for analysis included patient background (age, sex, type of disease, presence of complications with other autoimmune diseases, HLA, and IAIHG score), clinical parameters at presentation [alanine aminotransferase (ALT), alkaline phosphatase, IgG, anti-nuclear antibodies (ANA), and anti-smooth muscle antibodies (ASMA)], histology and therapy.
Serology and histology
ANA and ASMA were detected by indirect immunofluorescence using human epithelial (HEp-2) cells or frozen rat kidney sections. Elevated serum ALT (> 5x upper limit of normal) identified acute presentation of the disease. Patients with acute presentation of the disease with histological evidence of chronic hepatitis, such as the presence of fibrosis and inflammatory cell infiltrations in the portal tracts with interface hepatitis, were diagnosed acute exacerbation phase. Patients with acute presentation of disease but no history of any prior liver disease and no histological evidence of chronic hepatitis were diagnosed acute hepatitis phase. Using the simplified criteria, three characteristics are used for liver histology grading. Interface hepatitis, lymphocytic/lymphoplasmacytic infiltrates in portal tracts and extending into lobules, and emperipolesis and hepatic rosette formation are regarded as typical for AIH diagnosis. Therefore, the presence of all three features is required to be considered “typical” AIH liver histology. However, chronic hepatitis with lymphocytic infiltration but without all the features considered to be typical is compatible with AIH liver histology. Liver histology was considered “atypical” for AIH when showing signs of other diagnosis. Liver biopsy specimens diagnosed as chronic hepatitis underwent histological staging based on the classification of Desmet et al[17] as follows: No fibrosis (F0), fibrosis confined to portal tracts (F1), periportal or portal-portal septa but intact vascular relationships (F2), fibrosis with distorted structure but no obvious cirrhosis (F3) and probable or definite cirrhosis (F4). Confluent necrosis with inflammation in zone 3 was considered to be CN. Two pathologists blinded to the clinical data assessed histopathological findings. The clinicopathological features of AIH with CN were compared to AIH without CN.
Statistical analysis
We used the student’s t-test for comparing means, the Mann-Whitney U-test for non-normally distributed data and the χ2 test for differences in distributions between groups. A commercially available computer program (Prism version 4.0a; GraphPad Software, Inc.) was used for all statistical calculations and P < 0.05 (two tailed) was considered statistically significant.
RESULTS
In this study, thirty-six of 41 patients showed acute exacerbation phase and 5 of 41 showed acute hepatitis phase. CN was present in liver biopsies of 13 (31.7%) patients with acute presentation of AIH. Table 1 shows the clinicopathological features of AIH with CN compared to AIH without CN. Three of 13 patients showed acute hepatitis phase and the others were diagnosed as acute exacerbation phase from histological findings. Patients with CN had significantly lower serum IgG levels than patients without CN (mean: 2307 mg/dL vs 3126 mg/dL, P < 0.05). Patients with CN had significantly higher ANA-negative rates than patients without CN (30.7% vs 3.5%, P < 0.05 for all measures). However, other clinical features were similar between the two groups (mean age: 51.1 years vs 52.7 years, serum alkaline phosphatase: 498 IU/L vs 450 IU/L, steroid use: 84.6% vs 85.7%, azathioprine use: 15.4% vs 25.0%, other autoimmune diseases: 30.7% vs 25.0%).
Table 1.
Clinicopathological data of patients with autoimmune hepatitiswith and without centrilobular necrosis n (%)
| Abbreviations (normal values) | With CN (n = 13) | Without CN (n = 28) | P value |
| Mean age (range) (yr) | 51.1 (23-80) | 52.7 (21-77) | NS |
| Sex (M/F) | 2/11 | 1/27 | NS |
| ALT (IU/L) | 907 (228-3250) | 667 (224-1761) | NS |
| ALP (IU/L) | 498 (223-999) | 450 (76-883) | NS |
| IgG (mg/dL) | 2307 (1150-3913) | 3126 (1294-5449) | < 0.05 |
| ANA neg. | 4 (30.7) | 1 (3.5) | < 0.05 |
| Other autoimmune disease | 4 (30.7) | 7 (25.0) | NS |
| Steroid therapy | 11 (84.6) | 24 (85.7) | NS |
| Azathioprine therapy | 2 (15.4) | 7 (25.0) | NS |
| AIH score original | 12 (6-20) | 14 (6-18) | NS |
| (Median) simplified | 6 (3-8) | 7 (5-8) | < 0.05 |
| Interface hepatitis | 10 (76.9) | 26 (92.8) | NS |
| Portal inflammation | 13 (100) | 24 (85.7) | NS |
| Plasma cell infiltration | 7 (53.8) | 7 (25.0) | NS |
| Rosette formation | 3 (23.1) | 3 (12.0) | NS |
| Fibrosis | |||
| Stage F0-2 | 11 (84.6) | 10 (35.7) | < 0.05 |
| Stage F3-4 | 2 (15.4) | 18 (64.3) |
ALT: Alanineaminotransferase (8-42 IU/L); ALP: Alkaline phosphatase (115-359 IU/L); IgG: Immunoglobulin G (870-1700 mg/dL); ANA: Antinuclear antibody (< 40 ×); NS: Not significant; AIH: Autoimmune hepatitis; CN: Centrilobular necrosis. P values were calculated using the Mann-Whitney U test and the χ2 test.
