Abstract
Motor learning adjusts movement size and direction to keep movements accurate. A useful model of motor learning, saccade adaptation, uses intra-saccade target movement to make saccades seem inaccurate and elicit adaptive changes in saccades. In the most studied saccade adaptation procedure, which we call short-term saccade adaptation (STSA), monkeys decrease or increase the size of their saccades by tracking 1000 – 2000 adapting target movements in a single saccade session. STSA elicits rapid changes of limited size and duration. Larger, more persistent reduction in saccade size results from adapting saccades daily for 19 days, a procedure that we call long-term saccade adaptation (LTSA). LTSA mimics the demands of rehabilitation more closely than does STSA and, unlike STSA, produces changes that could maintain long-term accuracy. Previous work describes LTSA that reduces saccade size in monkeys. Though convenient to study, size-reducing LTSA is not a good model for rehabilitation because few injuries necessitate making movements smaller. Here we characterize size-increasing LTSA and compare it, in the same monkeys, to size-reducing LTSA. We found that size-increasing LTSA can double saccade gain in ~21 days, and is slower than size-decreasing LTSA. In contrast to a single size-decreasing STSA, a single size-increasing STSA does not prevent additional saccade size increase at the normal rate when a monkey continues to track adapting target movements. We conclude that size-increasing LTSA is slower than size-decreasing LTSA but can make larger changes in saccade size. Size-increasing and size-decreasing LTSA use distinct mechanisms with different performance characteristics.
Keywords: long term saccade adaptation, gain, eye movements, macaque
1
When movements are repeatedly inaccurate because of injury, aging, or growth, the brain changes motor commands to improve performance. In the lab we make voluntary rapid eye movements, saccades, seem to be inaccurate by providing visual feedback that these movements do not end on target. We do this by moving the target to a new location during each saccade. This feedback makes saccades change gradually so that they end closer to their targets. Monkeys usually asymptotically adapt within 1000–2000 saccades. We call this short-term saccade adaptation (STSA). STSA to decrease or increase saccade size is a common model for studying motor plasticity (e.g., Straube et al., 1997; Kojima et al., 2010).
Despite its utility for studying plasticity, STSA is not a good model of long-term maintenance of movement accuracy. It fades quickly and its size is limited, i.e. one large change precludes subsequent change (Robinson et al., 2006). Visual feedback that saccades are inaccurate elicits longer lasting changes in saccade size when it persists for ~3 weeks (Robinson et al., 2006). This long-term saccade adaptation (LTSA) does not impair subsequent STSAs, indicating that LTSA and STSA use separate mechanisms. It is not yet clear that LTSA is the mechanism for maintaining movement accuracy in natural settings, but its time course makes it a better model than STSA.
In previous work we characterize size-decreasing LTSA because, in monkeys, size-decreasing STSA is typically faster and larger than size-increasing adaptation (Straube et al., 1997; Robinson et al., 2003). However, despite its convenience, size-decreasing adaptation is less useful than size-increasing adaptation for correcting damaged movements. No injury makes saccades too large except damage to the caudal region of the cerebellar fastigial nucleus. Thus, there seems little use outside the laboratory for size-decreasing adaptation. In contrast, many dysfunctions of the oculomotor system, e.g., muscle weakness or nerve damage, make movements smaller, and are therefore potentially improved by size-increasing adaptation.
In summary, size-increasing LTSA is an example of motor learning that will help remedy hypometria, a common problem of impaired movements, and is likely to last long enough to keep movements accurate. Here we describe size-increasing LTSA in monkeys. We found that size-increasing LTSA changes saccade size at about one third the rate of size-decreasing LTSA but can double saccade gain in ~21 days. In contrast to a single size-decreasing STSA, a single size-increasing STSA does not prevent additional increase in saccade size if the monkey continues to track adapting target movements. Though it is slower, the size-increasing LTSA mechanism has more capacity to change saccade size than does size-decreasing LTSA. The difference in the time course of size-increasing and size-decreasing LTSA leads us to conclude that they use distinct mechanisms.
2: Experimental Procedures
2.1 Subjects and animal preparation
Three juvenile male rhesus monkeys (Macaca mulatta) were implanted with a three-turn coil of Teflon-coated wire in one eye in an aseptic surgery. This wire connected to a plug attached to the top of the monkey’s skull with metal screws and dental acrylic. In the same surgery we similarly attached head stabilizing hardware which was embedded in dental acrylic. The animals recovered from surgery in their home cages for at least one week after surgery.
2.2 Animal training
We used the search coil technique (Fuchs and Robinson, 1966; Robinson, 1963) to measure eye position. We trained animals to use saccades to track a small laser spot projected onto a screen 57 cm away. The spot was positioned by two computer-controlled galvanometers. Animals fixated the target for 0.6–2 s, then the target moved to a new position to the left or right. Monkeys received a small dollop of apple sauce reward for keeping their eyes within 2° of the target position as it moved to new locations. Training took place in a light-tight, sound-attenuating booth. Except for the laser spot, animals were otherwise in complete darkness.
2.3 Eliciting short-term saccade adaptation (STSA)
To make saccades seem to have missed their targets, and thereby drive adaptation, we moved targets during saccades (McLaughlin, 1967). Software detected a saccade when eye velocity exceeded 70°/s and moved the target before the saccade ended. To increase saccade size in these experiments we presented target movements of 8° to the right or left of current eye position. During saccades to these targets we made the target move an additional 8° in the same direction as the initial target movement. Therefore the target started at either a central fixation point between the animal’s eyes, or 16° to the right or left of this point on a horizontal line. When a monkey tracked this type of target movements its saccades to targets 8° to the right or left became larger than 8°.
