Abstract
Total syntheses of HMP-Y1, atrop-HMP-Y1, hibarimicinone, atrop-hibarimicinone, and HMP-P1 are described using a two-directional synthesis strategy. A novel benzyl fluoride Michael–Claisen reaction sequence was developed to construct the complete carbon skeleton of HMP-Y1 and atrop-HMP-Y1 via a symmetrical, two-directional, double annulation. Through efforts to convert HMP-Y1 derivatives to hibarimicinone and HMP-P1, a biomimetic mono-oxidation to desymmetrize protected HMP-Y1 was realized. A two-directional unsymmetrical double annulation and biomimetic etherification were developed to construct the polycyclic and highly-oxidized skeleton of hibarimicinone, atrop-hibarimicinone, and HMP-P1. The use of a racemic biaryl precursor allowed for the synthesis of both hibarimicinone atropisomers and provides the first confirmation of the structure of atrop-hibarimicinone. Additionally, this work documents the first reported full characterization of atrop-hibarimicinone, HMP-Y1, atrop-HMP-Y1, and HMP-P1. Lastly, a pH-dependent rotational barrier about the C2–C2' bond of hibarimicinone was discovered, which provides valuable information necessary to achieve syntheses of the glycosylated congeners of hibarimicinone.
INTRODUCTION
Background
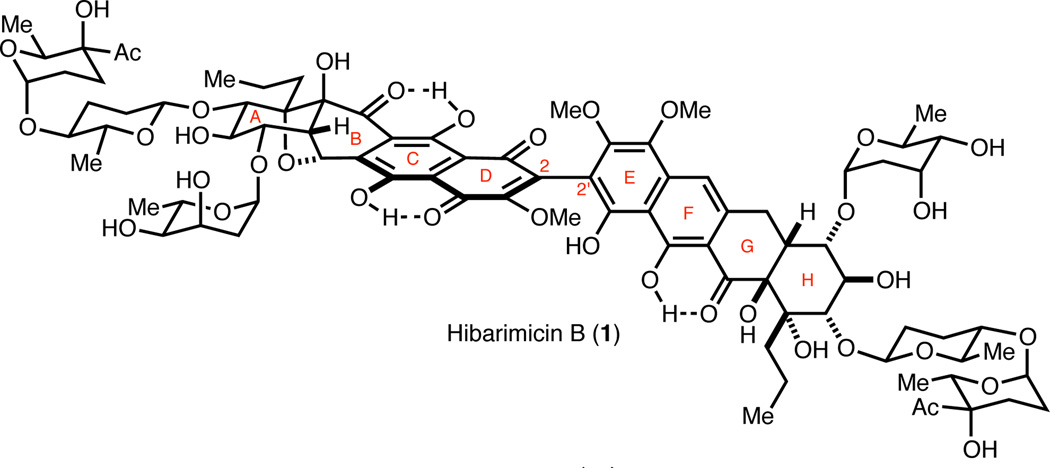
Hibarimicins A–G are complex pseudo-dimeric type-II polyketides isolated from the culture broth of the rare actinomycete Microbispora rosea subsp. hibaria TP-A0121.1 These metabolites inhibit proliferation and induce differentiation of numerous human cancer cell lines. In particular, hibarimicin B (1, Figure 1), which is identical to angelmicin B,1b,c has the most potent anti-proliferative activity in HL-60 cells (IC50 = 58 nM).1h The cellular target and biological mechanism of action of 1 remain undetermined. The hibarimicins are amongst the most complex and largest type-II polyketides known. Hibarimicins A–G share an unprecedented highly-oxidized aglycon, hibarimicinone (2a, Scheme 1). The C2-symmetry of 2a is broken by oxidation of the B-, C-, and D-rings relative to the G-, F-, and E-rings, respectively. More specifically, the B-ring contains a cyclic ether bridging C8' and C13', the C-ring contains a hydroxyl group at C6', and the D-ring is a quinone. Furthermore, 2a exhibits axial chirality about its highly congested C2–C2' bond and is isolated as a single atropisomer.1i,2 Altogether, the hibarimicins and hibarimicinone (2a) are challenging targets that have resisted total synthesis3 until earlier this year when Tatsuta et al. reported the first total synthesis of 2a.4 Herein, we report enantioselective total syntheses of hibarimicinone (2a) and atrop-hibarimicinone (2b), and the first total syntheses of the biosynthetically related natural product aglycons HMP-Y1 (3a), atrop-HMP-Y1 (3b) and HMP-P1 (6) (Scheme 1).
Figure 1.
Structure of hibarimicin B (1).
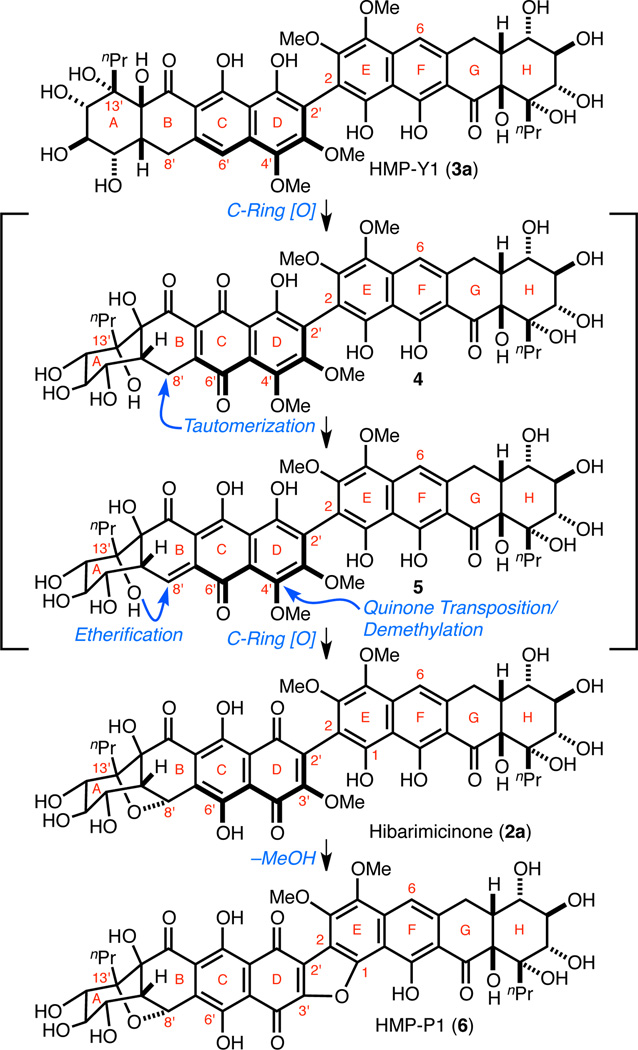
Scheme 1. Proposed Biosynthetic Conversion of HMP-Y1 (3a) to Hibarimicinone (2a) and HMP-P1 (6).
