INTRODUCTION
Aging is a complex process with multiple alterations in the physiological structure and functional responses of the organism. In recent years, aging has been suggested as an independent predictor of erectile dysfunction (ED) according to epidemiologic studies. The cavernosal angioarchitecture is modified with age. The decrease in smooth muscle cells and the considerable enlargement of vascular lumens in the penis may limit the basic function of the penile vascular tree in the elderly [1].
It is widely known that ED is predominantly a disease of vascular origin, directly associated with the ability of cavernosal tissue relaxation. Nitric oxide (NO) is a principal modulator of penile erection and a decrease in NO bioavailability is a central mechanism for ED [2]. Impaired endothelium-dependent and NO-mediated cavernous relaxation with aging are due to decreased NO production [2]. In addition, there is a close relationship among ED, aging and endothelial dysfunction, and data suggest that ED may be an early manifestation of endothelial dysfunction [3]. Structural changes and ischemia that occur with aging seem to play crucial roles in the pathophysiology of ED [4].
Aging in the penis is accompanied by an induction of inducible nitric oxide synthase (NOS) and peroxynitrite formation that may lead to an increase in apoptosis and proteololysis [5]. Since relaxation elicited by peroxynitrite were more persistent but less than those due to NO, peroxynitrite was thought to contribute to the pathogenesis of ED [6]. Furthermore, attenuated neuronal NOS (nNOS) protein expression has been described in impaired neurogenic relaxation of cavernosal tissue of aged rabbits [2]. Also, altered phosphorylation/constitutive activation of endothelial NOS by fluid shear stress may be a major determinant of compromised vascular homeostasis of the aged penis [7]. It seems that the majority of alterations contributing to age-associated ED are related to a decrease in NO availability leading to impaired cavernosal relaxation.
Two toxins from Phoneutria nigriventer spider venom, PnTx2–6 and PnTx2–5, have been isolated and characterized to be involved in the erectile function in experimental animals [8–10]. These toxins are both described according to their effects on Na+ channels [11], and depend on Na+ channel type, it may interfere on smooth muscle response in penile tissue [12]. It has been suggested that penile erection induced by PnTx2–6 was associated with two genes, both involved in the relaxation of the smooth muscle in the penis [13]. Also, PnTx2–6 injection in hypertensive rats was able to reverse the severe ED observed in these animals [9]. In addition, subcutaneous administration of the radio labeled (99m technetium)-PnTx2–6 in rats showed the presence of this toxin in the penis [14], as well as in testis of mice when the toxin was labeled with iodine-125 [15]. These results suggest that PnTx2–6 preferentially localizes in penile tissue, which makes this molecule a direct target for research into the mechanisms of erectile function and reversal of ED.
It has been described that PnTx2–6 causes neuronal depolarization. This toxin slows down the inactivation period of Na+ channels [16], leading to an increase in Ca+ influx, via activation of N-type Ca+ channels which in turn activates nNOS inducing NO production and improving relaxation of vascular smooth muscle causing penile erection [17].
Aim
This study investigates if PnTx2–6, a toxin from Phoneutria nigriventer spideris able to improve erectile function impaired by aging through mechanisms involving NO release. One of the symptoms noticed in patients bitten by this spider is priapism and previous studies demonstrated that PnTx2–6 was able to improve rat cavernosal relaxation [14]. Moreover, the effect of this toxin in the penis has been linked with NO release [9], which is impaired by aging.
Methods
Animals
Wistar rats (12–14 weeks-old and 69–70 weeks-old) were used in these studies. All procedures were carried out in accordance with the Guiding Principles in the Care and Use of Animals, approved by the Georgia Health Sciences University Committee on the use of animals in research and education. The animals were housed 4 per cage on a 12-h light/dark cycle and fed a standard rat chow diet and water ad libtum.
In vivo measurements of ICP/MAP
Rats were anesthetized with ketamine/xylazine (100:10 mg/kg, i.p.) and the erectile function was evaluated by comparing the ratio of intracavernosal pressure to mean arterial pressure (ICP/MAP) as described elsewhere [9]. Frequency–response curves were performed with continuous stimulation of the major pelvic ganglion (MPG) (30 s duration for each frequency) using a crescent range (1, 2, 4, 8, 12 and 32 Hz), a pulse duration of 5ms and a voltage of 2V. The effect of PnTx2–6 (12 µg/kg s.c.) on ganglionic stimulation-induced elevation of ICP/MAP was evaluated in aged and control rats. The effect of PnTx2–6 was also evaluated in the presence of sildenafil citrate (10 µg/kg, i.v) at sub-maximal ganglionic stimulation (8Hz).
Drugs and Solutions
Physiological salt solution of the following composition was used: 130mM NaCl, 14.9 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4.7H2O, 1.6 mM CaCl2. 2H2O, and 0.026 mM EDTA. Atropine, phenylephrine, sodium nitroprusside, bretylium tosylate [(o-bromobenzyl) ethyldimethylammonium p-toluenesulfonate], sildenafil citrate was purchased from Sigma Chemical Co. (St. Louis, MO, USA). All reagents used were of analytical grade. Stock solutions were prepared in deionized water, except sildenafil that was dissolved DMSO, and stored in aliquots at −20C; dilutions were made up immediately before use. The toxin PnTx2–6 from the venom of Phoneutria nigriventer spider was purified using HPLC as described [18] and provided by the collaborators of this work from Ezequiel Dias Foundation (Brazil). Its sequence was confirmed by MALDI-TOF mass spectrometry.
