Abstract
Purpose
To investigate both volume and length of the three muscle compartments of the normal and the affected leg in unilateral congenital clubfoot.
Methods
Volumetric magnetic resonance imaging (VMRI) of the anterior, lateral and postero-medial muscular compartments of both the normal and the clubfoot leg was obtained in three groups of seven patients each, whose mean age was, respectively, 4.8 months, 11.1 months and 4.7 years. At diagnosis, all the unilateral congenital clubfeet had a Pirani score ranging from 4.5 to 5.5 points, and all of them had been treated according to a strict Ponseti protocol. All the feet had percutaneous lengthening of the Achilles tendon.
Results
A mean difference in both volume and length was found between the three muscular compartments of the leg, with the muscles of the clubfoot side being thinner and shorter than those of the normal side. The distal tendon of the tibialis anterior, peroneus longus and triceps surae (Achilles tendon) were longer than normal on the clubfoot side.
Conclusions
Our study shows that the three muscle compartments of the clubfoot leg are thinner and shorter than normal in the patients of the three groups. The difference in the musculature volume of the postero-medial compartment between the normal and the affected side increased nine-fold from age group 2 to 3, while the difference in length increased by 20 %, thus, showing that the muscles of the postero-medial compartment tend to grow in both thickness and length much less than the muscles of the other leg compartments.
Keywords: Congenital clubfoot, Muscle atrophy, Volumetric MRI muscle study, Muscle growth, Pathogenesis of congenital clubfoot
Introduction
In a previous study [1], we showed that both foetuses and untreated newborns with unilateral congenital clubfoot (UCCF) have leg muscle atrophy on the clubfoot side. Muscle atrophy is primitive, since it is already present in the early stages of foetal development of congenital clubfoot and affected newborns before starting treatment [2–8]. We measured the area of leg muscles on histologic cross-sections of foetuses and on magnetic resonance imaging (MRI) cross-scans of babies, children and adults taken midway between the joint line of the knee and the ankle, using special computer software. Surface measurement data were obtained with that method; however, no information was given on the overall volume and length of the leg muscles within their respective compartments. Thanks to a volumetric MRI program in use at the Department of Radiology of our hospital, we were able to calculate the overall volume of the leg muscles and their length within the three leg compartments. The length of the distal tendon of the tibialis anterior and peroneus longus, as well as of the Achilles tendon, was also measured.
Materials and methods
Volumetric magnetic resonance imaging (VMRI) of the three leg muscle compartments was obtained in a cohort of 21 patients with treated UCCF. All the patients had been treated according to the Ponseti method [9], i.e. weekly manipulation and long-leg plaster cast application (a total of 4–5 casts), which started within 2 weeks after birth, followed by percutaneous Achilles tendon tenotomy at 6–8 weeks of age. Another plaster cast was applied after tenotomy, for 3 weeks. All the patients wore a Mitchell–Ponseti brace up to 3 years of age. All the patients had a Pirani score [10] ranging from 4.5 to 5.5 points. At the time of the MRI evaluation, all the feet were fully corrected. We divided the patients into three groups. Group 1 included seven patients ranging in age from 4 to 6 months; group 2, seven patients from 10 to 12 months of age; and group 3, seven patients from 4 to 6 years of age. Group 1 and 2 patients were still wearing a Mitchell–Ponseti brace at the time of the VMRI examination, while group 3 patients had already stopped wearing the brace when VMRI was performed. Group 1 and 2 patients were not ambulating, whereas group 3 patients were all ambulating at the time of the VMRI examination. No patient had had recurrence of the deformity. In no patient was the range of motion of the ankle evaluated.