Biosynthesis Hypothesis
Mutagenesis of Microbispora rosea subsp. hibaria TP-A0121 led to the identification of novel metabolites, including HMP-Y1 (3a), HMP-P1 (6), and their glycosylated derivatives (Scheme 1).1f Through 13C-acetate labeling studies, it was discovered that C2-symmetric 3a is a precursor to 2a, which is subsequently glycosylated to yield hibarimicins A–G. Ostensibly, this conversion (3a → 2a) proceeds by breaking the C2-symmetry of 3a via oxidation of the B-, C-, and D-rings and demethylation of the C4'–OMe methyl group. We postulated that a single desymmetrizing oxidation of the C-ring of 3a to hypothetical quinone 4 would be sufficient to relay oxidation to the B- and D-rings. This could be achieved via (1) tautomerization of quinone 4 to C8'-ortho-quinone methide 5 with subsequent oxy-Michael addition of the C13'–OH to install the B-ring cyclic ether, (2) re-oxidation of the C-ring, and (3) transposition of the C-ring quinone to the D-ring with concomitant demethylation to give 2a. HMP-P1 (6) arises from 2a via cyclization of C1–OH onto C3' of the D-ring quinone and subsequent expulsion of methanol.1f
Results and Discussion
Synthesis Plan
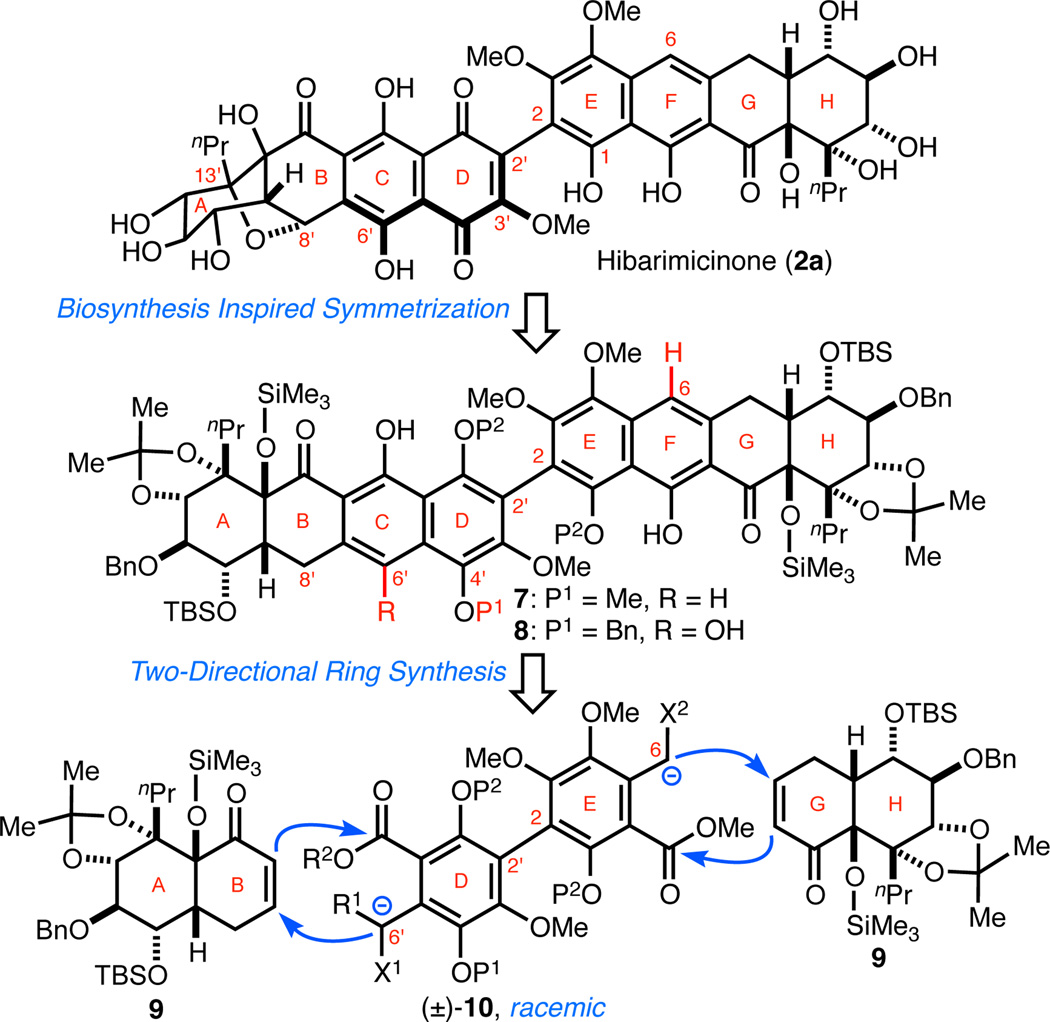
Inspired by our proposed biosynthetic relay oxidation scheme, we envisioned that a similar set of biomimetic retrosynthetic disconnections could simplify 2a to two plausible precursors, C2-symmetric octacycle 7 and pseudo-C2-symmetric octacycle 8 (Scheme 2). Targeting 7 was attractive for two reasons: (1) global deprotection would yield HMP-Y1 (3a) and (2) it would allow direct assessment of the feasibility of a biomimetic mono-oxidation to access a quinone analogous to 4. In contrast to 7, the C2-symmetry of 8 is perturbed by the presence of a benzyl group5 and the C6'–OH (both highlighted in red), the latter of which would facilitate chemoselective C-ring oxidation to a quinone. The most noteworthy feature shared by both 7 and 8 is the degeneracy of the AB- and HG-ring systems that result from the retrosynthetic excision of the B-ring cyclic ether bond. Next, it was envisioned that both octacyclic systems could be constructed in a single operation via a two-directional double annulation,6 where the dianion of biaryl 10 would react with two equivalents of the AB-/HG-enone (+)-9. The use of a symmetric biaryl annulation donor would lead to 7 whereas the employment of an unsymmetrical variant, with additional oxidation at C6', would result in 8. Both of these strategies are convergent and circumvent the need to construct the hindered C2–C2' bond at a late stage. Efforts by the Roush group to form the C2–C2' bond in simple model systems via cross-coupling were met with difficulty.3d At the outset of our study, the configuration of the stereochemical axis about the C2–C2' bond of hibarimicinone (2a) was ambiguous.1i Consequently, we elected to proceed with racemic biaryl annulation donor 10 to prepare and characterize both atropisomers of HMP-Y1 (3a) and hibarimicinone (2a).
Scheme 2. Biosynthesis-Inspired Retrosynthesis Analysis of HMP-Y1 (3a), Hibarimicinone (2a), and HMP-P1 (6).
Synthesis of the AB-/HG-Enone (+)-9
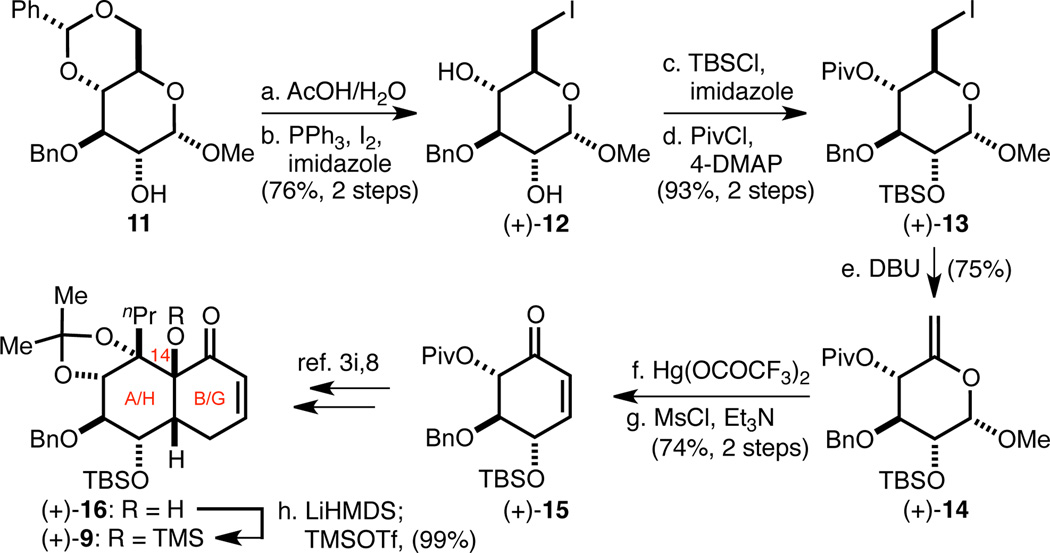
We previously reported a gram-scale enantiospecific synthesis of the AB-/HG-ring system corresponding to the unnatural enantiomer of 2a.3i The AB-/HG-enone (+)-16 (Scheme 3), with stereochemistry corresponding to the natural enantiomer of 2a, was prepared from key intermediate cyclohexenone (+)-15 following an analogous series of diastereoselective transformations. Notably, both enantiomers of (+)-15 were accessed from α-d-methyl-glucopyranoside by taking advantage of its latent C2-symmetry. The AB-/HG-enone synthesis commenced with AcOH-mediated deprotection of benzylidene acetal 11 followed by selective Wittig iodination of the resultant primary hydroxyl group to provide diol (+)-12. Next, chemoselective monosilylation of (+)-12 with TBSCl was accomplished by exploiting a subtle steric difference between its two secondary hydroxyl groups. The remaining secondary hydroxyl group was then pivoylated under forcing conditions to furnish differentially protected pyranose (+)-13. Exposure of (+)-13 to DBU promoted elimination of the primary iodide to generate exocyclic enol ether (+)-14, which underwent type-II Ferrier rearrangement upon treatment with catalytic Hg(OCOCF3)2.7 The resultant β-hydroxy-cyclohexanone was dehydrated to provide (+)-15. Following our previous procedures,3i (+)-15 was converted to (+)-168 and the tertiary C14–OH was ultimately TMS-protected to give annulation acceptor (+)-9.