The frequency of advanced fibrosis in patients with CN was significantly lower than in patients without CN (F0-2: 84.6% vs 35.7%, F3-4: 15.4% vs 64.3%, P < 0.05). Other histological features were similar between the two groups (interface hepatitis: 76.9% vs 92.8%, portal inflammation: 100% vs 85.7%, plasma cell infiltration: 53.8% vs 25.0%, hepatocyte rosette formation: 23.1% vs 12.0%). Although there was no significant difference between groups when evaluated using the revised original score (12 vs 14), the simplified AIH score of patients with CN was significantly lower (6 vs 7, P < 0.05).
Table 2 shows the clinical and serological features of the 13 AIH patients with CN. Three patients with CN (cases 6, 7 and 10) had non-diagnostic scores in both the original and simplified systems. Two patients (cases 6 and 10) had undetectable autoantibodies and normal serum IgG levels at initial presentation, and ANA became detectable at second determination, while the other patient (case 7) was positive for hepatitis C virus antibody and negative for hepatitis C virus-RNA. Corticosteroid therapy was effective in these patients. Of 12 patients with CN screened for class II human leukocyte antigen by PCR-rSSO Typing Tests (SRL, Tokyo, Japan), 7 patients (64%) had DR4, and 4 (33%) had DR9. One patient had both DR1 and DR15 while none had DR3. Frequency of DR4 was similar between patients with and without CN.
Table 2.
Clinical and serological features of 13 autoimmune hepatitis patients with centrilobular necrosis
| Case | Age (yr)/sex | ALT (IU/L) | ALP (IU/L) | IgG (mg/dL) | ANA (titer) | ASMA (titer) |
AIH score |
HLA |
Histology |
|||||
| (O) | (S) | DR4 | (I) | (L) | (R) | (E) | (F) | |||||||
| 1 | 56/F | 831 | 357 | 3330 | 5120 | 40 | 20 | 8 | DR4 | + | + | + | + | 3 |
| 2 | 68/F | 1230 | 479 | 3215 | 640 | Neg | 11 | 7 | - | + | + | + | - | 3 |
| 3 | 45/F | 3250 | 423 | 1800 | 160 | Neg | 14 | 6 | DR4 | + | + | - | - | 2 |
| 4 | 49/M | 713 | 528 | 2300 | 640 | 40 | 15 | 7 | DR4 | + | + | + | - | 1 |
| 5 | 23/F | 789 | 596 | 3913 | 160 | 640 | 17 | 7 | DR4 | + | + | - | - | 1 |
| 6 | 42/M | 1499 | 972 | 1150 | Neg | Neg | 9 | 3 | DR4 | - | + | - | - | 1 |
| 7 | 57/F | 471 | 223 | 2266 | Neg | 20 | 6 | 3 | DR4 | + | + | - | - | 1 |
| 8 | 80/F | 499 | 410 | 1410 | 40 | Neg | 11 | 4 | - | + | + | - | - | 1 |
| 9 | 52/F | 416 | 458 | 1280 | Neg | 160 | 11 | 5 | - | - | + | - | - | 1 |
| 10 | 44/F | 1067 | 377 | 1370 | Neg | Neg | 6 | 3 | DR4 | - | + | + | - | 1 |
| 11 | 44/F | 418 | 273 | 3503 | 2560 | Neg | 14 | 7 | - | + | + | - | - | 2 |
| 12 | 35/F | 383 | 335 | 3086 | 160 | 80 | 17 | 7 | ND | + | + | - | - | 1 |
| 13 | 69/F | 228 | 999 | 1286 | 160 | Neg | 13 | 5 | - | + | + | - | - | 2 |
Abbreviations (normal values): ALT: Alanine aminotransferase (8-42 IU/L); ALP: Alkaline phosphatase (115-359 IU/L); IgG: Immunoglobulin G (870-1700 mg/dL); ANA: Antinuclear antibody (< 40 ×); ASMA: Anti-smooth muscle antibody (negative); O: Original system; S: Simplified system; I: Interface hepatitis; L: Lymphocytic/lymphoplasmocytic infiltrates in portal tracts; R: Rosette formation; E: Emperipolesis; F: Fibrosis; Neg: Negative; ND: Not detected; F: Female; M: Male; AIH: Autoimmune hepatitis.