The VMRI examination lasted an average of 15 min, and it was performed in all the children under general anaesthesia, which was induced and maintained with sevoflurane 2–6 %. Premedication was done with midazolam p.o. at the dose of 0.05 mg/kg body weight. The examination was performed using a 1.5 T (Achieva, Philips Healthcare, Best, The Netherlands) MRI apparatus, equipped with an 8-channel reception dedicated coil. Patients were examined in the supine position with both legs positioned inside the coil, in a symmetric position with a strap holding the feet together and with the ankle in about 20° of plantar flexion. MRI images were acquired on axial planes. The MRI protocol consisted of the following sequences: T1 3D Wats-FFE (TSE) (TR/TE 25.00/4.63 ms; thickness, 3 mm, gap 0; matrix, 512 × 512). The MRI images were analysed using the software Carestream Solutions (version 11.0-med_im, Carestream Health, Rochester, NY, USA). Statistical analyses of the MRI images were performed using Stata software (version 11.0, StataCorp, College Station, TX, USA).
In each leg compartment of both the normal and the affected side, both muscular volume and length were measured. The length of the distal tendon of the tibialis anterior and peroneus longus, as well as of the Achilles tendon, was also measured. Each muscle compartment was evaluated and measured between the proximal muscle–tendon junction, where the borderline between the muscles and the brief tendons was clear-cut, and the distal muscle–tendon junction was midway between the beginning of the tendon body within the muscle belly and the end of the longest muscle belly. The length of the tibialis anterior and peroneus longus was measured between the beginning of the tendon body and the top of the talar dome, while the Achilles tendon was measured between the beginning of the tendon body and the distal calcaneal insertion. The MRI volumetric definition was not so accurate as to identify every single muscle–tendon unit within each leg compartment.
The investigation protocol was submitted to the ethical committee of our hospital. The protocol was approved, based on two comments and two conditions: (1) the study may provide new data to improve knowledge of the unknown pathogenesis of the deformity; (2) the chief anaesthesiologist of our hospital, who is the president of the ethical committee, stated that the MRI sedation protocol was absolutely safe for our patients; (3) all the children had to be carefully evaluated by a paediatrician, a paediatric cardiologist and an anaesthesiologist before the MRI examination; (4) informed consent had to be given by the parents after they had received a detailed description of the research protocol. Parents had to be aware that MRI was not mandatory for treatment, but it was very important for understanding the pathogenesis of congenital clubfoot.
The mean differences in volume (cm3) or length (cm) between normal and clubfoot legs were weighted up by dividing each difference by the volume (or length) of the normal leg. A positive mean difference indicates that the volume (length) of the normal leg is larger (longer) than that of the clubfoot leg.
Results
The muscle compartments were easily identified owing to their intermediate signal. Different colours were assigned to the three muscle compartments in order to better define them during the volumetric reconstructions: violet to the postero-medial, sky blue to the anterior and green to the lateral. Because of the VMRI’s resolving power, it was not possible to distinguish the single muscle–tendon units within the three compartments. Tendons were identified by their highly hypointense signal in T2. Peripheral nerves and blood vessels were not investigated.
The mean volume of each muscle compartment, the mean total volume of the three compartments and their difference between the normal and the clubfoot leg are given for each group of patients in Table 1. In each of the three groups, there was a considerable variation between the minimum and the maximum volume of each muscle compartment, as well as of the total volume of the leg muscles in relation to the age, height and weight of each single patient in both the normal and the affected leg. All the muscle compartments were thinner on the clubfoot side and the mean difference in volume between the anterior, the lateral and the postero-medial compartments of both the normal and the affected leg is reported in Table 1. The difference was more marked for the muscles of the postero-medial compartment, with the exception of group 2 patients (Fig. 1).
Table 1.