Scheme 3. Synthesis of the AB-/HG-enone (+)-8.
aConditions: (a) AcOH/H2O, 80 °C; (b) PPh3, I2, imidazole, PhMe/CH2Cl2, RT → 45 °C, 76% (two steps); (c) TBSCl, imidazole, CH2Cl2, 0 °C → RT, 99%; (d) PivCl, 4-DMAP, ClCH2CH2Cl, 50 °C, 94%; (e) DBU, MeCN, 80 °C, 75%; (f) 30 mol% Hg(OCOCF3)2, Me2CO/H2O; (g) MsCl, Et3N, CH2Cl2, 0 °C → RT, 74% (two steps); (d) LiHMDS, THF, 0 °C; then TMSOTf, 0 °C → RT, 99%.
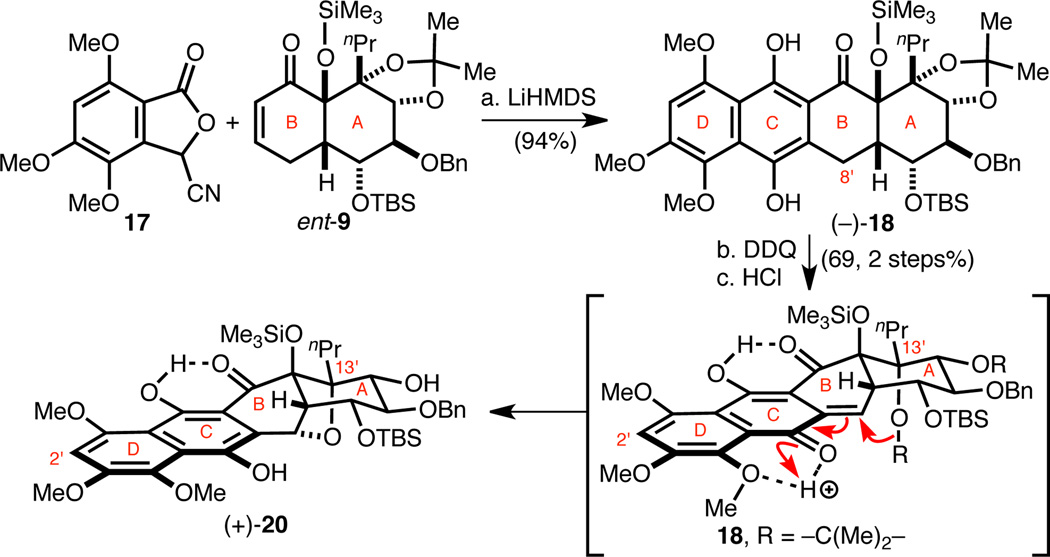
Demonstration of a Biomimetic Etherification on a Model ABCD-Ring System
A key transformation in our synthesis plan is a late-stage biomimetic etherification that retrosynthetically symmetrizes hibarimicinone (2a) and HMP-P1 (6). Consequently, a model ABCD-ring system was first investigated to test the viability of this proposed reaction. Kraus annulation9 of cyanophthalide 1710 with ent-AB-/HG-enone ent-98 under rigorously oxygen-free conditions gave ABCD-tetracycle (−)-18 (Scheme 4). Deoxygenation was critical for the success of the annulation, as adventitious oxygen caused decomposition. (−)-18 was then oxidized with DDQ to the corresponding C-ring quinone, which upon exposure to anhydrous HCl led to clean formation of pentacyclic ether (+)-20. This transformation presumably occurs through the intermediacy of ortho-quinone methide 19,11 which is trapped by the proximal acetonide oxygen with concomitant acetonide cleavage. Notably, this acid-mediated etherification required high dilution in order to avoid formation of (−)-18 and decomposition products, potentially via intermolecular processes.11c We propose that acetonide cleavage is triggered by its proximity to the reactive ortho-quinone methide intermediate due to the failure to isolate any acetonide cleavage products that lack the cyclic ether bond. This concept is further justified by later observations on the dimeric system, where only the acetonide of A-ring is cleaved whereas the G-ring acetonide remains intact (vide infra). Overall, the model oxidation-etherification sequence afforded excellent precedence for our subsequent studies.
Scheme 4. Synthesis of an ABCD-Pentacyclic Model System.
aConditions: (a) LiHMDS, THF, −78 → 0 °C, 94%; (b) DDQ, CH2Cl2, −20 °C; (c) HCl, CH2Cl2, 0 °C, 69% (two steps).
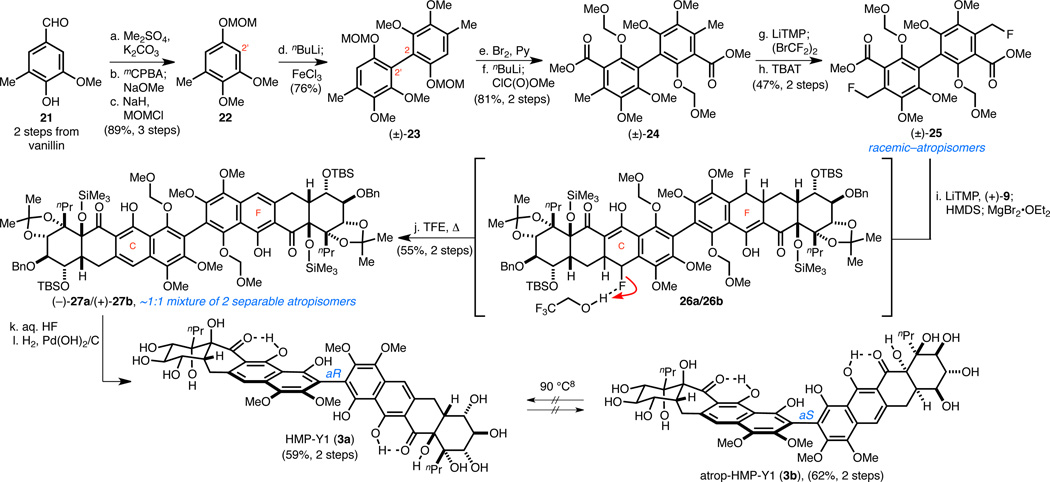
Development of a Benzyl Fluoride Michael–Claisen Reaction Sequence to Achieve the First Total Synthesis of HMP-Y1 (3a) and atrop-HMP-Y1 (3b)
Our synthesis plan for HMP-Y1 (3a) involved a symmetric two-directional double annulation to generate C2-symmetric octacycle 7, which could potentially be desymmetrized through a biomimetic mono-oxidation to access hibarimicinone (2a) and HMP-P1 (6). Such an ambitious double annulation strategy required a flexible synthesis of symmetric biaryl annulation donors in which different substituents at C6/C6' could be introduced, due to the lack of robust annulation sequences to generate naphthols (1-hydroxynaphthalenes rather than 1,4-dihydroxynaphthalenes, i.e., hydroquinones).12 Our biaryl synthesis began with 5-methylvanillin 2113 (Scheme 5), which was converted to trialkoxytoluene 22 in a three step process involving: (1) O-methylation, (2) Dakin oxidation followed by in situ formate methanolysis, and (3) MOM protection of the resultant phenol. Next, regioselective ortho-lithiation of 22 at C2' and FeCl3-mediated oxidative dimerization of the resultant aryllithium species delivered biaryl (±)-23. Carbomethoxy groups were then installed in a two-step sequence involving bromination and lithium–halogen exchange followed by acylation to afford bis-ortho-toluate (±)-24.
Scheme 5. Synthesis of HMP-Y1 (3a) and atrop-HMP-Y1 (3b) via a Benzyl Fluoride Michael–Claisen Reaction Sequence.
aConditions: (a) Me2SO4, K2CO3, Me2CO, 98%; (b) mCPBA, NaHCO3, CH2Cl2; then Na2CO3, MeOH; (c) NaH, MOMCl, DMF, 0 °C → RT, 91% (two steps); (d) nBuLi, TMEDA, THF, −78 → 0 °C; then FeCl3, 0 °C → RT, 76%; (e) Br2, Py, CH2Cl2, 0 °C, 91%; (f) nBuLi, THF, −78 °C; then ClC(O)OMe, −78 °C → RT, 89%; (g) LiTMP, THF, −78 °C; then (BrCF2)2, −78 °C, 62%; (h) TBAT, MeCN, 82 °C, 75%; (i) (+)-9, LiTMP, THF, −78 °C; then HMDS, −78 → −35 °C; then MgBr2•OEt2, −35 → 0 °C; (j) CF3CH2OH/H2O, NaHCO3, 80 °C, 55% (two steps); (k) aq. HF, MeCN/THF, 50 °C; (l) H2, Pd(OH)2/C, THF; for 3a, 59% (two steps); for 3b, 62% (two steps).