To decrease saccade size we presented the 16° target movements. During the saccade that tracked this movement the target moved back 8° in the opposite direction so that it ended 8° away from initial eye position. The target started either at a central fixation point between the animal’s eyes, or 8° to the right or left of this point on a horizontal line. When a monkey tracked this type of target movements its saccades to targets 16° to the right or left became smaller than 16°. In a single adaptation session we adapted saccades to both the left and the right. Adapting saccades in one direction does not influence saccades in the opposite direction (Albano, 1996; Deubel et al., 1986; Frens and van Opstal, 1997; Miller et al., 1981; Straube et al., 1997; Weisfeld, 1972, but see Rolfs et al, 2010: Simultaneously adapting saccades in opposite directions results in a slower time course for human STSA than adapting in one-direction). We therefore treated adaptation of leftward and rightward saccades in the same session as two independent adaptations.
2.4 Eliciting long-term saccade adaptation (LTSA)
To elicit long-term saccade adaptation we presented size-increasing intra-saccade target movements as described above for about two hours each day on consecutive days. Typically a monkey made 1000–2000 saccades in each direction during each day’s adaptation. When they were not inside the experimental booth, animals wore opaque blindfolding goggles. Except for a ~5 second period at the beginning and end of each daily adaptation session, a monkey’s only visual experience was while it tracked the target with intra-saccade target movements. We ended a long-term adaptation when saccades at the beginning and end of the same session were statistically equivalent in at least one direction according to Schuirmann’s (1987) TOST equivalence test with P<= to 0.05 as the criterion for equivalence.
To evaluate any size-increasing capacity remaining after the end of a long-term adaptation we presented intra-saccade target movements of an additional 4° on the first day after we ended an LTSA. On the second day after an LTSA ended we presented the same intra-saccade target movements that we did during the LTSA. This allowed the monkey’s saccades to return to the size that they were at the end of the LTSA, i.e., ~16°. Beginning on the third day after LTSA end, we presented normal 8° target movements, i.e., not followed by intra-saccade target movements. We did this on consecutive days until the size of the monkey’s saccades returned to normal.
To compare size-increasing to size-decreasing LTSA in the same animal we also elicited size-decreasing LTSA using 16° to 8° intra-saccade target movement. At the end of size-decreasing LTSA we presented normal 16° target movements for two hours each day on consecutive days until the monkeys’ gains recovered to normal. The monkeys were exposed to normal target movements for a minimum of two months between size increasing and size decreasing LTSA experiments.
2.5 Data collection and analysis
We used a CED Power 1401 laboratory interface (Cambridge Electronic Design, Cambridge UK) to digitized voltages proportional to horizontal and vertical eye and target positions at 1kHz. A custom MATLAB (Mathworks Inc, Natick, MA) program identified target and eye movements and measured their properties. This program initially detected large, rapid changes in target position and then scanned forward within the next 500 ms to the nearest eye movement with a peak velocity greater than 100°/s. It then identified the times before and after the time of peak velocity where the velocity dropped below 20°/s. These were the start and end times of the saccade. The program measured saccade size and divided it by target movement size to calculate gain. We assessed gain change during the course of an LTSA with two measures:
Starting gain: the average gain of the first fifty saccades for each day of LTSA and recovery.
Absolute gain change: the absolute value of the difference between the mean of the first and last 50 saccades of each day’s adaptation and recovery.
We then fitted an exponential to both of these sets of values, minimizing the sum-squared error. Parameter A in the following equation is a multiplier that describes the difference between the first point and the asymptotic value of the fit curve. We use this variable to quantify the degree of adaption. g is the gain measurement calculated for each day using either method one or two from above. b is the bias; τ is the rate constant and n is the number of days the adaptation took.
To test for similarity of gain at the start and end of a single day’s session, we used the first and last 50 saccades of the session in the two one-sided tests (TOST) procedure of Schuirmann (1987) and considered P <= 0.05 to be significant.
All surgical and behavioral training procedures were approved by the Animal Care and Use Committee at the University of Washington. The animals were cared for by the veterinary staff of the Regional Primate Research Center. They were housed under conditions that comply with National Institutes of Health standards as stated in the Guide for the Care and Use of Laboratory Animals (DHEW Publication NIH85-23 1985) and with recommendations from the Institute of Laboratory Animal Resources and the American Association for Accreditation of Laboratory Animal Care.
3: Results
3.1 Gain change during long-term saccade adaptation and long-term recovery
Rightward and leftward saccades adapted very similarly in these experiments. For brevity we show the gains of only leftward saccades in Figure 1 and subsequent figures. Figure 1 shows the gains of leftward saccades for all three monkeys during one size-increasing LTSA. Table 1 summarizes gain changes during adaptation and recovery for both directions of LTSA. It includes information about the total number of days and trials, the initial and final gain values, the absolute change in gain, and multiplier and rate constant for the exponential fits of starting gains (SG, see Figure 3) and the rate constant for the fit of absolute change in gain (ACG, see Figure 4). All monkeys have initial gains above 1. Straube et al (1997) documented a similar small but very rapid change in saccade size during the initial trials of adaptation. This may represent a small strategic component of the monkey’s saccade planning.