The negative mean difference value shows a decrease in volume of the leg muscular compartments of the clubfoot side compared to the non-affected side in the three groups of patients
| Patient group | Leg muscular compartment | Non-affected leg muscular compartment mean volume (cm3 ± SD) | Clubfoot leg muscular compartment mean volume (cm3 ± SD) | Mean difference between non-affected and clubfoot leg muscular compartment mean volumes (% ± SD) |
|---|---|---|---|---|
| Group 1 (age 4–6 months) | Anterior | 12.2 ± 3.1 | 7.3 ± 1.0 | −39.5 ± 5.8 |
| Lateral | 8.3 ± 2.4 | 5.1 ± 1.7 | −39.0 ± 6.4 | |
| Postero-medial | 28.1 ± 12.7 | 18.1 ± 9.0 | −36.4 ± 2.5 | |
| Total | 48.3 ± 17.4 | 30.4 ± 11.2 | −37.0 ± 3.0 | |
| Group 2 (age 10–12 months) | Anterior | 29.5 ± 8.6 | 21.9 ± 5.2 | −24.0 ± 8.3 |
| Lateral | 18.8 ± 5.6 | 14.2 ± 4.0 | −23.4 ± 6.1 | |
| Postero-medial | 37.2 ± 5.6 | 31.1 ± 5.5 | −16.9 ± 2.7 | |
| Total | 85.2 ± 19.2 | 67.2 ± 14.5 | −20.8 ± 3.2 | |
| Group 3 (age 4–6 years) | Anterior | 60.0 ± 19.0 | 54.1 ± 18.3 | −10.4 ± 5.20 |
| Lateral | 32.7 ± 7.4 | 29.8 ± 7.6 | −9.2 ± 2.7 | |
| Postero-medial | 208.5 ± 118.7 | 112.7 ± 66.8 | −46.4 ± 1.2 | |
| Total | 301.1 ± 137.1 | 196.2 ± 86.6 | −34.6 ± 2.1 |
SD standard deviation
Fig. 1.
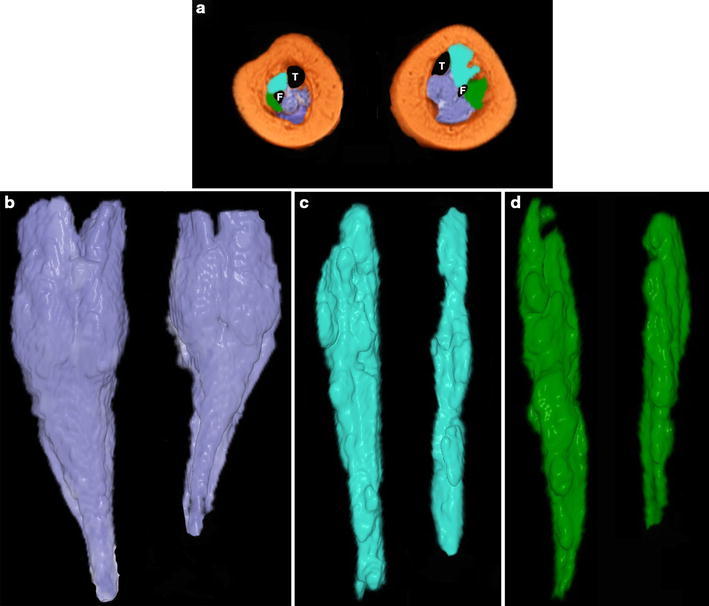
a Magnetic resonance imaging (MRI) cross-section taken at the middle third of the legs in a 4-month-old patient with unilateral congenital right clubfoot (the plantar aspect of the foot faces the reader). The left leg is normal. The postero-medial muscle compartment is coloured in violet, the anterior in sky-blue and the lateral in green. T tibia, F fibula. b The postero-medial compartment is thinner and shorter on the clubfoot side than on the normal side, and the thinnest part starts below the lower end of the gastrocnemius. Both the anterior c and the lateral d muscle compartments are also thinner and shorter than normal on the clubfoot side
All the muscle compartments were shorter on the clubfoot side. Table 2 reports the mean length of each muscle compartment, as well as the mean length of the distal tendon of the tibialis anterior, the peroneus longus and the Achilles tendon. The difference between the normal and the affected side for each group of patients is also reported (Fig. 1).
Table 2.