Functional studies in cavernosal strips
Functional studies in rat cavernosal strips were performed as described previously by Nunes et al, 2010 [14].
Determination of cGMP levels
Rat cavernosal strips were equilibrated for 20min in oxygenated physiological salt solution (130mM NaCl, 14.9 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.18 mM KH2PO4, 1.17 mM MgSO4.7H2O, 1.6 mM CaCl2. 2H2O, and 0.026 mM EDTA) at 37 °C. Tissues were then stimulated for 10min with L-NAME (10−4M), in the presence and absence of PnTx2–6 (10−8M). Next, strips were collected immediately by freezing the segments in liquid nitrogen. Some tissues were frozen following the addition of vehicle to obtain baseline readings. Frozen cavernosal tissue were pulverized, homogenized in trichloroacetic acid (TCA; 5% w/v) and then centrifuged at 1500 g for 10min at 4°C. The TCA was extracted from samples with three washes of water-saturated ether. The weights of the dried pellets were used to standardize the different samples. cGMP was extracted and quantified using a cGMP enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI). Assays were performed in duplicate using different dilutions of samples.
NOS Activity Assay
NOS enzyme activity was determined in homogenized fractions of the young and old cavernosal tissue samples by the conversion of L-arginine to L-citrulline in the presence of PnTx2–6 (10−8M). Briefly, samples were pulverized, homogenized in buffer A (50 mM Tris-HCl, pH 7.4, EDTA (0.1 mM), EGTA (0.1 mM), and PMSF (1 mM) and then sonicated. The homogenized fraction (60 µg of protein) was incubated in buffer B containing NADPH (1 mM), calmodulin (100 nM), tetrahydrobiopterin (10 µM), and L-[3H] arginine (0.6 µCi/µl) for 30 min at 37 °C. The reaction was stopped with Hepes (50 µM: pH 7.4) and EDTA (0.1 mM). The radioactivity of the samples was measured by liquid scintillation counting. Enzyme activity of NOS is expressed as pmol L-citrulline/min/mg protein.
Statistical analyses
Results were expressed as mean±SEM. Statistical analyses were performed using Two-way analyses of variance (ANOVA) followed by Bonferroni post hoc test for multiple comparisons. Systolic blood pressure and fluorescence intensity data were analyzed by Student’s t- test for unpaired comparison. A value of p≤0.05 was considered statistically significant.
Results
PnTx2–6 potentiates aged rats erectile function after subcutaneous injection and this effect is further increased by sildenafil
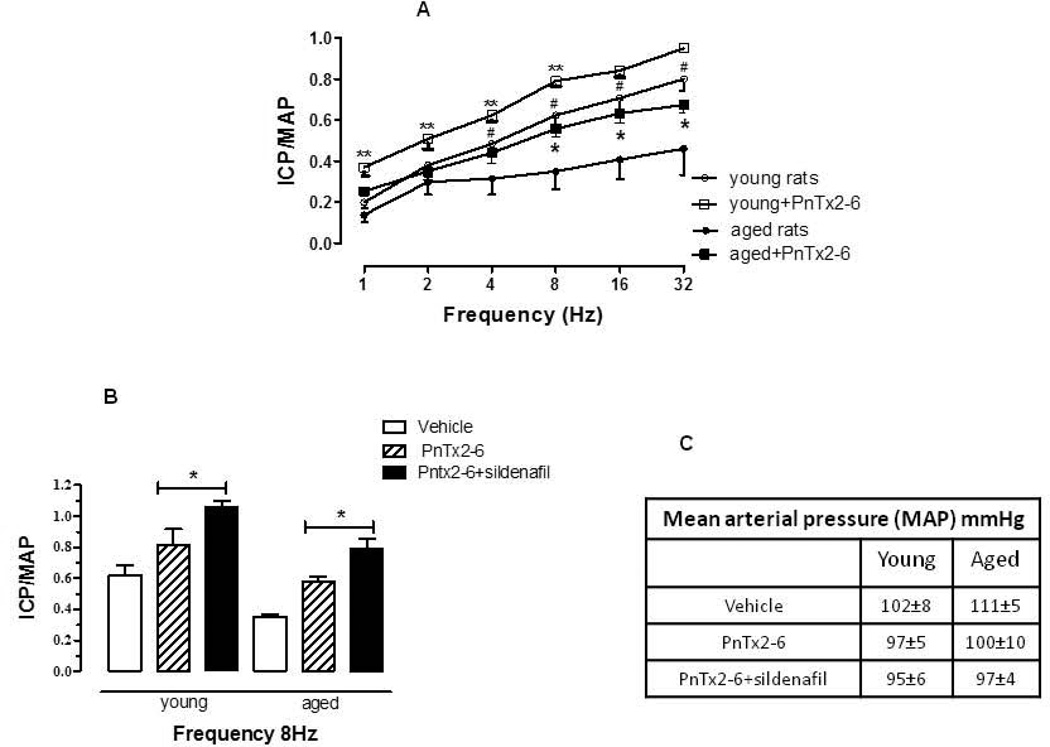
To examine the effect of PnTx2–6 on penile erectile function of aging and young control rats, voltage– response curves (1–32 Hz, 0.1 ms, 30 s each step) were performed before and 15–20 min after injection of the toxin (12 µg/kg, s.c.). The dose used has been shown to cause a significant effect in normotensive rat erectile function as described previously by our group [9]. The erectile response, represented as the ICP/MAP ratio, was significantly potentiated after subcutaneous injection (Figure 1A) demonstrating that PnTx2–6 may contribute significantly to improved age-related erectile function. Also, in another group of animals sildenafil citrate (10 µg/kg, i.v) was administrated (0.5 ml/Kg) in the presence of PnTx2–6 and sub-maximal stimulation (8Hz) was performed 15–20 min after injection. Result showed an additional increase in the erectile function of aged and control rats compared to administration of PnTx2–6 only (Figure 1B). The blood pressure is not affected by this toxin (Figure C).