Figure 1.
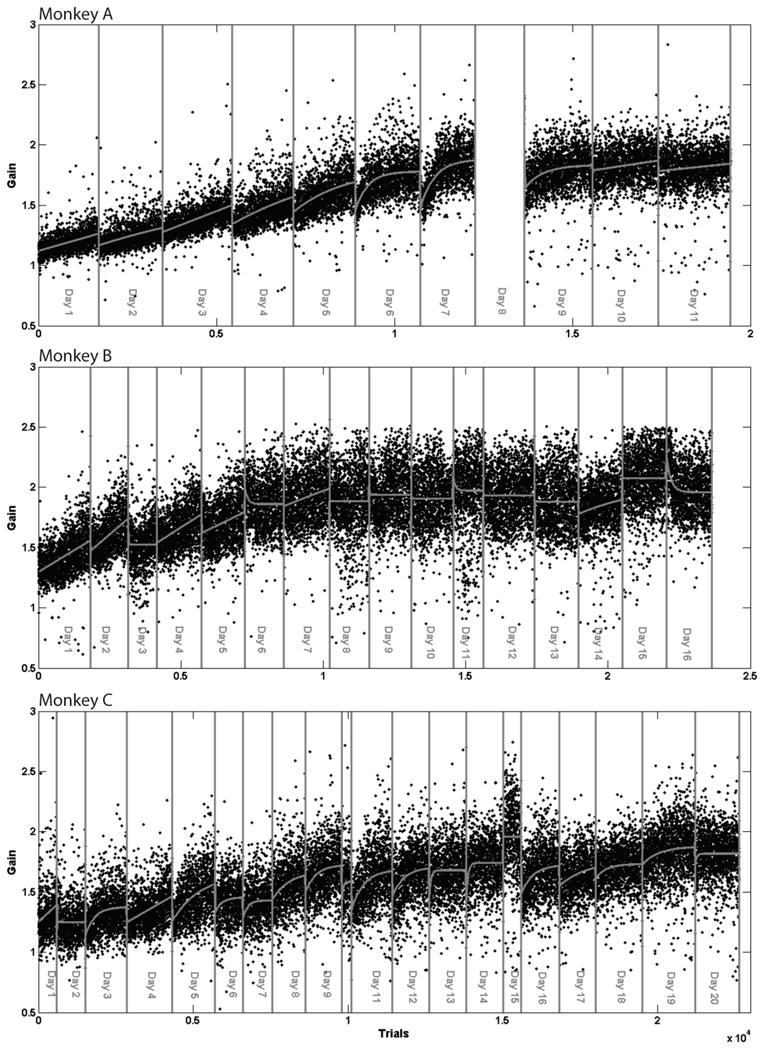
Size-increasing adaption. Gains of leftward saccades as monkeys tracked targets that moved from 8° leftward to 16° leftward during each saccade. Thus, complete adaptation would have a saccade gain of 2. Here, as in Figures 2 and 5, saccade gain for each trial is in sequential order. There was an error in data collection during day 8 of monkey A’s adaption, therefore it is not shown.
Table 01.
| Monkey A | Adaptation | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|
| Forward | Backward | Forward | Backward | |||||
| Left | Right | Left | Right | Left | Right | Left | Right | |
| Number of Trials | 22,673 | 21,787 | 48,824 | 49,801 | 11,618 | 11,535 | 16,664 | 16,673 |
| Initial Gain | 1.11 | 1.07 | 0.99 | 0.9 | 1.64 | 1.72 | 0.69 | 0.58 |
| End Gain | 1.85 | 1.81 | 0.52 | 0.51 | 1.02 | 0.96 | 1.02 | 0.98 |
| Absolute Change | 0.74 | 0.74 | 0.47 | 0.39 | 0.62 | 0.76 | 0.33 | 0.4 |
| SG Multiplier | 1.71 | 1.49 | −0.48 | −0.52 | −1.28 | −1.19 | 0.55 | 0.62 |
| SG Rate constant | 17.48 | 9.51 | 4.81 | 3.66 | 1.32 | 2.72 | 3.19 | 3.9 |
| ACG Rate constant | 4743.83 | −4.3 | 29.85 | 6.16 | 1.38 | 4.49 | 250 | 714.29 |
| Number of Days | 13 | 13 | 25 | 25 | 7 | 7 | 7 | 7 |
|
Monkey B
|
||||||||
| Number of Trials | 23,643 | 22,566 | 23,967 | 24,374 | 16,074 | 15,589 | 19,244 | 19,464 |
| Initial Gain | 1.37 | 1.37 | 1.05 | 1.17 | 1.9 | 1.61 | 0.86 | 0.73 |
| End Gain | 2.03 | 1.71 | 0.62 | 0.55 | 1.08 | 1.11 | 1.07 | 1.02 |
| Absolute Change | 0.66 | 0.34 | 0.43 | 0.62 | 0.82 | 0.5 | 0.21 | 0.29 |
| SG Multiplier | 0.89 | 0.82 | −0.45 | −0.74 | −1.35 | −1.03 | 0.41 | 0.7 |
| SG Rate constant | 4.77 | 9.67 | 4.95 | 2.48 | 1.62 | 1.42 | 1.73 | 1.19 |
| ACG Rate constant | 2.72 | 0.08 | 588.24 | 2.57 | 1.45 | 1.6 | 1.49 | 1.03 |
| Number of Days | 16 | 16 | 15 | 15 | 8 | 8 | 9 | 9 |
|
Monkey C
|
||||||||
| Number of Trials | 22,638 | 23,848 | 11,405 | 11,355 | 16,767 | 17,751 | ||
| Initial Gain | 1.3 | 1.39 | 0.96 | 1.04 | 1.64 | 1.67 | ||
| End Gain | 1.75 | 1.87 | 0.52 | 0.56 | 1 | 1.05 | ||
| Absolute Change | 0.45 | 0.48 | 0.44 | 0.48 | 0.64 | 0.62 | ||
| SG Multiplier | 13.46 | 0.67 | −0.78 | −0.7 | −1.77 | −0.99 | ||
| SG Rate constant | 476.19 | 8.68 | 1.65 | 2.02 | 0.96 | 1.93 | ||
| ACG Rate constant | 3870.86 | 909.09 | 1.52 | 2.64 | 0.88 | 1.93 | ||
| Number of Days | 20 | 20 | 11 | 11 | 10 | 10 | ||
Figure 3.