The tibialis anterior, peroneus longus and triceps surae tendons were measured as representative of the corresponding muscular compartment
| Patient group | Leg muscular compartments: distal tendon of the tibialis anterior, peroneus longus, triceps surae | Non-affected leg muscular compartment and tendon mean length (cm ± SD) | Clubfoot leg muscular compartment and tendon mean length (cm ± SD) | Mean difference between non-affected and clubfoot muscular compartment and tendon mean lengths (% ± SD) |
|---|---|---|---|---|
| Group 1 (age 4–6 months) | Anterior muscular compartment Tibialis anterior tendon |
Muscles: 6.9 ± 0.4 Tendon: 2.2 ± 0.4 |
Muscles: 5.7 ± 0.3 Tendon: 3.3 ± 0.6 |
Muscles: −17.8 ± 4.0 Tendon: 48.2 ± 12.3 |
| Lateral muscular compartment Peroneus longus tendon |
Muscles: 6.7 ± 0.5 Tendon: 3.4 ± 0.4 |
Muscles: 5.2 ± 0.7 Tendon: 4.6 ± 0.5 |
Muscles: −22.7 ± 10.5 Tendon: 36.4 ± 3.4 |
|
| Postero-medial muscular compartment Triceps surae tendon |
Muscles: 6.8 ± 0.7 Tendon: 4.5 ± 0.9 |
Muscles: 5.7 ± 0.3 Tendon: 5.0 ± 1.0 |
Muscles: −16.0 ± 5.8 Tendon: 12.9 ± 6.4 |
|
| Group 2 (age 10–12 months) | Anterior muscular compartment Tibialis anterior tendon |
Muscles: 8.2 ± 0.4 Tendon: 2.8 ± 0.4 |
Muscles: 7.0 ± 0.8 Tendon: 4.2 ± 1.0 |
Muscles: −13.8 ± 10.1 Tendon: 44.5 ± 10.7 |
| Lateral muscular compartment Peroneus longus tendon |
Muscles: 8.2 ± 1.0 Tendon: 3.3 ± 1.1 |
Muscles: 7.0 ± 1.3 Tendon: 4.6 ± 1.5 |
Muscles: −15.0 ± 7.2 Tendon: 40.7 ± 5.7 |
|
| Postero-medial muscular compartment Triceps surae tendon |
Muscles: 11.3 ± 1.6 Tendon: 3.6 ± 0.4 |
Muscles: 10.6 ± 1.5 Tendon: 4.3 ± 0.4 |
Muscles: −6.0 ± 1.2 Tendon: 19.9 ± 2.9 |
|
| Group 3 (age 4–6 years) | Anterior muscular compartment Tibialis anterior tendon |
Muscles: 14.2 ± 0.5 Tendon: 3.5 ± 0.4 |
Muscles: 11.3 ± 0.4 Tendon: 6.5 ± 0.4 |
Muscles: −20.6 ± 1.0 Tendon: 83.3 ± 9.2 |
| Lateral muscular compartment Peroneus longus tendon |
Muscles: 9.4 ± 0.4 Tendon: 7.2 ± 0.4 |
Muscles: 9.2 ± 0.4 Tendon: 7.4 ± 0.3 |
Muscles: −2.1 ± 0.6 Tendon: 3.0 ± 1.3 |
|
| Postero-medial muscular compartment Triceps surae tendon |
Muscles: 14.6 ± 0.4 Tendon: 6.9 ± 0.5 |
Muscles: 12.1 ± 0.4 Tendon: 8.7 ± 0.4 |
Muscles: −17.7 ± 0.9 Tendon: 26.1 ± 2.4 |
The negative mean difference values show a decrease in muscle length on the clubfoot side compared to the non-affected side. The positive mean difference values show an increase in tendon length on the clubfoot side compared to the non-affected side
SD standard deviation
The distal tendon of the tibialis anterior, peroneus longus and triceps surae (Achilles tendon) were longer than normal on the clubfoot side. The mean difference in the tendon length between the anterior, the lateral and the postero-medial compartments of both the normal and the affected side is reported in Table 2.