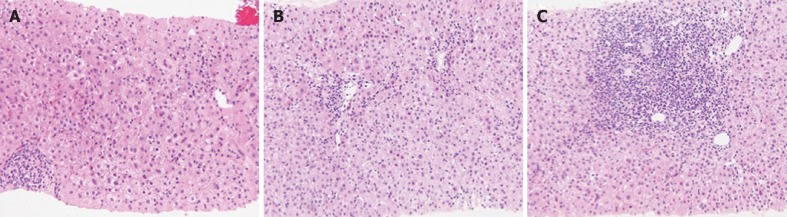
Three of the 13 CN cases showed acute hepatitis without interface hepatitis (case 6, 9 and 10), but two patients (cases 6 and 10) progressed to classic AIH. Although the liver histology of case 6 with acute hepatitis of unknown cause showed CN without portal inflammation (Figure 1A), a liver biopsy specimen from the same patient 2 mo later revealed interface hepatitis with CN (Figure 1B and C). Patients with CN responded better to corticosteroid treatment than those without.
Figure 1.
Pathological findings of liver biopsy. A: Liver biopsy from a case of centrilobular necrosis without classic histological features of autoimmune hepatitis that progressed to classic autoimmune hepatitis [hematoxylin staining (HE), 200 ×]; B, C: Centrilobular necrosis with interface hepatitis in liver biopsy (HE, 200 ×).
DISCUSSION
AIH is a form of hepatitis associated with autoantibodies and hypergammaglobulinemia, histologically characterized by chronic active hepatitis showing portal inflammation with fibrosis, interface hepatitis, hepatocyte rosette formation and prominent plasma cell infiltration[18,19]. Cases satisfying the definition of AIH but not fitting the classical disease presentation usually have acute severe presentation, few or no symptoms, atypical histological findings, absent or variant serological markers, concurrent cholangiographic changes, or are male and non-Caucasian[20]. Rare cases of AIH with CN as the dominant finding have been reported. In 1997, Pratt et al[11] reported four cases of steroid-responsive hepatitis, presumably AIH with CN. Patients with CN have a higher frequency of acute disease onset and lower frequency of cirrhosis than those without CN[7]. Some of these patients have recurrent CN or progress to classic AIH, although others do not[7,8,11,21]. If CN with autoimmune features represents an early stage of classic AIH, one would expect to encounter it more frequently in patients with acute onset of AIH. In a study of 26 patients with recent onset AIH, only one patient had hepatitis with CN[22]. Therefore, acute onset AIH may simply be a sign of an acute exacerbation of pre-existing chronic AIH[6,22]. Indeed, Miyake et al[23] reported that 47 of 160 patients (29%) had CN, and found CN not only in patients with acute onset AIH, but also acute exacerbation of pre-existing chronic AIH. Another possibility is that AIH with CN represents a different form of AIH.
In this study, we observed CN in 31.7% of our AIH patients with acute presentation and the frequency of advanced fibrosis was significantly different between patients with and without CN. Serum IgG levels of patients with CN were significantly lower while ANA-negative rates were significantly higher than in patients without CN. These findings indicate that CN reflects an early lesion in AIH. Moreover, two of the three CN cases without classic histological features of AIH progressed to classic AIH. In addition, none of the patients with CN presented with liver cirrhosis at diagnosis. However, it is difficult to identify CN, as the area of zone 3 is unclear in cases of liver cirrhosis. Similarly, in a recent study of six AIH patients with CN, none presented with liver cirrhosis and only one had elevated serum IgG levels[20]. In another study, serum ALT levels were higher in patients with CN than in patients without CN, and two of 20 patients with CN had undetectable autoantibodies and normal serum IgG levels at initial presentation[7].