The reaction kinetics and ultimate success of Michael–Claisen reaction sequences14 hinges on numerous factors. These include, but are not limited to: (1) the stability and nucleophilicity of the reacting carbanion,15 (2) the stability of the electrophilic acceptor to the base required to deprotonote the donor,16 and (3) the steric bulk of the donor (substituents at C6/C6' and the ester side chain) and of the acceptor. Furthermore, the slow step of the tandem reaction sequence will change based on the particular donor and acceptor used. We found that (±)-24 could be deprotonated twice by LiTMP and that the corresponding dianion underwent two-directional bis-Michael–Claisen reaction sequence with various 2-cyclohexenones, including the AB-/HG-enone ent-9. However, the slow rate of both the Michael and Claisen reactions17 of the sequence with sterically encumbered ent-9 versus simpler 2-cyclohexenones coupled with the finite lifetime of the dianion of (±)-24 led to very low yields (<10%) of the desired octacyclic dihydronaphthalene products. Attempts to facilitate the Claisen step of the reaction sequence by utilizing activated ester analogues (i.e., phenyl and 2,2,2-trifluoroethyl) were particularly problematic since the sterically larger activated esters slowed the initial Michael reaction and the dianions were more prone to decomposition. Most importantly, aromatization of the C-/F-rings of the octacyclic products was met with difficulty and led us to consider alternative approaches.18 Benzyl sulfoxide19 and benzyl sulfone20 substituted ortho-toluates were also evaluated to achieve the desired naphthol annulation with (+)-9 or ent-9, but ultimately proved unsuccessful in the context of a two-directional double annulation (vide infra).
We next envisaged a benzyl fluoride Michael–Claisen reaction sequence to generate naphthalenes after subsequent dehydrohalogenation.21 Although there was no precedence for such a strategy, a benzyl fluoride annulation donor was attractive for several reasons: (1) the electronegative fluorine atom should stabilize the biaryl dianion, (2) the small atomic radius of fluorine should provide minimal steric hinderance to the initial Michael reaction, (3) the strength of C–F bonds would disfavor α-elimination and SN2 displacement of fluoride, and (4) despite the strength of C–F bonds, elimination of the benzyl fluoride under appropriate conditions could lead to C- and F-ring aromatization.22 The dianion of (±)-24 was brominated with (BrCF2)2 to yield the bis-benzyl bromide, which upon heating with TBAT23 afforded bis-benzyl fluoride (±)-25. After significant experimentation, the desired protected HMP-Y1 derivatives (−)-27a and (+)-27b24 were accessed from (+)-9 and (±)-25 in a two-step procedure involving: (1) a bis-Michael–Claisen reaction sequence promoted by LiTMP and MgBr2•OEt2 to afford octacycles 26a and 26b and (2) the formal elimination of HF by heating the unpurified reaction product in 2,2,2-trifluoroethanol (TFE) to achieve aromatization of the C- and F-rings and provide atropisomers (−)-27a and (+)-27b, which were readily separated and carried forward independently. Several features of this sequence deserve comment. As we had hoped, the use of a bis-benzyl fluoride (±)-25 allowed for the initial bis-Michael addition to occur at −78 °C, thus minimizing decomposition of the dianion intermediate and of (+)-9. Addition of MgBr2•OEt2 mid annulation sequence was critical to promote the final intramolecular Claisen reactions and obviated the need to use an activated ester analogue. This discovery should help expand the substrate scope of the Michael–Claisen reaction sequence. Finally, the unique ability of TFE to promote the desired elimination is presumably due to its ability to strongly hydrogen bond with fluorine, and thus activate it for mild solvolysis. Indeed, use of ethanol in place of TFE only led to trace elimination. The employment of a benzyl fluoride annulation-elimination sequence to generate naphthalene derivatives is without precedence and may prove to be a general method for the synthesis of naphthols.
Global deprotection of (−)-27a and (+)-27b with aqueous HF followed by hydrogenolysis afforded HMP-Y1 (3a) and atrop-HMP-Y1 (3b), respectively.25 Heating 3a or 3b to 90 °C led to no detectable isomerization about the C2–C2' bond.8 With no authentic CD-spectra for natural 3a available, synthetic 3a and 3b were designated based on comparison to the CD spectrum of the glycosylated derivative of 3a, HMP-Y6.8 The axial stereochemistry of HMP-Y1 (3a) has not been rigorously determined, although model studies and the CD-spectra of HMP-Y6 and hibarimicinone (2a) suggest 3a possesses the aR configuration by the CD exciton chirality method.2,26 Additionally, 3a, 2a, and hibarimicin A–G are all isolated as single atropisomers.26 We show that the axial stereochemistry of 3a and 2a are not the result of thermodynamic equilibration (vide infra), and thus their biosynthetic relationship also argues that they possess the same relative configuration about the C2–C2' bond. Therefore, since the axial chirality of 2a was unambiguously determined,4 3a can be assigned the aR configuration.
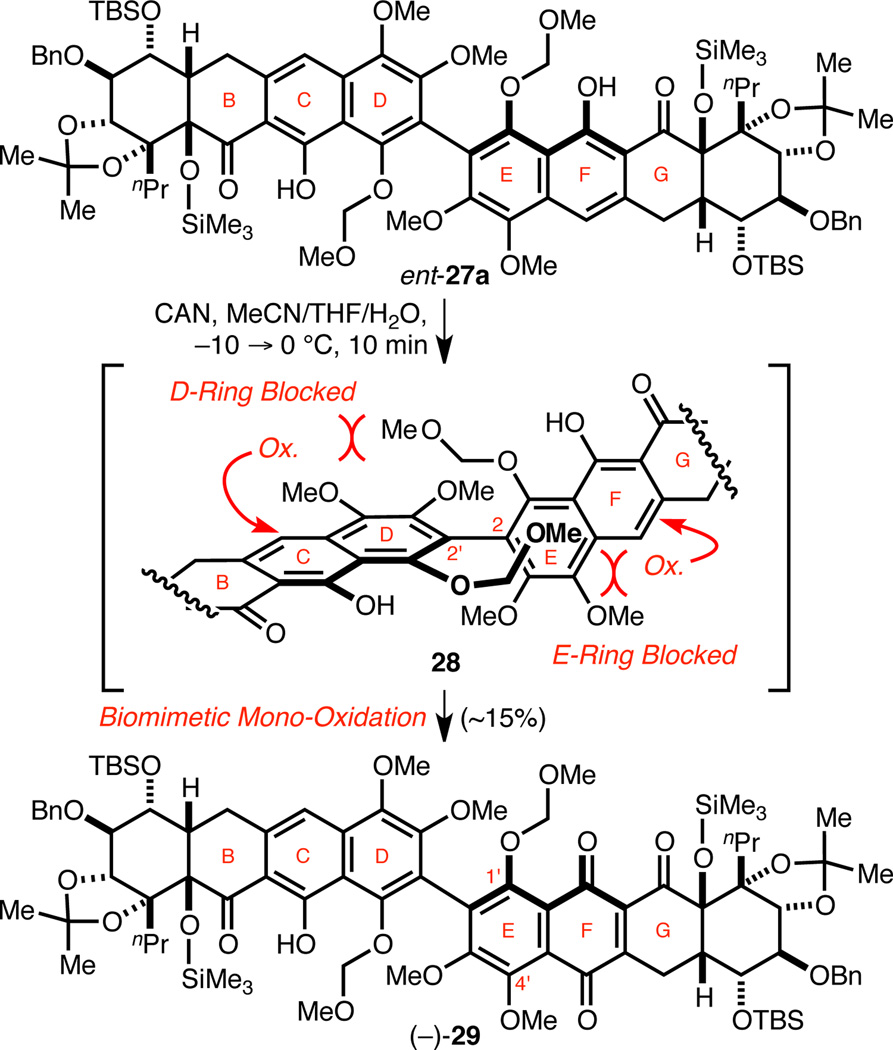
Biomimetic Mono-Oxidation of Protected HMP-Y1
With a route to HMP-Y1 (3a) and atrop-HMP-Y1 (3b) established, we next attempted the biomimetic mono-oxidation of protected HMP-Y1 derivatives. We discovered that the desired oxidation of ent-27a to the C-ring quinone (−)-29 could be achieved in low yield with CAN (Scheme 6), demonstrating the plausibility of our proposed biomimetic desymmetrizing oxidation. We speculate that the congested biaryl may sterically occlude the approach of oxidants to the otherwise easily oxidized D-/E-ring system,27 allowing oxidation of the more electron-deficient C-/F-rings. Despite this initial success, our attempts to optimize the CAN oxidation were met with difficulty due to bis-oxidation and formation of nitrated byproducts. A survey of other oxidants also proved fruitless.