Figure 1.
Erection induced by ganglionic stimulation in young control and aged rats after subcutaneous injection of PnTx2–6. Aged rats showed decreased erectile function (ICP/MAP) induced by ganglionic stimulation (1–32 Hz) compared to young rats. Subcutaneous injection of PnTx2–6 (12 µg/kg) significantly improves erectile function, in both young and aged animals (A). Administration of sildenafil (i.v.) plus toxin resulted in further increased erectile function in aged rats, at submaximal stimulation of 8Hz (B). Blood pressure is not affected neither by PnTx2–6, sildenafil or both (C).In 1A P≤0.05 (*) compared to aged+PnTx2–6 vs. aged rats, (#) compared to young vs. aged rats, (**) compared to young+PnTx2–6 vs. young rats (two-way ANOVA followed by Bonferroni test), n= 5 per group. In 1B *P≤0.05 compared treatment with PnTx2–6 in the presence in absence of sildenafil in both young and aged rats.
EFS-mediated relaxation in aged rat cavernosal tissue is improved by PnTx2–6 toxin
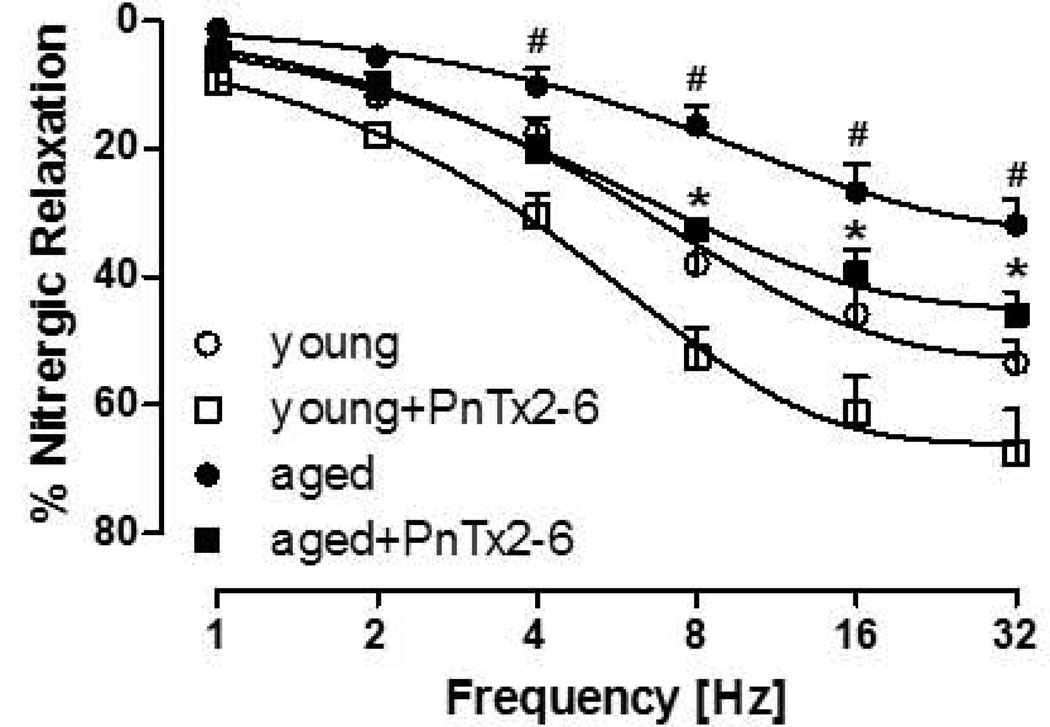
Tissues were incubated with PnTx2–6 or vehicle for 4 min, contracted to PE and a relaxation response curves to EFS was performed. As shown in Figure 2, aged rat cavernosal strips incubated with PnTx2–6 exhibited a dose-dependent increase in cavernosal relaxation, reaching significance at 8Hz to 32Hz (n=6, p<0.05), suggesting that PnTx2–6 induces an increase in NANC mediated relaxation (16.3±2 vs. 32.7±0.8 compared to PnTx2–6 treated). Relaxation of cavernosal strips from aging rats, were decreased approximately 40%at the maximum EFS compared to young animals (32%±3 vs. 54%±2 respectively). When the strips were incubated with PnTx2–6, both young and aged rats exhibited a significantly increased relaxation response (Figure 2). Interestingly, there is no statistical difference between the relaxation response curves obtained from PnTx2–6 treated aged rats and young control rats. This data suggests that PnTx2–6 can reverse dysfunctional erectile function due to aging.
Figure 2.
PnTx2–6 improves nitrergic relaxation in cavernosum strips from aged and young rats. Aged cavernosal strips showed decreased EFS-induced relaxation compared to young (#p<0.05). Incubation of PnTx2–6 (10−8M) for 4 min significantly enhanced cavernosal relaxation in both aged and young animals (n=9, *p<0.05). As observed in the figure, impaired cavernosal relaxation in aged rat strips were reversed by PnTx2–6 toxin.