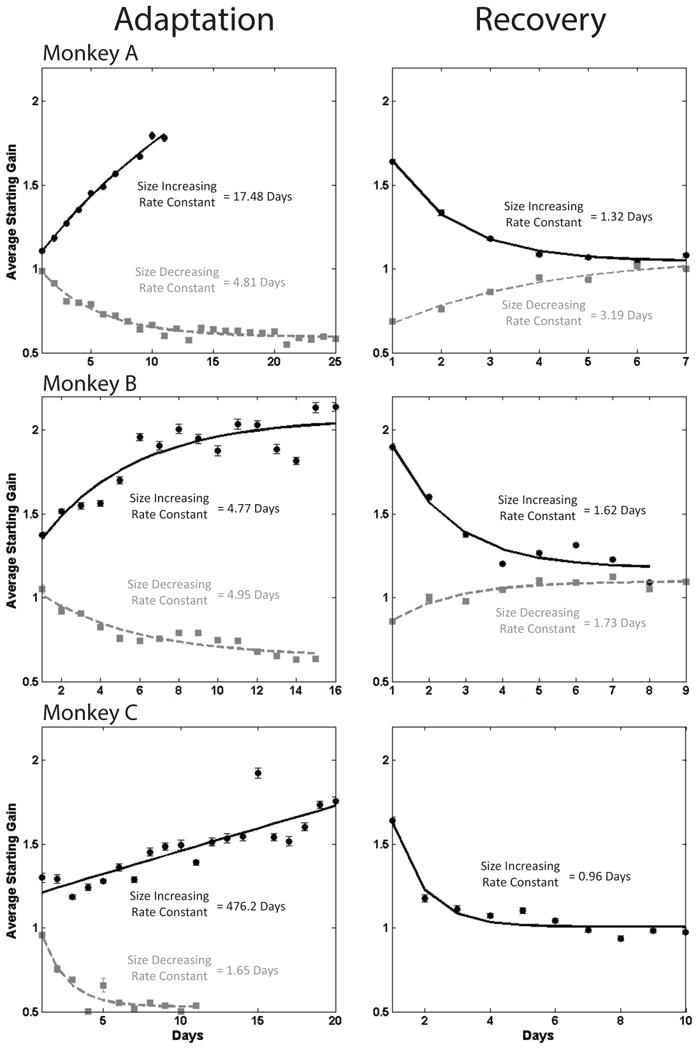
Starting gains on each day of size-increasing (black) and size-decreasing (gray) LTSA. Least-squares exponential are fit to each relationship. We did not collect the same recovery data of size-decreasing adaptation for monkey C, therefore it is not shown.
Figure 4.
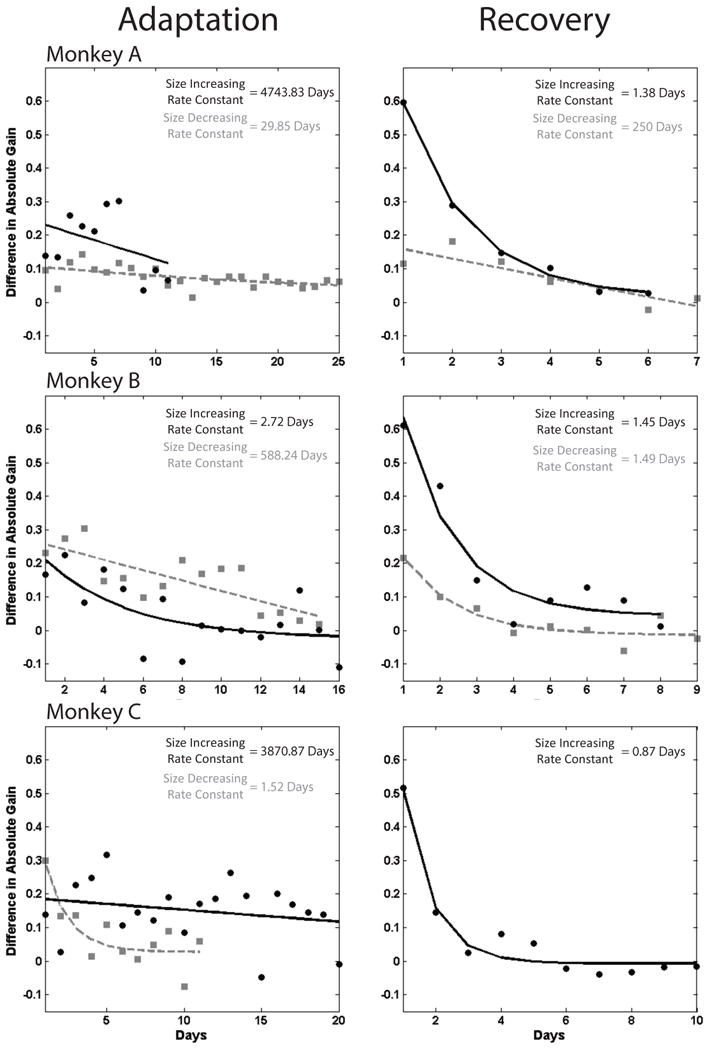
Absolute gain change on each day of size-increasing (black) and size-decreasing (gray) LTSA. Least-squares exponential are fit to each relationship. Again, we did not collect the same recovery data of size-decreasing adaptation for monkey C, therefore it is not shown.