Scheme 6. Biomimetic Mono-Oxidation of Protected HMP-Y1.
Nevertheless, with naphthazarin (−)-29 in hand we next investigated the desired biomimetic etherification reaction. Unfortunately, exposure of (−)-29 to the optimized conditions developed on our model system led to no observable etherification but rather only rapid MOM group cleavage. A screen of various acids and conditions also proved unsuccessful. The resistance of (−)-29 to undergo the desired etherification in contrast to the C-ring quinone derivative of (−)-18 was surprising. Since the major difference between the two systems is the lability of the MOM groups of (−)-29 relative to the methyl group of (−)-18, we postulated that a free phenol at C1' might disfavor either acetonide decomposition or formation of the necessary ortho-quinone methide intermediate. This prompted us to replace the MOM group with a more acid-stable protecting group.
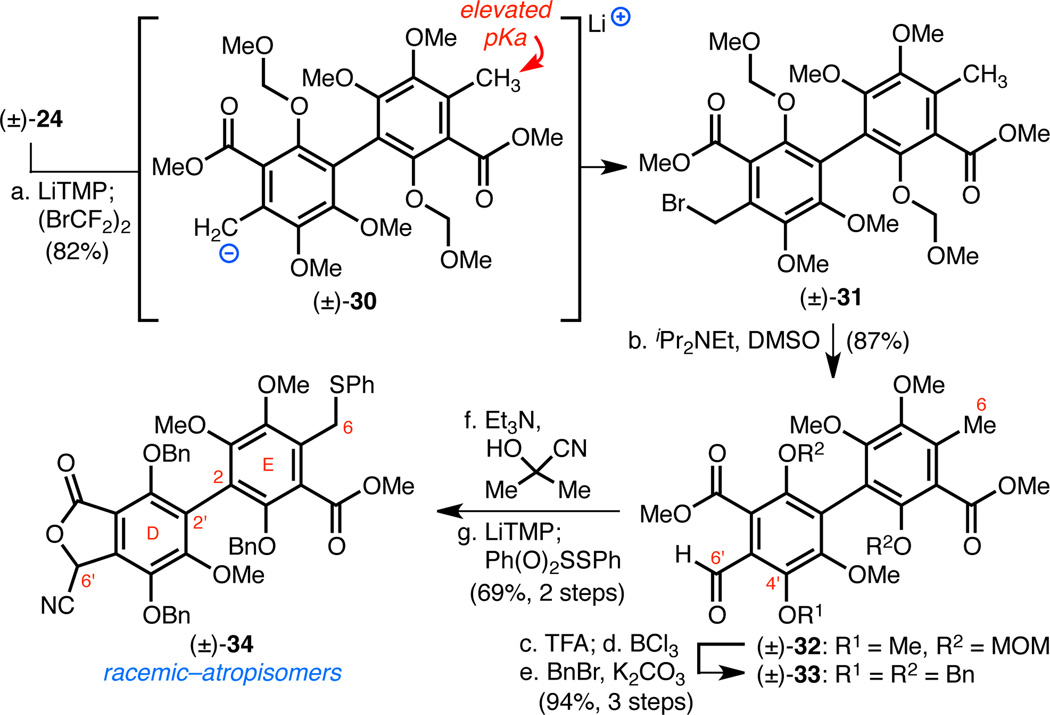
Additionally, our current biomimetic strategy would inevitably require a late-stage demethylation of the C4'–OMe methyl group. Ideally, one would remove the C4'–OMe methyl group as late in an eventual synthesis of hibarimicin B (1) as possible to protect the sensitive and stereochemically labile binaphthyl core (vide infra). However, the acidic conditions necessary to effect demethylation would be incompatible with the sensitive 2-deoxy- and 2,3-dideoxyglycosides of 1. The aforementioned reasons prompted us to investigate our alternative strategy for the synthesis of hibarimicione (2a) and HMP-P1 (6) utilizing an unsymmetrical two-directional annulation reaction with biaryl (±)-34 (Scheme 7).
Scheme 7. Synthesis of Unsymmetrical Biaryl (±)-34 via a Selective Mono-Deprotonation of (±)-24.
aConditions: (a) LiTMP, THF, −78 °C; then (BrCF2)2, 82%; (b) iPr2NEt, DMSO, 70 °C, 87%; (c) TFA, CH2Cl2, 0 °C → RT; (d) BCl3, CH2Cl2, −78 → 0 °C; (e) BnBr, K2CO3, DMF, 0 → 60 °C, 94% (three steps); (f) Me2C(OH)CN, Et3N, CHCl3, 97%; (g) LiTMP, THF, −78 °C; then Ph(O)2SSPh, 71%.
Synthesis of the Unsymmetrical Biaryl Annulation Donor (±)-34
The unsymmetrical fully substituted biaryl (±)-34 presents unique synthesis challenges that are shared with the hibarimicins; cross-coupling technology to form such sterically hindered biaryls from electron-rich aromatics is limited.3d In contrast, dimerization reactions to form hindered biaryls are robust and reliable (e.g., 22 → (±)-23). Thus we imagined that a practical synthesis approach to (±)-34 would necessitate the desymmetrization of (±)-24. A strategy to mono-functionalize (±)-24 involving radical bromination would inevitably result in an inefficient statistical mixture of benzylic bromides. However, we hypothesized that selective mono-deprotonation of (±)-24 would be feasible since the initial carbanion would elevate the pKa of the remaining ortho-toluate due to a field effect. Indeed, we found that selective mono-deprotonation of (±)-24 at C6' could be achieved with 1.25 equiv of LiTMP (Scheme 7). The resultant anion (±)-30 was then brominated with (BrCF2)2 to give benzyl bromide (±)-31 in 82% yield (Scheme 7). This single element of asymmetry was sufficient to introduce the remaining differential functionality of (±)-34. (±)-31 was oxidized to aldehyde (±)-32,28 which was then converted to tri-benzyl-protected biaryl (±)-33 by (1) acid-promoted removal of the MOM groups, (2) chemoselective cleavage of the C4'–OMe methyl group with BCl3, and (3) global reprotection with BnBr. Treatment of (±)-33 with a controlled source of hydrogen cyanide afforded a cyanophthalide intermediate.29 Finally, double deprotonation of this intermediate with LiTMP followed by a short exposure to S-phenyl benzenethiosulfonate chemoselectively installed the phenyl sulfide moiety21 at C6 to provide biaryl (±)-34. The observed chemoselectivity in this reaction is a result of the much higher reactivity of the ortho-toluate anion relative to the cyanophthalide anion.