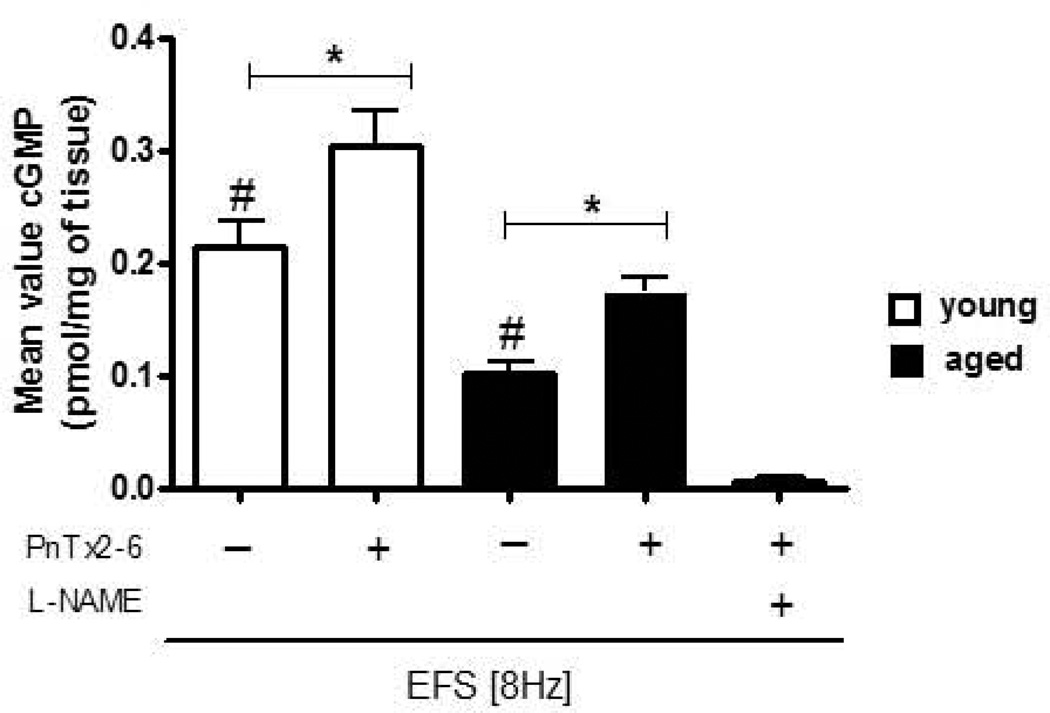
Enhanced cGMP level evoked by PhTx2–6 in aged cavernosal tissue was suppressed by L-NAME
Incubation with PnTx2–6 induced an increase in cGMP levels, as compared to basal levels, further confirming an increase in NO by PnTx2–6. EFS leads to the release of NO, and cGMP levels were evaluated in cavernosal tissue from young and aged rats under this condition (n=5, Figure 3). Cavernosal tissue incubated with L-NAME, the non-specific NOS inhibitor, and PnTx2–6 showed decreased levels of cGMP, which were lower than baseline. These data suggest that this toxin increases NO produced by NOS and corroborate with in vivo studies showing that PnTx2–6 improves the erectile function in aged rats.
Figure 3.
Increased cGMP levels by PnTx2–6 in aged cavernosal tissue were suppressed by L-NAME. Aged rats showed reduced cGMP levels in cavernosal tissue compared to young rats (# p<0.05). Cavernosal tissue from young and aged rats treated with toxin showed enhanced cGMP levels compared to non-treated. L-NAME (10−4 M) abolished cGMP levels increased by PnTx2–6 incubation. These results point to improved relaxation mediated by NO/cGMP pathway due to PnTx2–6 (n=5 per group, *p<0.05).
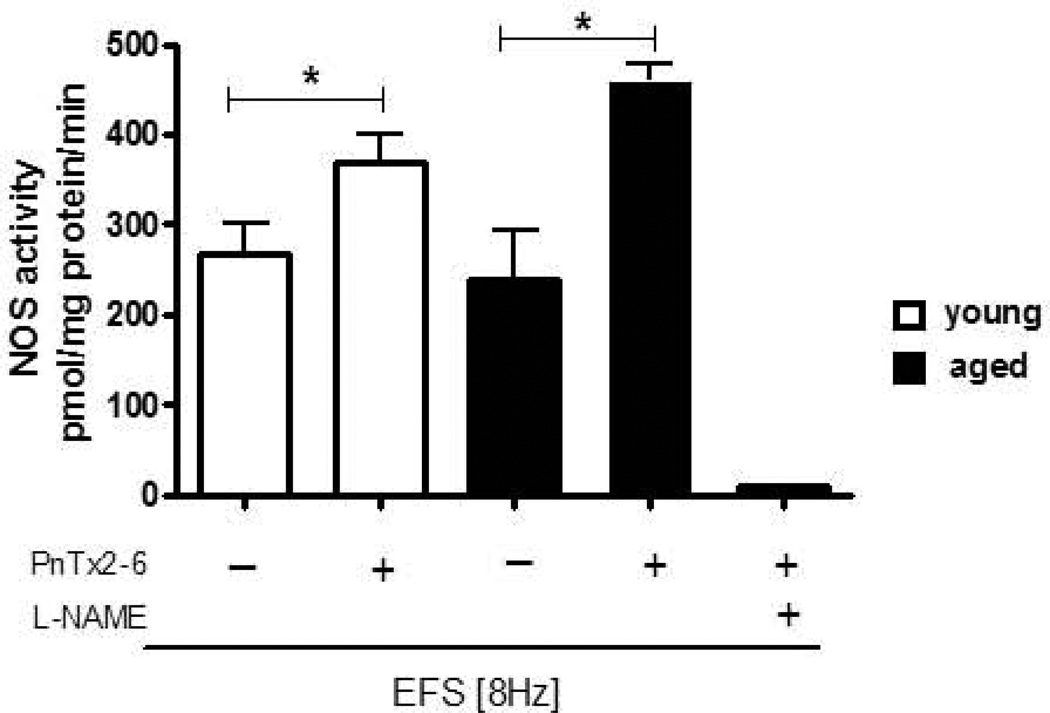
NOS activity in aged cavernosal tissue is enhanced by PnTx2–6
To investigate NOS enzyme activity in tissue from aged rats in the presence of PnTx2–6, the conversion of L-arginine to L-citrulline was determined. Pulverized and homogenized aged rat cavernosal tissue showed no difference in NOS activity compared to young. However, the NOS activity was increased after 10 minutes incubation with PnTx2–6 in cavernosal tissue from both animals, aged and young (Figure 4). In addition, we observed a greater increase in NOS activity in aged rats as compared to young. This result suggests that PnTx2–6 action in cavernosal tissue relaxation involves NO release by increase NOS activity, probably from nitrergic nerves.