During size-increasing LTSA all three monkeys achieve gains close to 2. Previous work with STSA (Straube et al., 1997; Robinson et al., 2003) indicates that monkeys have less size-increasing capacity than size-decreasing capacity. Our findings here indicate that size-increasing LTSA is capable of nearly doubling saccade size. We arbitrarily limited the LTSA that we elicited here to achieving a gain of 2. We have no indication that this is the limit and it seems likely that size-increasing LTSA could more than double saccade gain.
As apparent in Figure 1, during the first several daily sessions of LTSA, saccade size usually increased from the start to the end of the session. Overnight gain usually decreased so that gain at the start of each day was smaller than the final gain for the previous day. All three monkeys exhibited this pattern. Saccade gains for monkeys A, B, and C stabilized by day 11, 16 and 20, respectively.
Figure 2 shows, the gains of leftward saccades in all three monkeys during recovery from the size-increasing LTSA in Fig. 1. As apparent in Figure 2 saccade gain decreased quickly on the first day of recovery. At the beginning of the next day, and several subsequent days, gains were larger than at the end of the previous day. This pattern was the same for all three monkeys. By the end of recovery, mean saccade gains at the start and end of the session were equivalent and there was no further decay overnight. In one animal we left the goggles on for 35 days after a forward adaptation and measured the recovery without presentation of normal targets. In this case we blanked the target as soon as the saccade was initiated and placed it at the position that the eye landed within 20ms of saccade completion (Robinson et al., 2003). This indicates zero error to the animal and minimizes feedback. Although there was some recovery (10.6%, 16.6%, 30.6% and 6.2% for four different experiments), eye movements did not fully recover to a gain of 1. In contrast, after a single short term adaptation (day 2 in figure 1 for all monkeys), there is a substantial shift of the eye movements back toward the baseline gain of 1 overnight.
Figure 2.
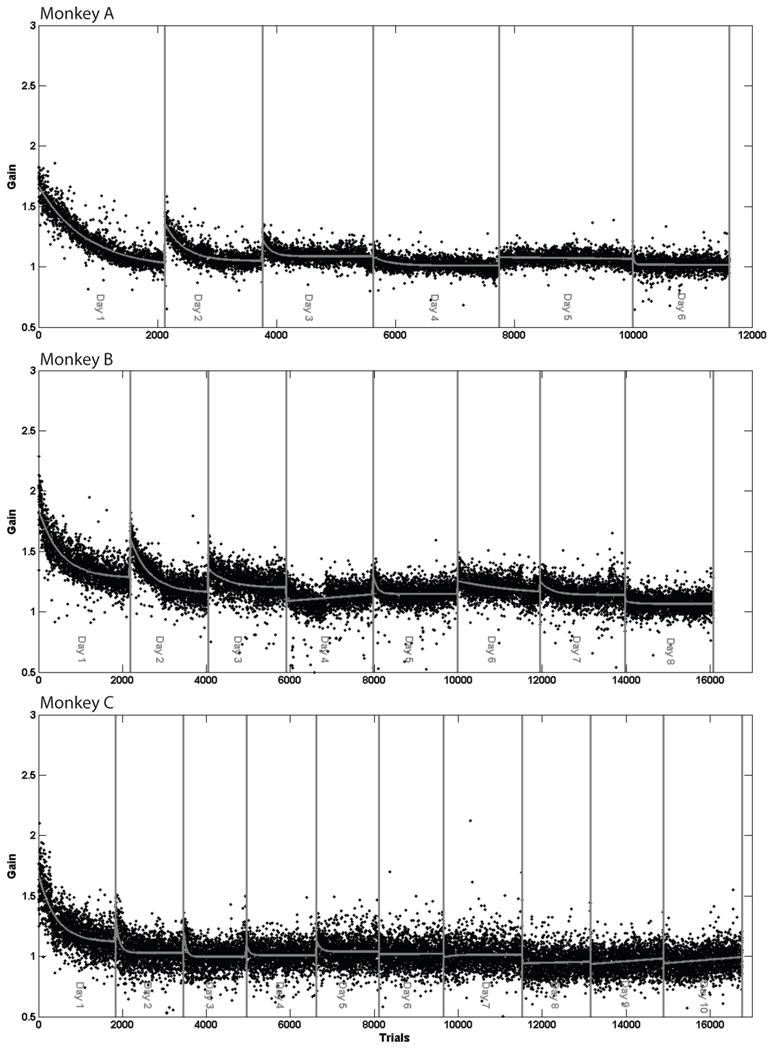
Recovery from adaption. Gains of leftward saccades as monkeys recovered from the size-increasing LTSA in Figure 1 by tracking normal 8° leftward target movements.