Completion of Hibarimicinone (2a) and atrop-Hibarimicinone (2b) via an Unsymmetrical Two-Directional Double Annulation
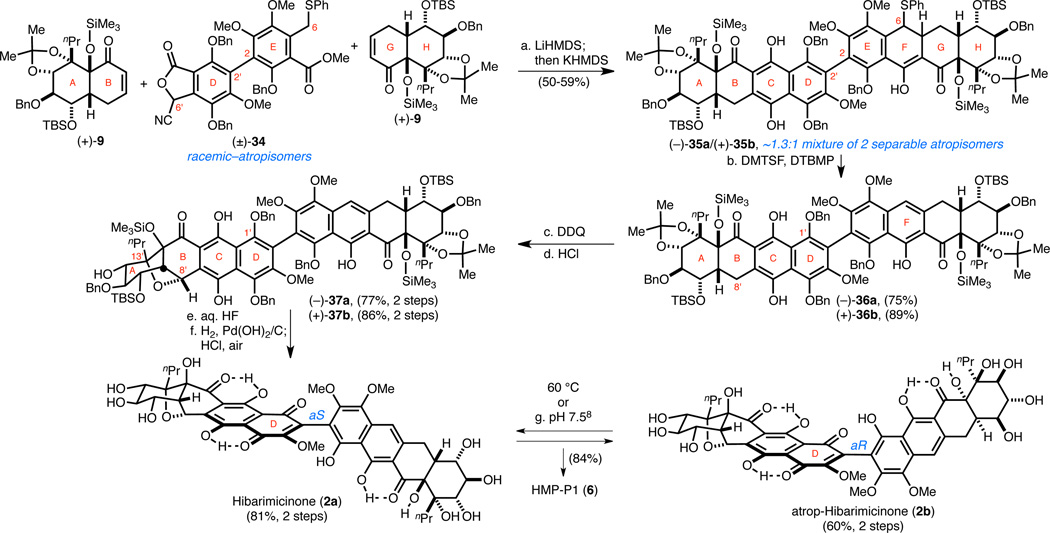
We anticipated that reaction of the lithiated cyanophthalide of (±)-34 with (+)-9 would directly construct the C-ring hydroquinone via a Kraus annulation, and reaction of the lithiated benzyl phenyl sulfide of (±)-34 with a second equivalent of (+)-9 would lead to the F-ring via a Michael–Claisen reaction sequence. We found that the desired transformations could be achieved by treating a mixture of (±)-34 and (+)-9 with LiHMDS followed by subsequent addition of KHMDS mid annulation sequence under rigorously oxygen-free conditions to yield octacycle (−)-35a and (+)-35b as a ~1.3:1 mixture of atropisomers (Scheme 8). The addition of KHMDS was crucial to facilitate the final intramolecular Claisen reaction to construct the F-ring.30 At this stage, atropisomers (−)-35a and (+)-35b were separated and carried forward independently. Elimination of the C6-benzylic phenyl sulfide was accomplished with dimethyl(methylthio)sulfonium tetrafluoroborate (DMTSF) to yield binaphthalenes (−)-36a and (+)-36b. It is worth reiterating at this point that the corresponding C6-benzyl sulfoxide and sulfone derivatives of (±)-34 ultimately proved unsuccessful in a two-directional annulation,31 highlighting the difficulty to achieve naphthol annulations in the context of complex molecule synthesis. To the best of our knowledge, this is the first example of a benzyl sulfide Michael–Claisen reaction sequence to generate naphthalenes, and together with the benzyl fluoride Michael–Claisen reaction sequence reported offer two new alternatives to approach challenging naphthol annulations.
Scheme 8. Completion of Hibarimicinone (2a), atrop-Hibarimicinone (2b), and HMP-P1 (6).
aConditions: (a) LiHMDS, THF, −78 → 0 °C; then KHMDS, 0 °C → RT, 50–59%; (b) DMTSF, DTBMP, MeCN, 0 °C → RT; for (−)-35a, 75%; for (+)-35b, 89%; (c) for (−)-35a: DDQ, PhMe, 0 °C; for (+)-35b: DDQ, PhMe, 0 °C → RT; (d) HCl, ClCH2CH2Cl, 5 °C; for (−)-37a, 77% (two steps); for (+)-37b, 86% (two steps); (e) aq. HF, MeCN/THF; (f) H2, Pd(OH)2/C, EtOAc; then HCl, MeOH, air; for 2a, 81% (two steps); for 2b, 60% (two steps); (g) aq. pH 7.5 NaH2PO4/NaOH buffer, MeOH, RT; from 2a, 84%; from 2b, 84%.
Oxidation of (−)-36a and (+)-36b with DDQ produced the corresponding C-ring quinones. Exposure of the respective quinones to anhydrous HCl promoted the desired biomimetic etherification to yield nonacycles (−)-37a and (+)-37b. This successful etherification of the benzyl protected naphthazarins, in contrast to MOM-protected (−)-29, confirmed our suspicion that the nature of the C1'-phenol has far-reaching stereoelectronic effects on this system. With the complete skeletons of 2a and 2b in hand, all that remained to complete the syntheses was global deprotection and oxidation of the D-ring. Deprotection of the acid-labile protecting groups was accomplished upon exposure to HF. Finally, the benzyl groups were removed via hydrogenolysis, and after addition of acidic methanol, filtering, and exposure to air, hibarimicinone (2a) and atrop-hibarimicinone (2b) were formed. All of the spectroscopic data for 2a and 2b match those reported4,8,26 and thereby confirmed the structure of 2b.
Discovery of a pH-Dependent Rotational Barrier About the C2–C2' Bond in Hibarimicinone (2a) and atrop-Hibarimicinone (2b)
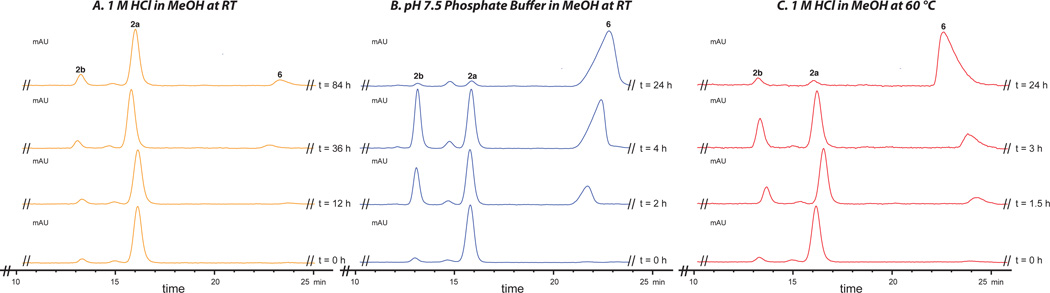
The addition of acidic methanol prior to aerobic oxidation was crucial to prevent isomerization between 2a and 2b and formation of HMP-P1 (6).25,32 Upon prolonged handling or standing at ambient temperatures in acidic methanol (1 M HCl), we observed minor interconversion between 2a and 2b minimal formation of 6 (Figure 2A). However, simple exposure of 2a and 2b, independently, to pH 7.5 buffered methanol led to the formation of 6 in 84% yield in both cases (Scheme 8). Monitoring these transformations by HPLC revealed that nearly complete isomerization about the C2–C2' bond (2a ↔ 2b) had occurred within 4 hours while formation of 6 was almost complete after 24 hours (Figure 2B).8 Together, these observations suggest that the rotational barriers about C2–C2' for 2a and 2b are pH-dependent.
Figure 2.
(A) Upon standing in acidic methanol (1 M HCl) at RT, hibarimicinone (2a) and atrop-hibarimicinone (2b) undergo minor interconversion and minimal conversion to HMP-P1 (6) (orange HPLC traces). (B) Exposure of 2a to pH 7.5 aqueous phosphate buffer at RT (blue HPLC traces) or (C) acidic methanol (1 M HCl) at 60 °C (red HPLC traces) resulted in isomerization to 2b and eventual formation of 6. See SI for HPLC timecourses for 2b.