Figure 4.
Augmented NOS activity by PnTx2–6 toxin in young and aged cavernosal tissue. NOS activity was determined in the absence and presence of the PnTx2–6 toxin (10−8M) in corpus cavernosum (CC) from control young and aged rats. A non-specific NOS inhibitor (L-NAME) was used as a control. Data represents the mean ± S.E.M. of 4 experiments per group. *p < 0.05; compared to young vs. aged rats.
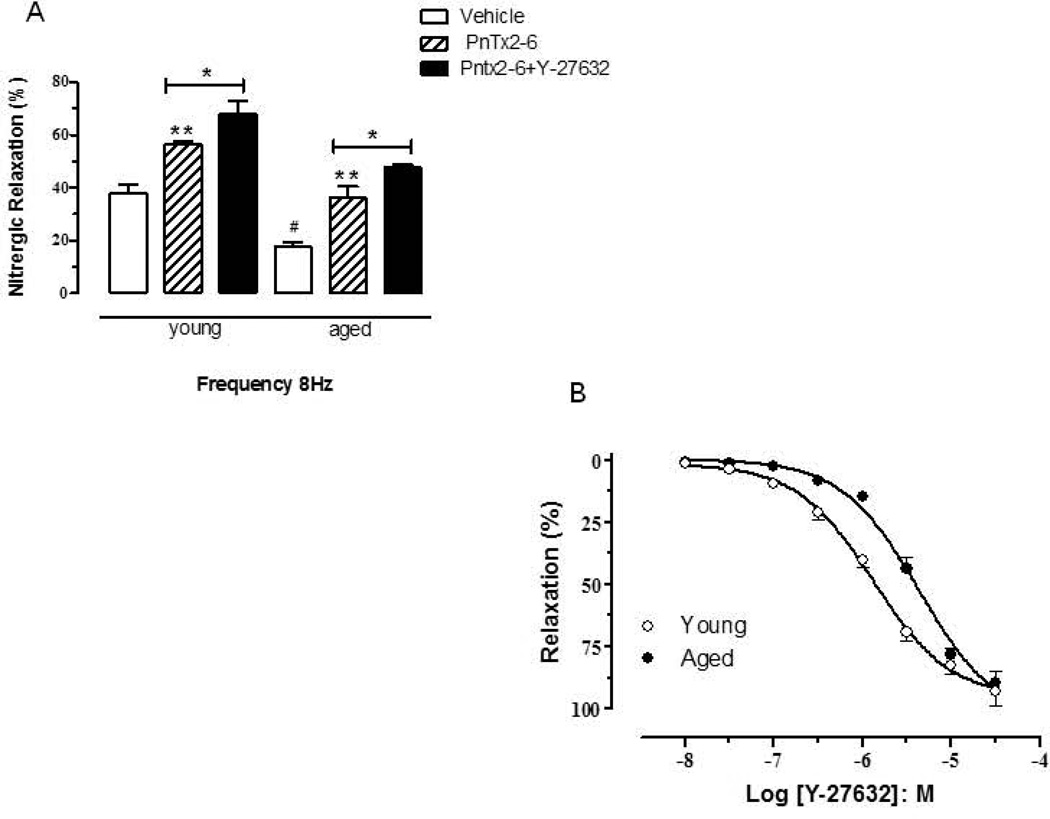
Rho-kinase inhibitor augments the relaxation effect of PnTx2–6 in aged rats
We verified if the relaxation effect evoked by PnTx2–6 could be improved in the presence of Rho-kinase inhibitor (Y-27632, 10−6M, 20 minutes incubation). Previously, a cumulative Y-27632 relaxation-response curve was performed to confirm the dose able to evoke an approximate 40% of relaxation (Figure 5A). Nitrergic relaxation at 8Hz showed a further increase in the relaxation of both strips; young and aged, incubated with Rho-kinase inhibitor, as compared to only PnTx2–6 (Figure 5B).
Figure 5.