We also evaluated the ability of a simplified scoring system to identify AIH with and without CN compared with the revised original diagnostic criteria. Although there was no significant difference between groups evaluated using the revised original score, the simplified AIH score of patients with CN was significantly lower. Miyake et al[24] reported that 30% of male patients, 23% of patients with acute presentation, 50% of patients showing histological acute hepatitis and 46% of patients negative for antinuclear antibodies at presentation who were not diagnosed with AIH according to the simplified criteria. The revised original scoring system has greater value in diagnosing patients with few or atypical features of AIH, especially in patients with cryptogenic or autoantibody-negative chronic hepatitis. However, the simplified scoring system can exclude the diagnosis more frequently in patients with etiologically distinctive disease who have concurrent immune manifestations[25,26].
On the other hand, the American Association for the Study of Liver Disease practice guidelines state that in patients with CN, sequential liver tissue examinations demonstrate transition from CN to interface hepatitis[27]. Our study indicated that 3 of 13 cases of AIH with acute presentation showed CN without portal inflammation (interface hepatitis) and 10 of 13 showed CN with portal inflammation. In addition, 26 of 41 cases of AIH with acute presentation showed portal inflammation without CN. Thus, portal inflammation with or without CN is considered to be an acute exacerbation phase of AIH. CN without portal inflammation is the most important histological finding which relates to the acute hepatitis phase of AIH. Taken together, CN is observed in both patients with acute hepatitis phase and acute exacerbation phase of AIH, although AIH with CN often shows clinical features of the genuine acute form.
COMMENTS
Background
Recently, it has been reported that there are two types of the acute presentation in patients with autoimmune hepatitis (AIH) in Japan. Typical liver histology of AIH is interface hepatitis with lymphocytic/lymphoplasmacytic infiltrates in portal tracts and extending into lobules, although centrilobular necrosis (CN) has been observed without the classic histological features of AIH.
Research frontiers
Recent reports have showed that it may be possible to distinguish the acute hepatitis phase from the acute exacerbation phase of AIH by the existence of both CN and portal inflammation.
Innovations and breakthroughs
CN is a non-specific histological finding caused by hepatotoxins, such as acetaminophen, paracetamol, thioacetamide, tetrachloride, congestive hepatic injury and cardiac hepatopathy due to acute right side cardiac failure. Other reports have highlighted that CN probably reflects early stage AIH detected primarily in patients with an acute onset.
Applications
This study may suggest a future strategy for differentiating between the acute hepatitis phase and the acute exacerbation phase of AIH based on the existence of both CN and portal inflammation.
Terminology
Confluent necrosis with inflammation in zone 3 was considered to be CN. Elevated serum alanine aminotransferase (> 5x upper limit of normal) identified acute presentation of the disease. Patients with acute presentation of the disease with histological evidence of chronic hepatitis, such as the presence of fibrosis and inflammatory cell infiltrations in the portal tracts with interface hepatitis, were diagnosed as acute exacerbation phase. Patients with acute presentation of disease with no history of any prior liver disease and no histological evidence of chronic hepatitis were diagnosed as acute hepatitis phase.
Peer review
The authors compared the clinicopathological features of acute presentation of AIH with and without CN in series of well-characterized cases affected by AIH in a single hospital in Japan. They conclude that CN is a feature both in patients with acute AIH or acute exacerbation of AIH. The study is well designed and performed. Although the number of cases is quite small, they are well-characterized so the conclusions of this study are solid and of importance.