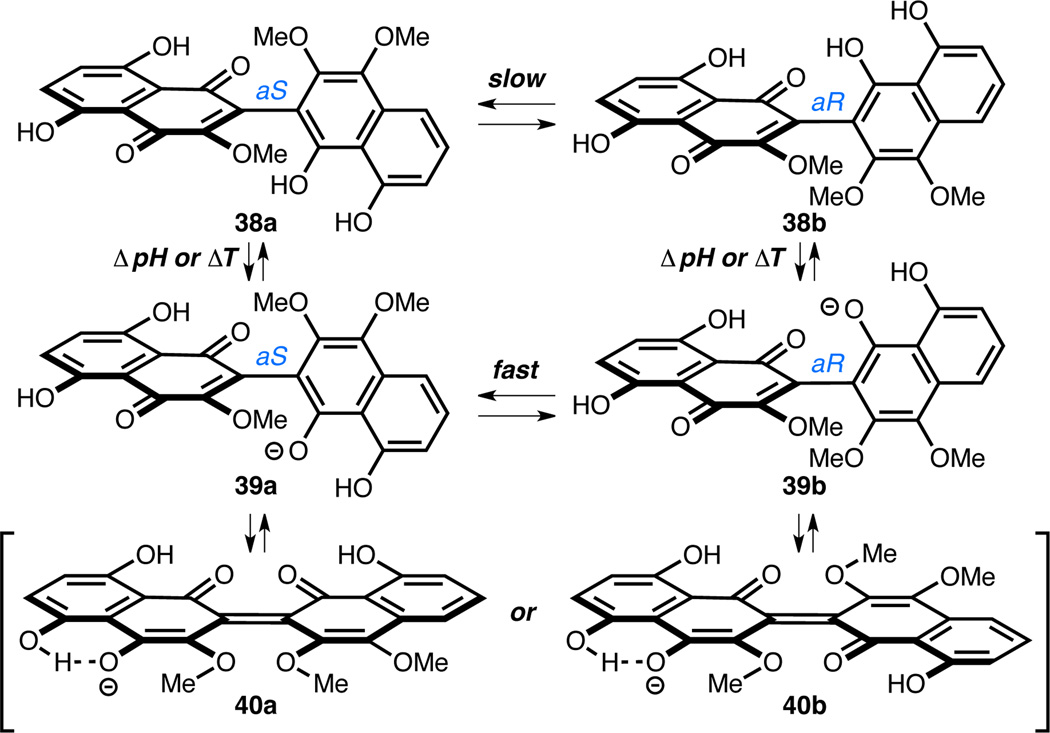
These findings are particularly interesting owing to prior observations that heating 2a in neutral methanol at 60 °C leads to nearly complete interconversion to 2b in 30 minutes and ultimately complete cyclization to yield 6 after 90 minutes.1i,2,26 However, we found that heating either 2a or 2b to 60 °C in acidic methanol (1 M HCl) led to only partial interconversion between 2a and 2b and minor conversion to 6 after 90 minutes (Figure 2C). This suggests that the observed rapid rotation at 60 °C in neutral methanol has less to do with providing the necessary thermal energy to surpass the intrinsic activation barrier about C2–C2' in the uncharged forms of 2a/2b (38a/38b in Figure 3), but rather enables access to the deprotonated form of 2a and 2b (39a/39b in Figure 3) via inter- or intramolecular proton transfer. Rapid interconversion between 37a and 37b can then follow through a transition state that is stabilized by π-electron overlap,1b,33 as depicted in cross-conjugated resonance structures 40a and 40b. Consequently, variables that affect the equilibrium between 38a and 39a, and 38b and 39b will affect the rate of isomerization. The addition of acid to the media inhibits access to species 39a and 39b by driving the equilibrium toward 38a and 38b, and thus disfavors isomerization. In contrast, heat (60 °C) should promote equilibration between the protonation states and thus facilitate isomerization. Our discovery of the pH-dependent barrier demonstrates the delicate nature of the C2–C2' which must be accommodated in an eventual synthesis of hibarimicin B (1).32
Figure 3.
A proposed model to explain the pH-dependent rotational barrier about the C2–C2' bond of 2a and 2b. Only the CDEF-ring system is depicted for brevity.
Conclusion
Enantioselective syntheses of hibarimicinone (2a) and atrophibarimicionone (2b), and the first total syntheses of HMP-Y1 (3a), atrop-HMP-Y1 (3b), and HMP-P1 (6) have been accomplished. The complete carbon skeleton of each natural product was assembled via a convergent two-directional annulation strategy. The use of a racemic biaryl in conjunction with the two-directional annulation strategy enabled both atropisomers of the natural products to be separately constructed and fully characterized, thus providing the first reported full characterization of 2b, 3a, 3b, and 6. Additionally during the pursuit of this annulation strategy, we encountered numerous challenges when conducting naphthol annulation reactions. Consequently, we developed two valuable Michael–Claisen reaction sequences to construct complex naphthols that might find use as general methods. The mild conditions needed to dehydrohalogenate the benzyl fluoride intermediates are particularly noteworthy given the strength of C–F bonds.
The plausibility of our proposed biosynthesis was also validated by the demonstration that a desymmetrizing mono-oxidation of the C-ring can be conducted on protected HMP-Y1 derivatives. Over-oxidation to the bis-C-/F-ring quinone was also observed, but natural products corresponding to such a double oxidation have not been isolated in nature or during mutagenesis studies. This perhaps suggests that an enzyme mediates this key biosynthetic transformation, but how 3a is only mono-oxidized remains unclear.
After the key two-directional annulations, only three and five steps were needed to complete HMP-Y1 (3a) and hibarimicinone (2a), respectively. In the case of 2a, these steps include a biomimetic etherification to install the B-ring cyclic ether via an ortho-quinone methide intermediate. The success of this reaction required an acid-stable protecting group on the C1'-phenol owing to subtle yet far-reaching stereoelectronic effects imparted by the naphthazarin-naphthalene system. The peculiarities and sensitivity of this system are also highlighted by our discovery of the pH-dependent rotational barrier about the C2–C2' bond. These particular observations provide crucial information that will facilitate an eventual synthesis of hibarimicin B (1).
Lastly, the intermediate (−)-37a will be highly useful in an eventual total synthesis of 1; it is suitably protected with orthogonal protecting groups to allow for the sequential installation of the 2-deoxy- and 2,3-dideoxyglycosides prior to deprotection of the sensitive binaphthyl core of the molecule. Future studies toward the total synthesis of 1 will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
We thank Prof.’s Y. Igarashi, H. Hori, and G. Sulikowski for communication regarding atropisomerism and for providing authentic spectroscopic data. B.B.L. acknowledges Amy S. Lee for helpful discussions.
Funding Sources
No competing financial interests have been declared. We acknowledge financial support from NIH (R016M090068). B.B.L. acknowledges a NSF Predoctoral Fellowship and Bristol-Myers Squibb. B.C.M. acknowledges Eli Lilly and AstraZeneca.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental procedures, spectroscopic data, and copies of CD, UV–vis, 1H and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Uehara Y, Li PM, Fukazawa H, Mizuno S, Nihei Y, Nishio M, Hanada M, Yamamoto C, Furumai T, Oki T. J. Antibiot. 1993;46:1306–1308. doi: 10.7164/antibiotics.46.1306. [DOI] [PubMed] [Google Scholar]; (b) Hori H, Higashi K, Ishiyama T, Uramoto M, Uehara Y, Oki T. Tetrahedron Lett. 1996;37:2785–2788. [Google Scholar]; (c) Kajiura T, Furumai T, Igarashi Y, Hori H, Higashi K, Ishiyama T, Uramoto M, Uehara Y, Oki T. J. Antibiot. 1998;51:394–401. doi: 10.7164/antibiotics.51.394. [DOI] [PubMed] [Google Scholar]; (d) Hori H, Igarashi Y, Kajiura T, Furumai T, Higashi K, Ishiyama T, Uramoto M, Uehara Y, Oki T. J. Antibiot. 1998;51:402–417. doi: 10.7164/antibiotics.51.402. [DOI] [PubMed] [Google Scholar]; (e) Hori H, Kajiura T, Igarashi Y, Furumai T, Higashi K, Ishiyama T, Uramoto M, Uehara Y, Oki T. J. Antibiot. 2002;55:46–52. doi: 10.7164/antibiotics.55.46. [DOI] [PubMed] [Google Scholar]; (f) Kajiura T, Furumai T, Igarashi Y, Hori H, Higashi K, Ishiyama T, Uramoto M, Uehara Y, Oki T. J. Antibiot. 2002;55:53–60. doi: 10.7164/antibiotics.55.53. [DOI] [PubMed] [Google Scholar]; (g) Igarashi Y, Kajiura T, Furumai T, Hori H, Higashi K, Ishiyama T, Uramoto M, Uehara Y, Oki T. J. Antibiot. 2002;55:61–70. doi: 10.7164/antibiotics.55.61. [DOI] [PubMed] [Google Scholar]; (h) Cho SI, Fukazawa H, Honma Y, Kajiura T, Hori H, Igarashi Y, Furumai T, Oki T, Uehara Y. J. Antibiot. 2002;55:270–278. doi: 10.7164/antibiotics.55.270. [DOI] [PubMed] [Google Scholar]; (i) Hori H, Igarashi Y, Kajiura T, Sato S, Furumai T, Higashi K, Ishiyama T, Uehara Y, Oki T. Tennen Yuki Kagobutsu Toronkai Koen Yoshishu. 2004;46:49–54. [Google Scholar]
- 2.Romaine IM, Hempel JE, Shanmugam G, Hori H, Igarashi Y, Polavarapu PL, Sulikowski GA. Org. Lett. 2011;13:4538–4541. doi: 10.1021/ol2017005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.For studies towards the hibarimicins or their related natural products, see: Lee C-S, Audelo MQ, Reibenpies J, Sulikowski GA. Tetrahedron. 2002;58:4403–4409. Maharoof US, Sulikowski GA. Tetrahedron Lett. 2003;44:9021–9023. Kim K, Mahroof US, Raushel J, Sulikowski GA. Org. Lett. 2003;5:2777–2780. doi: 10.1021/ol034823f. Narayan S, Roush WR. Org. Lett. 2004;6:3789–3792. doi: 10.1021/ol048436x. Lambert WT, Roush WR. Org. Lett. 2005;7:5501–5504. doi: 10.1021/ol052321r. Lee WD, Kim K, Sulikowski GA. Org. Lett. 2005;7:1687–1689. doi: 10.1021/ol050105c. Li J, Todaro LJ, Mootoo DR. Org. Lett. 2008;10:1337–1340. doi: 10.1021/ol703036y. Li J, Todaro L, Mootoo DR. Eur. J. Org. Chem. 2011:6281–6287. doi: 10.1002/ejoc.201100815. Milgram BC, Liau BB, Shair MD. Org. Lett. 2011;13:6436–6439. doi: 10.1021/ol202728v.