Relaxation evoked by PnTx2–6 is enhanced by Rho-kinase inhibitor in aged and young cavernosal strips. Nitrergic relaxation is significantly decreased compared to aged vs. young rats (#). Treatment with PnTx2–6 improves nitrergic relaxation at 8Hz, in aged and young cavernosal strips compared to non-treated (**), which is further increased by incubation with Y-27632 (*), a Rho-kinase inhibitor (10−6M), as observed in figure A (n=5 per group, **, #, *p<0.05). Cumulative Rho-kinase inhibitor relaxation-response curve is showed in figure B.
Discussion
In this study we investigated the effect of PnTx2–6, a toxin isolated from P. nigriventer venom in corpus cavernosum from aged rats. Our results demonstrate that this toxin was able to improve the debilitated erectile function in aged rats (in vivo experiments). Also, in vitro experiments suggest an important role of NO release in the cavernosal relaxation evoked by PnTx2–6. Erectile function in aged rats was significantly reduced around 50% compared to young animals at 8Hz (0.35±0.01 vs. 0.62±0.07 respectively). The reduced erectile function in aged rats was normalized 15–20 minutes after PnTx2–6 injection (Figure 1A). Administration of sildenafil further increased the PnTx2–6 effect (Figure 1B) in aged rats, suggesting that PnTx2–6 action does not involve PDE-5 inhibition. PnTx2–6 toxin does not affect the arterial blood pressure in anesthetized rats (1C). Functional experiments showed impaired nitrergic relaxation in cavernosal tissue from aged rats compared to young (32%±3 vs. 54%±2 respectively, 32Hz). Incubation with PnTx2–6 was able to reverse the ED in aged rats (Figure 2). Although PnTx2–6 was able to improve the erectile function and neurogenic-mediated cavernosal relaxation in young rats, priapism has been described only when the whole venom is administered. In addition, the effect of this toxin in vivo is not longer than 1 hour.
We speculate that the mechanism of action of PnTx2–6 in these animals involves NO release [9]. To address this issue, cGMP levels and NOS activity were evaluated in aged and young animals in the presence and absence of PnTx2–6. Activation of NOS is increasingly recognized as an essential factor in the vascular homeostasis and erectile physiology of the penis. This mechanism occurs mainly by Ca +2 binding to calmodulin, leading to neurogenic NO production [19]. Data in both humans and experimental animals indicate that impaired endothelial dysfunction with aging is mediated by a decrease in NO bioavailability (25). Moreover, it has been shown that the prevalence and severity of ED increases with the advance of age [20–21], and vasculogenic ED is recognized as the major etiology which refers to the impairment of the vascular perfusion to the corpora cavernosa [3]. Aging is recognized to alter endothelial cell function, and erectile impairment with aging has been attributed to decreased NOS activity, decreased NO bioavailability, and reduced activation of protein kinase G (PKG) by cGMP, resulting in reduced endotheliaum-dependent cavernosal smooth muscle relaxation [22–23]. Treatment with PnTx2–6 toxin increases cGMP levels in both, aged and young rat cavernosal tissue, and this effect was suppressed by L-NAME (Figure 3). These results support our hypothesis that PnTx2–6 is able to improve impaired erectile function by NO/cGMP pathway in aged rats.
NOS activity is fundamental in the erectile process to generate NO leading to initiation cavernosal tissue relaxation and maintenance of full erection [24]. Our results did not show altered NOS activity in cavernosal tissue from aged compared to young rats. However, PnTx2–6 toxin increased NOS activity in both tissues, aged and young (Figure 4). There is modification in the NOS expression resulting from different changes in eNOS and nNOS expression in ED associated with ageing. It has been reported that eNOS protein expression is increased in aged rat cavernosal tissue [25], and up-regulated in aged rabbit cavernosal strips [26]. Nevertheless, unchanged NOS activity has been reported in corpus cavernosum from aged rabbits [27], and probably this can be explained by compensatory mechanisms between nNOS and eNOS expression. Also, age decreases NOS-containing nerve fibers in the rat penis [22], which can contribute to modifications in the NOS isoforms expressions. We verified if PnTx2–6 would be able to alter the NOS expression and there is no difference between PnTx2–6 treated and non-treated tissues (data not shown), suggesting that the fast action of PnTx2–6 in the cavernosal relaxation is not long enough to alter NOS protein levels. On the other hand, the penile erection induced by PnTx2–6 has been associated with two genes, both involved in the relaxation of the smooth muscle in the penis [10].
RhoA/Rho-kinase signaling pathway maintains constriction of the cavernosal arterioles and sinuses, keeping the penis in the flaccid state [28–29]. Increased RhoA/Rho-kinase activity has been causes increased vascular tone. Also, Rho-kinase mediated Ca +2 sensitizing pathway play a role in age-associated ED [30] because a Rho-kinase inhibitor, Y27632, improved erectile function in aged rats [31]. The role of Rho-kinase signaling in age-related ED has not been completely elucidated. However, increased Rho-kinase activity has been described in aged rat penile tissue [30]. This data is supported by molecular studies showing that MYPT1 phosphorylation is notable increased in aged rat penes [32]. In order to investigate if the potential effect of PnTx2–6 on erectile function would be related with Rho-kinase pathway, Y27632 was used in the presence of the toxin. Results from our experiments showed that increased nitrergic relaxation evoked by PnTx2–6 was augmented in presence of Y27632 (30 min incubation), suggesting that PnTx2–6 would be acting in a Rho-kinase- independent manner (Figure 5B). Cumulative Rho-kinase inhibitor relaxation-response curve was performed to confirm the Y-27632 dose able to evoke approximately 40 % of relaxation (Figure 5A).