3.2 Rates of long-term saccade adaptation and recovery
We characterized the rate of long-term adaptation with two measures of each day’s adaptation: 1) mean starting gain and 2) absolute gain change. Mean starting gain is the average gains of a day’s first fifty saccades. We graphed the value of each day’s average against days of adaptation and fit an exponential curve to this relationship. Figure 3 shows these graphs for size-increasing (black) and size-decreasing (grey) LTSAs. Note that size-increasing (8° to 16°) show gains changing from 1 to 2 and size-decreasing (16° to 8°) adaptation shows gains changing from 1 to 0.5. Thus, although the movements are being adapted by the same magnitude, the conversion of the amplitude values into gains causes an apparent compression of size-increasing LTSA compared to size-decreasing LTSA. Changes in mean starting gain during LTSA are orderly in all monkeys for both size-increasing and size-decreasing LTSA. We measured LTSA rate as the rate constant of the exponential curve fit to mean starting gain. Size-increasing LTSA was significantly slower than size-decreasing LTSA in Monkeys A and C. In Monkey B size-increasing and size-decreasing LTSA occurred at approximately the same rate. In all three animals, recovery from size-increasing LTSA was faster than the preceding LTSA.
Our second measure, absolute gain change, is the absolute value of the difference between the average gains of the first and last fifty saccades on the same day. Figure 4 shows absolute gain change on successive days of adaptation for both size-increasing (black) and size-decreasing (gray) LTSAs. Absolute gain change did not change as smoothly as mean starting gain during LTSA but the qualitative results are similar. Size-increasing LTSA is much slower than size-decreasing LTSA in monkeys A and C. In Monkey B, size-increasing LTSA is faster than size-decreasing LTSA. Measured with absolute gain change, recovery from size-increasing LTSA was faster than the preceding LTSA in all three animals.
3.3 STSA interactions with previous LTSA and STSA
Previous work shows that a multi-day size-decreasing LTSA does not impair additional gain decrease during a subsequent one-day STSA, i.e., size-decreasing STSA is normal after size-decreasing LTSA. In contrast, the gain decrease elicited by an STSA is strongly impaired if that STSA immediately follows a previous size-decreasing STSA. The fact that STSA is normal after LTSA but not after STSA indicates that the LTSA and STSA mechanisms for reducing saccade gain are independent of one another (Robinson et al., 2006).
Here we used a similar strategy to test whether or not size-increasing LTSA and STSA are similarly independent. We elicited size-increasing STSA after a previous size-increasing STSA or LTSA. Figure 5 shows data for all three monkeys in the different experiments. The left column shows saccade gains elicited by two consecutive STSAs. During the first, the target moved from 8° to 16° from initial eye position during each saccade. During the second, the target moved an additional 4°, from 8° to 20°. The center column shows saccade gains elicited on the last day of an 8° to 16° LTSA followed by an STSA during which the target moved from 8° to 20°. The right column shows gains elicited during a simple 8° to 16° STSA.
Figure 5.
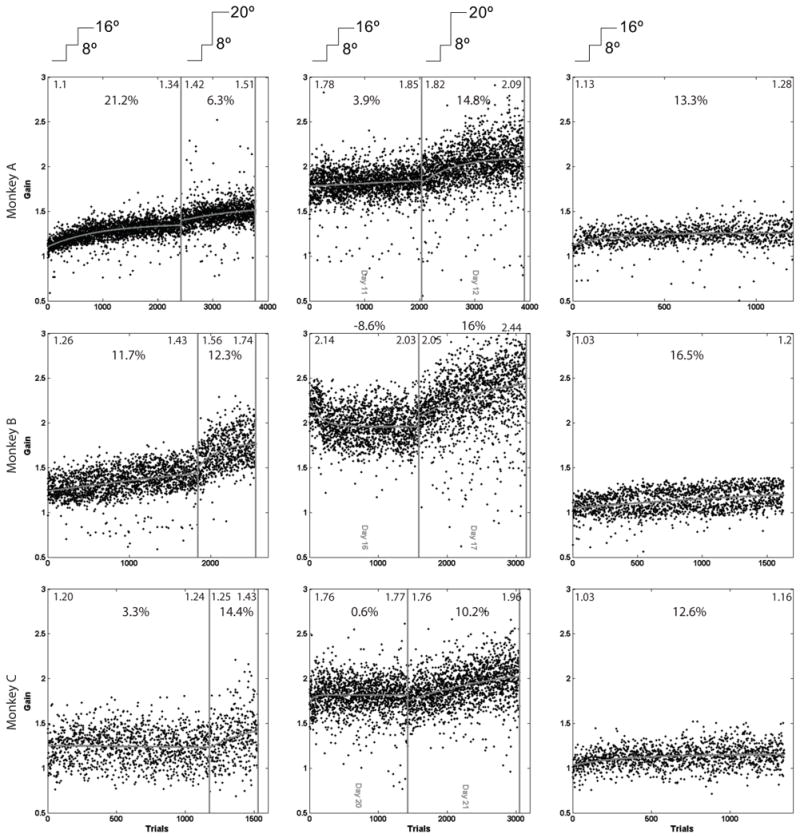
Short-term and long-term adaptations. Left, Gains of saccades during two consecutive size-increasing STSAs. During the first, the target moved from 8° to 16° during saccades. During the second, the target moved from 8° to 20°. Center, Gains of saccades during the last day of an 8° to 16° LTSA immediately followed by an 8° to 20° STSA. Right, Gains during an 8° to 16° STSA. Gray curves are least square fits of increasing exponentials to saccade gains. Numbers at the top of each panel show the percentage increase in the curve between the beginning and end of the panel. Inserts on the top of each panel illustrate target movement. Numbers at the top of each pane show the average of the 50 trials at the start or end of that epoch.