- 4.Tatsuta K, Fukuda T, Ishimori T, Yachi R, Yoshida S, Hashimoto H, Hosokawa S. Tetrahedron Lett. 2012;53:422–425. [Google Scholar]
- 5.The use of a benzyl group was a strategic concession to protect the sensitive core of 1 up until the last step of an eventual synthesis of 1.
- 6.For other examples of two-directional double annulation reactions, see: Hauser FM, Gauuan PJ. Org. Lett. 1999;1:671–672. doi: 10.1021/ol990758r. (b) Ref. 2. (c) Ref. 4, and references therein.
- 7.Ferrier RJ. J. Chem. Soc. Perkin Trans. 1979;1:1455–1458. [Google Scholar]
- 8.See SI for full details.
- 9.Kraus GA, Sugimoto H. Tetrahedron Lett. 1978;19:2263–2266. [Google Scholar]
- 10.Wendt JA, Gauvreau PJ, Bach RD. J. Am. Chem. Soc. 1994;116:9921–9926. [Google Scholar]
- 11.For related transformations utilizing ortho-quinone methidesm, see: (a) Ref. 4. Hart DJ, Huang H-C. J. Am. Chem. Soc. 1988;110:1634–1635. Nicolaou KC, Lim YH, Piper JL, Papageorgiou CD. J. Am. Chem. Soc. 2007;129:4001–4013. doi: 10.1021/ja0685708.
- 12.For reviews, see: Mal D, Pahari P. Chem. Rev. 2007;107:1892–1918. doi: 10.1021/cr068398q. Rathwell K, Brimble MA. Synthesis. 2007;5:643–662.
- 13.5-Methylvanillin (S15) was prepared from vanillin in two steps on multigram scale following a literature procedure: Sinhababu AK, Borchardt RT. Syn. Comm. 1983;13:677–683.
- 14.(a) For other uses of Michael–Claisen reaction sequences to construct naphthalene derivatives, see: Sun C, Wang Q, Brubaker JD, Wright PM, Lerner CD, Noson K, Charest M, Siegel DR, Wang Y-M, Myers AG. J. Am. Chem. Soc. 2008;130:17913–17927. doi: 10.1021/ja806629e. and references therein. (b) For a related approach to 3a, see ref. 2.
- 15.(a) ortho-Toluate and related carbanions will suffer from competitive bimolecular self-condensation reactions with the ester moiety if the Michael addition is not fast enough. For an interesting discussion on the stability of ortho-toluate and related carbanions, see Brubaker JD. Ph.D. Thesis. Harvard University; 2007. and references therein. (b) In the case of a single annulation process, the instability of the deprotonated annulation donor can often be partially circumvented through the use of excess donor. However, due to the inherent stoichiometry of the two-directional double annulation, the biaryl donor is used as the limiting reactant and thus the stability of its dianion is critical to the success of the reaction
- 16.We observed that ent-9 is stable to LiTMP and LDA at −78 °C, and LiHMDS at 0 °C in THF. At higher respective temperatures for prolonged reaction times, significant decomposition occurred.
- 17.Simple 2-cyclohexenones will undergo the Michael addition within seconds at −78 °C and eventual Claisen reaction at −10 °C with the ortho-toluate carbanion corresponding to the D-/E-ring. In contrast, ent-9 underwent Michael addition after approximately 1 hour and the Claisen reaction was never driven to completion with the dianion of (±)-24.
- 18.DDQ or PhSeCl with pyridine could successfully be employed to aromatize dihydronaphthalenes of simple BCD-ring model systems but proved unsuccessful on binaphthyl systems.
- 19.(a) Hauser FM, Rhee RP. J. Org. Chem. 1978;43:178–180. [Google Scholar]; (b) Lee HG, Ahn J-Y, Lee AS, Shair MD. Chem. Eur. J. 2010;16:13058–13062. doi: 10.1002/chem.201002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Wildeman J, Borgen PC, Pluim H. Tetrahedron Lett. 1978;25:2213–2216. [Google Scholar]; (b) Huang X, Xue J. J. Org. Chem. 2007;72:3965–3968. doi: 10.1021/jo070241q. [DOI] [PubMed] [Google Scholar]
- 21.Benzyl phenylsulfide substituted ortho-toluates were concurrently found to be useful partners for naphthol annulations and were ultimately employed in our synthesis of 2a and 6 due to the inability to incorporate a benzyl fluoride at C6 of (±)-34.
- 22.Bitha P, Hlavka JJ, Boothe JH. J. Med. Chem. 1970;13:89–92. doi: 10.1021/jm00295a023. [DOI] [PubMed] [Google Scholar]
- 23.Pilcher AS, Ammon HL, DeShong P. J. Am. Chem. Soc. 1995;117:5166–5167. [Google Scholar]
- 24.(a) For brevity, each atropisomer is depicted as a single structure lacking stereochemistry about the C2–C2' bond. See SI for full details. (b) The regioisomer of the enolized 1,3-diketone is arbitrarily depicted.
- 25.No NMR or CD spectra for 3a and 6 have been previously recorded according to ref. 26. See SI for full details.
- 26.Described in a personal communication with Professor Y. Igarashi.
- 27.(a) Preferential oxidation of the D-ring occurs in simpler BCD-ring model systems. (b) The 1H NMR signal of the methoxymethyl groups of ent-27a and ent-27b are shifted over 0.6 ppm up-field relative to the corresponding monomer, suggesting that they are positioned over the naphthyl ring systems and subject to anisotropic magnetic field effects.
- 28.Kornblum N, Jones WJ, Anderson GJ. J. Am. Chem. Soc. 1959;81:4113–4114. [Google Scholar]
- 29.Sakulsombat M, Angelin M, Ramström O. Tetrahedron Lett. 2010;51:75–78. [Google Scholar]
- 30.Kummer DA, Li D, Dion A, Myers AG. Chem. Sci. 2011;2:1710–1718. doi: 10.1039/C1SC00303H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The small coupling constant between the C6' and C7' hydrogen atoms of (−)-35a and (+)-35b suggest a syn relationship of the hydrogen atoms with respect to the ring system. This relative stereochemistry would preclude syn-elimination to aromatize the F-ring. Indeed, one diastereomer of the corresponding sulfoxide of (±)-34 underwent two-directional annulation but failed to eliminate to aromatize the F-ring.
- 32.The carbohydrates of the hibarimicin natural products are cleaved with acidic methanol (1 M HCl, 30 °C). These conditions are similar to those we employ during the benzyl deprotection and oxidation of (−)-37a and (+)-37b. However, milder acidic conditions (i.e. aq. pH 3.5 phosphate buffer) in methanol may potentially be substituted during the analogous deprotection and oxidation of 1 since these conditions are employed in the HPLC purification of 1 and hibarimicin related natural products. See ref 1f–g for the conditions used for carbohydrate cleavage and purification of the hibarimicin natural products.
- 33.Eliel EL, Wilen SH. Stereochemistry of Organic Compounds. New York: John Wiley & Sons, Inc.; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.