In summary, our data showed that PnTx2–6 toxin improves the erectile function in aged rats, through cavernosal relaxation enhanced via NO/cGMP signaling pathway. This toxin is not able to modify the NOS protein levels in the experimental period we tested it, but it seems that it increases cGMP levels and NOS activity in both cavernosal tissues from aged and young rats. In addition inhibition of Rho-kinase in aged cavernosal tissue augments PnTx2–6-induced relaxation.
Conclusion
This study is the first to investigate the effect of PnTx2–6 in age-associated erectile dysfunction. Taken together, our results suggest this toxin as a potential pharmacological tool to investigate ED. Moreover, an association of this toxin with usual drugs for the treatment of ED might be of interest, since PnTx2–6 seems to act in different point of the NO/cGMP cascade compared to conventional treatments, such as PDE5 inhibitors.
Acknowledgments
This work was supported by grants from NIH (RO1-HL083685) and AHA in USA and grants from CAPES, CNPq, FAPEMIG and INCTTOX-FAPESP in Brazil. Dr. Nunes is supported by AHA Post-Doctoral fellowship.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Costa C, Vendeira P. Does erectile tissue angioarchitecture modify with aging? An immunohistological and morphometric approach. J Sex Med. 2008 Apr;5(4):833–840. doi: 10.1111/j.1743-6109.2007.00753.x. [DOI] [PubMed] [Google Scholar]
- 2.Toda N, Ayajiki K, Okamura T. Nitric oxide and penile erectile function. Pharmacol Ther. 2005 May;106(2):233–266. doi: 10.1016/j.pharmthera.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Aversa A, Bruzziches R, Francomano D, Natali M, Gareri P, Spera G. Endothelial dysfunction and erectile dysfunction in the aging man. Int J Urol. 2010 Jan;17(1):38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 4.Wespes E. The ageing penis. World J Urol. 2002 May;20(1):36–39. doi: 10.1007/s00345-002-0256-5. [DOI] [PubMed] [Google Scholar]
- 5.Ferrini M, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadavid NF. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod. 2001 Mar;64(3):974–982. doi: 10.1095/biolreprod64.3.974. [DOI] [PubMed] [Google Scholar]
- 6.Khan MA, Thompson CS, Mumtaz FH, Mikhailidis DP, Morgan RJ, Bruckdorfer RK, et al. The effect of nitric oxide and peroxynitrite on rabbit cavernosal smooth muscle relaxation. World J Urol. 2001 Jun;19(3):220–224. doi: 10.1007/s003450000162. [DOI] [PubMed] [Google Scholar]
- 7.Musicki B, Palese MA, Crone JK, Burnett AL. Phosphorylated endothelial nitric oxide synthase mediates vascular endothelial growth factor-induced penile erection. Biol Reprod. 2004 Feb;70(2):282–289. doi: 10.1095/biolreprod.103.021113. [DOI] [PubMed] [Google Scholar]
- 8.Yonamine CM, Troncone LR, Camillo MA. Blockade of neuronal nitric oxide synthase abolishes the toxic effects of Tx2-5, a lethal Phoneutria nigriventer spider toxin. Toxicon. 2004 Aug;44(2):169–172. doi: 10.1016/j.toxicon.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Nunes KP, Costa-Goncalves A, Lanza LF, Cortes SF, Cordeiro MN, Richardson M, et al. Tx2-6 toxin of the Phoneutria nigriventer spider potentiates rat erectile function. Toxicon. 2008 Jun 1;51(7):1197–1206. doi: 10.1016/j.toxicon.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villanova FE, Andrade E, Leal E, Andrade PM, Borra RC, Troncone LR, et al. Erection induced by Tx2-6 toxin of Phoneutria nigriventer spider: expression profile of genes in the nitric oxide pathway of penile tissue of mice. Toxicon. 2009 Nov;54(6):793–801. doi: 10.1016/j.toxicon.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Matavel A, Fleury C, Oliveira LC, Molina F, de Lima ME, Cruz JS, et al. Structure and activity analysis of two spider toxins that alter sodium channel inactivation kinetics. Biochemistry. 2009 Apr 14;48(14):3078–3088. doi: 10.1021/bi802158p. [DOI] [PubMed] [Google Scholar]
- 12.Capel RO, Monica FZ, Porto M, Barillas S, Muscara MN, Teixeira SA, et al. Role of a novel tetrodotoxin-resistant sodium channel in the nitrergic relaxation of corpus cavernosum from the South American rattlesnake Crotalus durissus terrificus. J Sex Med. 2011 Jun;8(6):1616–1625. doi: 10.1111/j.1743-6109.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 13.Andrade E, Villanova F, Borra P, Leite K, Troncone L, Cortez I, et al. Penile erection induced in vivo by a purified toxin from the Brazilian spider Phoneutria nigriventer. BJU Int. 2008 Sep;102(7):835–837. doi: 10.1111/j.1464-410X.2008.07762.x. [DOI] [PubMed] [Google Scholar]