In the left column we see that gain increases substantially during the first adaptation and also during the second. The first gain increase does not preclude the second. In the center column we see that gain increases during an STSA that immediately follows a completed LTSA (i.e., gains are high and stable). Again, previous gain increases do not impair subsequent increases. In the right column, we see gain increases during an STSA in the same monkey. These increases are similar to the increases during the STSAs in the right panels of the left and center columns, indicating that those STSAs were normal.
Table 2 shows the details of the different measurements for these experiments, including the parameters calculated with an exponential fit similar to that described in the method section. In this case the fit is of individual gain values for subsequent trials within a session, not descriptive gain measurements for subsequent sessions. In some cases, the data proved too noisy to be fit with this method and in those cases we have not included the parameters (NA).
Table 02.
| Monkey A | Monkey B | Monkey C | ||||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | |
| 1-Day | ||||||
| First Adaptation | ||||||
| Number | 2,420 | 2,419 | 1,840 | 1,820 | 1,173 | 1,173 |
| Initial Gain | 1.11 | 1.08 | 1.28 | 1.15 | 1.2 | 1.25 |
| End Gain | 1.34 | 1.3 | 1.41 | 1.31 | 1.24 | 1.27 |
| Absolute Change | 0.23 | 0.22 | 0.14 | 0.16 | 0.04 | 0.02 |
| Multiplier | 0.26 | 0.23 | 0.88 | 0.19 | 0.1 | −0.2 |
| Rate Constant | 909.09 | 833.33 | 6,993.99 | 833.33 | 56.18 | 10.91 |
| Second Adaptation | ||||||
| Number | 1,350 | 1,386 | 710 | 494 | 355 | 405 |
| Initial Gain | 1.39 | 1.33 | 1.55 | 1.31 | 1.25 | 1.21 |
| End Gain | 1.5 | 1.5 | 1.76 | 1.43 | 1.4 | 1.4 |
| Absolute Change | 0.12 | 0.17 | 0.21 | 0.12 | 0.15 | 0.18 |
| Multiplier | 0.17 | 0.32 | 0.29 | 0.2 | 0.32 | NA |
| Rate Constant | 1,157.70 | 1,720.10 | 256.41 | 106.38 | 370.37 | NA |
| STSA Post-LTSA | ||||||
| Number | 1,866 | 1,821 | 1,561 | 1,509 | 1,624 | 1,597 |
| Initial Gain | 1.82 | 1.97 | 2.05 | 1.68 | 1.76 | 1.76 |
| End Gain | 2.08 | 2.28 | 2.44 | 1.95 | 1.98 | 2.06 |
| Absolute Change | 0.26 | 0.31 | 0.39 | 0.27 | 0.23 | 0.3 |
| Multiplier | 0.3 | 0.4 | 0.51 | 0.26 | 0.4 | 0.41 |
| Rate Constant | 625 | 476.19 | 1,165.42 | 243.9 | 1,388.43 | 1,385.67 |
| Normal | ||||||
| Number | 1,199 | 1,190 | 1,626 | 1,580 | 1,343 | 1,177 |
| Initial Gain | 1.13 | 1.11 | 1.03 | 1.01 | 1.03 | 1.06 |
| End Gain | 1.27 | 1.29 | 1.2 | 1.13 | 1.16 | 1.12 |
| Absolute Change | 0.15 | 0.18 | 0.18 | 0.13 | 0.13 | 0.06 |
| Multiplier | 0.14 | 0.17 | 0.12 | 0.09 | 0.12 | NA |
| Rate Constant | 200 | 256.41 | 270.27 | 270.27 | 344.83 | NA |
4: Discussion
The major result of this study is that daily adaptation for ~21 days elicits large, persistent increases in saccade gain when we restrict a monkey’s visual experience almost exclusively to adapting target movements. With this procedure, both size-decreasing and size-increasing LTSA cause similarly large, long-lasting gain changes but size-increasing LTSA is slower than size-decreasing LTSA in five out of six experiments and produces larger changes in saccade size. These differences are consistent with, though do not prove, the proposal that size-increasing and size-decreasing LTSA are distinct mechanisms.