Footnotes
Peer reviewer: Dr. Pietro Invernizzi, IRCCS Istituto Clinico Humanitas, via Manzoni 113, Rozzano 20089, Italy
S- Editor Wu X L- Editor Hughes D E- Editor Zheng XM
References
- 1.Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 2.Czaja AJ, Freese DK. Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36:479–497. doi: 10.1053/jhep.2002.34944. [DOI] [PubMed] [Google Scholar]
- 3.Gardner CR, Laskin JD, Dambach DM, Chiu H, Durham SK, Zhou P, Bruno M, Gerecke DR, Gordon MK, Laskin DL. Exaggerated hepatotoxicity of acetaminophen in mice lacking tumor necrosis factor receptor-1. Potential role of inflammatory mediators. Toxicol Appl Pharmacol. 2003;192:119–130. doi: 10.1016/s0041-008x(03)00273-4. [DOI] [PubMed] [Google Scholar]
- 4.Alison MR, Sarraf CE. Liver cell death: patterns and mechanisms. Gut. 1994;35:577–581. doi: 10.1136/gut.35.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers RP, Cerini R, Sayegh R, Moreau R, Degott C, Lebrec D, Lee SS. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. 2003;37:393–400. doi: 10.1053/jhep.2003.50062. [DOI] [PubMed] [Google Scholar]
- 6.Okano N, Yamamoto K, Sakaguchi K, Miyake Y, Shimada N, Hakoda T, Terada R, Baba S, Suzuki T, Tsuji T. Clinicopathological features of acute-onset autoimmune hepatitis. Hepatol Res. 2003;25:263–270. doi: 10.1016/s1386-6346(02)00274-7. [DOI] [PubMed] [Google Scholar]
- 7.Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol. 2006;59:246–249. doi: 10.1136/jcp.2005.029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zen Y, Notsumata K, Tanaka N, Nakanuma Y. Hepatic centrilobular zonal necrosis with positive antinuclear antibody: a unique subtype or early disease of autoimmune hepatitis. Hum Pathol. 2007;38:1669–1675. doi: 10.1016/j.humpath.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Lefkowitch JH, Apfelbaum TF, Weinberg L, Forester G. Acute liver biopsy lesions in early autoimmune (“lupoid”) chronic active hepatitis. Liver. 1984;4:379–386. doi: 10.1111/j.1600-0676.1984.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 10.Abe M, Onji M, Kawai-Ninomiya K, Michitaka K, Matsuura B, Hiasa Y, Horiike N. Clinicopathologic features of the severe form of acute type 1 autoimmune hepatitis. Clin Gastroenterol Hepatol. 2007;5:255–258. doi: 10.1016/j.cgh.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Pratt DS, Fawaz KA, Rabson A, Dellelis R, Kaplan MM. A novel histological lesion in glucocorticoid-responsive chronic hepatitis. Gastroenterology. 1997;113:664–668. doi: 10.1053/gast.1997.v113.pm9247489. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H, Zeniya M. Acute presentation of autoimmune hepatitis: Does it exist A published work review. Hepatol Res. 2011;41:498–504. doi: 10.1111/j.1872-034X.2011.00808.x. [DOI] [PubMed] [Google Scholar]
- 13.Onji M. Proposal of autoimmune hepatitis presenting with acute hepatitis, severe hepatitis and acute liver failure. Hepatol Res. 2011;41:497. doi: 10.1111/j.1872-034X.2011.00810.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 16.Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 17.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 18.Czaja AJ. Autoimmune liver disease. Curr Opin Gastroenterol. 2006;22:234–240. doi: 10.1097/01.mog.0000218959.48064.7f. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter HA, Czaja AJ. The role of histologic evaluation in the diagnosis and management of autoimmune hepatitis and its variants. Clin Liver Dis. 2002;6:685–705. doi: 10.1016/s1089-3261(02)00022-3. [DOI] [PubMed] [Google Scholar]
- 20.Czaja AJ, Bayraktar Y. Non-classical phenotypes of autoimmune hepatitis and advances in diagnosis and treatment. World J Gastroenterol. 2009;15:2314–2328. doi: 10.3748/wjg.15.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misdraji J, Thiim M, Graeme-Cook FM. Autoimmune hepatitis with centrilobular necrosis. Am J Surg Pathol. 2004;28:471–478. doi: 10.1097/00000478-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Burgart LJ, Batts KP, Ludwig J, Nikias GA, Czaja AJ. Recent-onset autoimmune hepatitis. Biopsy findings and clinical correlations. Am J Surg Pathol. 1995;19:699–708. doi: 10.1097/00000478-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Takaguchi K, Ikeda H, Makino Y, Kobashi H, Sakaguchi K, et al. Clinical features of Japanese type 1 autoimmune hepatitis patients with zone III necrosis. Hepatol Res. 2007;37:801–805. doi: 10.1111/j.1872-034X.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 24.Miyake Y, Iwasaki Y, Kobashi H, Yasunaka T, Ikeda F, Takaki A, Yamamoto K. Clinical features of autoimmune hepatitis diagnosed based on simplified criteria of the International Autoimmune Hepatitis Group. Dig Liver Dis. 2010;42:210–215. doi: 10.1016/j.dld.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology. 2008;48:1540–1548. doi: 10.1002/hep.22513. [DOI] [PubMed] [Google Scholar]
- 26.Czaja AJ, Manns MP. Advances in the diagnosis, pathogenesis, and management of autoimmune hepatitis. Gastroenterology. 2010;139:58–72.e4. doi: 10.1053/j.gastro.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 27.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]