- 14.Nunes KP, Cordeiro MN, Richardson M, Borges MN, Diniz SO, Cardoso VN, et al. Nitric oxide-induced vasorelaxation in response to PnTx2-6 toxin from Phoneutria nigriventer spider in rat cavernosal tissue. J Sex Med. 2010 Dec;7(12):3879–3888. doi: 10.1111/j.1743-6109.2010.01978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yonamine CM. Biodistribuition studies of bee venom and spider toxin using radiotracers. J Venom Animal Toxins inclu Tropical Diseases. 2005;11(1):39–50. [Google Scholar]
- 16.Matavel A, Cruz JS, Penaforte CL, Araujo DA, Kalapothakis E, Prado VF, et al. Electrophysiological characterization and molecular identification of the Phoneutria nigriventer peptide toxin PnTx2-6. FEBS Lett. 2002 Jul 17;523(1–3):219–223. doi: 10.1016/s0014-5793(02)02988-5. [DOI] [PubMed] [Google Scholar]
- 17.Nunes KP, Wynne BM, Cordeiro MN, Borges MH, Richardson M, Leite R, et al. Increased cavernosal relaxation by Phoneutria nigriventer toxin, PnTx2-6, via activation at NO/cGMP signaling. Int J Impot Res. 2011 Oct 6; doi: 10.1038/ijir.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cordeiro MN. The purification and amino acid sequences of four Tx2 neurotoxins from the venom of the Brazilian "armed" spider Phoneutria nigriventer (keys) FEBS Lett. 1995;32(28):153–156. doi: 10.1016/0014-5793(92)81318-g. [DOI] [PubMed] [Google Scholar]
- 19.Okamura T, Fujioka H, Ayajiki K. Effects of calcium antagonists on the nitrergic nerve function in canine corpus cavernosum. Jpn J Pharmacol. 2001 Nov;87(3):208–213. doi: 10.1254/jjp.87.208. [DOI] [PubMed] [Google Scholar]
- 20.Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, Moreira ED, Jr, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010 Apr;7(4 Pt 2):1598–1607. doi: 10.1111/j.1743-6109.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 21.Lewis RW, Fugl-Meyer KS, Bosch R, Fugl-Meyer AR, Laumann EO, Lizza E, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004 Jul;1(1):35–39. doi: 10.1111/j.1743-6109.2004.10106.x. [DOI] [PubMed] [Google Scholar]
- 22.Carrier S, Nagaraju P, Morgan DM, Baba K, Nunes L, Lue TF. Age decreases nitric oxide synthase-containing nerve fibers in the rat penis. J Urol. 1997 Mar;157(3):1088–1092. [PubMed] [Google Scholar]
- 23.Lin CS, Liu X, Chow S, Lue TF. Cyclic AMP and cyclic GMP activate protein kinase G in cavernosal smooth muscle cells: old age is a negative factor. BJU Int. 2002 Apr;89(6):576–582. doi: 10.1046/j.1464-410x.2002.02643.x. [DOI] [PubMed] [Google Scholar]
- 24.Burnett AL. Novel nitric oxide signaling mechanisms regulate the erectile response. Int J Impot Res. 2004 Jun;16(Suppl 1):S15–S19. doi: 10.1038/sj.ijir.3901209. [DOI] [PubMed] [Google Scholar]
- 25.Bakircioglu ME, Sievert KD, Nunes L, Lau A, Lin CS, Lue TF. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001 Aug;166(2):734–738. [PubMed] [Google Scholar]
- 26.Haas CA, Seftel AD, Razmjouei K, Ganz MB, Hampel N, Ferguson K. Erectile dysfunction in aging: upregulation of endothelial nitric oxide synthase. Urology. 1998 Mar;51(3):516–522. doi: 10.1016/s0090-4295(97)00715-2. [DOI] [PubMed] [Google Scholar]
- 27.Numao N, Masuda H, Sakai Y, Okada Y, Kihara K, Azuma H. Roles of attenuated neuronal nitric-oxide synthase protein expression and accelerated arginase activity in impairing neurogenic relaxation of corpus cavernosum in aged rabbits. BJU Int. 2007 Jun;99(6):1495–1499. doi: 10.1111/j.1464-410X.2007.06860.x. [DOI] [PubMed] [Google Scholar]
- 28.Chitaley K, Wingard CJ, Clinton Webb R, Branam H, Stopper VS, Lewis RW, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001 Jan;7(1):119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 29.Waldkirch ES, Uckert S, Sohn M, Kuczyk MA, Hedlund P. Rho Kinase (ROK)-Related Proteins in Human Cavernous Arteries: An Immunohistochemical and Functional Approach. J Sex Med. 2012 Feb 29; doi: 10.1111/j.1743-6109.2012.02662.x. [DOI] [PubMed] [Google Scholar]
- 30.Jin L, Liu T, Lagoda GA, Champion HC, Bivalacqua TJ, Burnett AL. Elevated RhoA/Rho-kinase activity in the aged rat penis: mechanism for age-associated erectile dysfunction. FASEB J. 2006 Mar;20(3):536–538. doi: 10.1096/fj.05-4232fje. [DOI] [PubMed] [Google Scholar]
- 31.Rajasekaran M, White S, Baquir A, Wilkes N. Rho-kinase inhibition improves erectile function in aging male Brown-Norway rats. J Androl. 2005 Mar-Apr;26(2):182–188. doi: 10.1002/j.1939-4640.2005.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 32.Jin L, Burnett AL. RhoA/Rho-kinase in erectile tissue: mechanisms of disease and therapeutic insights. Clin Sci (Lond) 2006 Feb;110(2):153–165. doi: 10.1042/CS20050255. [DOI] [PubMed] [Google Scholar]