If so, then LTSA is similar to STSA and the vestibulo-ocular reflex (VOR) in consisting of at least two different mechanisms. Kojima et al. (2004) argue, based on consecutive size-decreasing and size-increasing STSAs, that size-increasing and size-decreasing STSA rely on distinct mechanisms. Our data support this idea by documenting another difference. A single size-decreasing STSA impairs further gain reductions while a single size-increasing STSA does not impair subsequent size increases. Others have documented a similar dichotomy for gain-up and gain-down VOR adaptation. Boyden et al. (2003) showed different time courses for adapted increases and decreases in VOR gain. They also showed differences in the persistence of the adaptations: recovering from gain-down VOR adaptation with gain-up adaptation was not as successful as the reverse condition. Also, Kimpo et al. (2005) showed that adapted decreases in VOR gain generalized across different frequencies of rotation more than increases did. Together, these findings indicate that the brain uses different mechanisms to increase and decrease movements in several distinct oculomotor behaviors.
We argue (Robinson et al., 2006) that size-decreasing STSA and LTSA are distinct from one another. This is based on our observation that size-decreasing STSA is impaired after previous STSA but not after previous LTSA. We propose that STSA makes a rapid, but temporary, correction to saccade hypermetria, analogous to first aid, while LTSA makes a slower and longer-lasting correction, analogous to a cure. Such an arrangement would provide both rapid and persistent correction for inaccuracy.
Our current observations indicate that we cannot make the same arguments about size-increasing STSA and LTSA. Size-increasing STSA proceeds normally after either STSA or LTSA. Thus, there is no indication that size-increasing STSA and LTSA are distinct. They may rely on the same slow, but very capable mechanism to increase saccade size. If so, then the difference between size-increasing STSA and LTSA is only in how long that common mechanism is engaged by adapting target movements.
Our and previous (Robinson et al., 2006) data show that animals recover from LTSA much more quickly than they adapt. This indicates that LTSA in either direction does not change a pre-configured adaptation state to which the brain defaults more quickly than it adapts. (Note that there remains the logical possibility that by adapting to a single size, we are not engaging the default state.) This putative default state, setting gains at ~1, may be plastic given a long enough exposure to adapting stimuli. No current data indicates whether or not it is possible to permanently reset the default state.
Highlights.
We adapt monkeys to double of halve their saccade size over apx. 3 weeks.
Long term saccade adaptations (LTSA) are more persistent than short term (1 day).
It takes fewer days to recover from LTSA than it does to finish long term adapting.
Size-increasing LTSA is slower than size-decreasing LTSA.
Size-increasing and size-decreasing LTSA use distinct mechanisms.
Acknowledgments
We would like to acknowledge Jonathan Garlid and Amy Nowack for excellent technical support. We would also like to acknowledge members of the eye movement group at the Washington National Primate Center. This work was funded by the following grants: R01 EY018585, 3R01EY018585-01A1S1 and 3R01EY018585-02S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam J Davis, Email: davisaj1@uw.edu.
F. Ric Robinson, Email: robinsn@uw.edu.
References
- Albano JE. Adaptive changes in saccade amplitude: oculocentric or orbitocentric mapping. Vision Res. 1996;36:2087–2098. doi: 10.1016/0042-6989(96)89627-1. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Raymond JL. Active reversal of motor memories reveals rules governing memory encoding. Neuron. 2003;39:1031–1042. doi: 10.1016/s0896-6273(03)00562-2. [DOI] [PubMed] [Google Scholar]
- Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Hum Neurobiol. 1986;5:245–253. [PubMed] [Google Scholar]
- Frens MA, Van Opstal AJ. Monkey superior colliculus activity during short term saccadic adaptation. Brain Res Bull. 1997;43:473–483. doi: 10.1016/s0361-9230(97)80001-9. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movements chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Kimpo RR, Boyden ES, Katoh A, Ke MC, Raymond JL. Distinct patterns of stimulus generalization of increases and decreases in VOR gain. J Neurophysiol. 2005;94:3092–3100. doi: 10.1152/jn.00048.2005. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Yoshida K. Memory of learning facilitates saccadic adaptation in the monkey. J Neurosci. 2004;24:7531–7539. doi: 10.1523/JNEUROSCI.1741-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010;30:3715–3727. doi: 10.1523/JNEUROSCI.4953-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. Parametric adjustment in saccadic eye movements. Percept Psychophys. 1967;2:359–362. [Google Scholar]
- Miller JM, Anstis A, Templeton WB. Saccadic plasticity: parametric adaptive control of retinal feedback. J Exp Psycyol. 1981;7:356–366. doi: 10.1037//0096-1523.7.2.356. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a sclera search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Noto CT, Bevans SE. Effect of visual error size on saccade adaptation in monkey. J Neurophysiol. 2003;90:1235–1244. doi: 10.1152/jn.00656.2002. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Soetedjo R, Noto C. Distinct short-term and long-term adaptation to reduce saccade size in monkey. J Neurophysiol. 2006;96:1030–1041. doi: 10.1152/jn.01151.2005. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Knapen T, Cavanagh P. Global saccadic adaptation. Vision Research. 2010;50(18):1882–1890. doi: 10.1016/j.visres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Schuirmann DJ. A comparison of the two onesided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokin Biopharm. 1987;15:657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- Straube A, Fuchs AF, Usher S, Robinson FR. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol. 1997;77:874–895. doi: 10.1152/jn.1997.77.2.874. [DOI] [PubMed] [Google Scholar]
- Weisfeld GE. Parametric adjustment to a shifting target alternating with saccades to a stationary reference point. Psychonom Sci. 1972;28:72–74. [Google Scholar]