Discussion
In a population of UCCF ranging in age from 4 months to 6 years, we showed, by VMRI, that the three muscle compartments of the leg were smaller and shorter than normal on the clubfoot side, whereas their corresponding distal tendons were longer than normal. We divided our patients into three groups so as to have data on patients who had had plaster cast correction and percutaneous tenotomy just recently, as in group 1, or several months earlier, as in group 2, with both groups of patients still wearing the brace and not ambulating. On the other hand, the children of the third group had already discontinued any kind of immobilisation 1–2 years previously, and their leg muscles had been exposed to full motion and weight-bearing.
In a previous histologic and MRI study on UCCF [1], we measured the leg muscle surface on either histologic cross-sections of foetus legs or MRI cross-scans of the legs of untreated newborns and treated children and adults. We showed that leg muscle atrophy is a primitive defect in congenital clubfoot rather than a consequence of treatment, in agreement with previous pathologic observations in foetuses and stillborns with congenital clubfoot [2–8]. We also showed that leg muscle atrophy increases with the patient’s age, and, therefore, a mechanism of muscle growth impairment was suggested as a possible pathogenetic factor of congenital clubfoot [1].
This study has several limitations. The three groups of patients were different from each other, rather than being one group of patients followed prospectively from 4 months to 6 years of age. Percutaneous tenotomy of the Achilles tendon altered its original length in relation to the muscle belly; manipulation, plaster cast immobilisation and bracing might also have negatively influenced muscle trophism. Moreover, the VMRI program does not yet allow clearly distinguishing single muscle units within each leg compartment, with the sole exception of the triceps surae, which is superficial and separated from the other muscles of the postero-medial compartment by a thick aponeurotic septum.
In each group of patients, there was a significant difference between the average volume of the three muscle compartments of the normal and the clubfoot leg, and the difference tended to increase with the age of the patients and the time from the end of treatment. All had had 4–5 weeks of immobilisation in plaster cast, percutaneous tenotomy and 3 weeks of further immobilisation in plaster cast prior to switching to a full-time Mitchell–Ponseti brace. The significant difference observed in muscle volume among the patients of each age group is due to age, weight and height differences. This finding is not surprising if we consider the rapid growth of the body in the first several years of life that may explain the variation in weight and height from patient to patient within a few months of one another agewise. Although we may assume that immobilisation might have somehow influenced the amount of atrophy of the clubfoot leg, we have to consider that that difference was also present in untreated newborns [1] and that, 1–3 years after the discontinuation of any treatment, the difference between the normal and the affected leg was further increased instead of being partially or fully resolved, as might be expected after exposure to full motion and weight-bearing.
The difference in volume between the normal and the clubfoot side in the anterior and lateral compartments, although significant, tended to be stable until 6 years of age, whereas in the postero-medial compartment, it increased almost nine-fold from group 2 (10–12 months of age) to group 3 (4–6 years of age). This finding may explain why clubfoot leg atrophy becomes clinically apparent in children of that age, with the postero-medial compartment contributing maximally to the overall leg atrophy. Moreover, up to 6 years of age, atrophy of both the anterior and the lateral compartments did not increase, with the exception of the second group of patients, in which the difference in volume and length of the anterior compartment was greater than the same difference between the muscles of the postero-medial compartment. Since we may assume that an increase in muscular volume and length is an expression of muscle growth in this context of patients, we could speculate that muscles of the postero-medial compartment tend to grow less than the muscles of the other compartments.
By measuring muscle–tendon length, we found that the triceps surae was shorter than normal, whereas the Achilles tendon was consistently longer than normal on the clubfoot side. This observation corresponds to the macroscopic pathological finding of the authors, who showed that the muscle–tendon ratio was constantly decreased in both the triceps surae and tibialis posterior tendon in dissected foetuses and stillborns [2, 6–8]. The mechanisms which regulate the growth ratio between muscle belly and adjacent tendons are not very clearly known. On the basis of our findings and of previous pathological studies, we may assume that, whenever muscular length growth is decreased, there is a relative increase in growth of its distal tendon, as typically shown for the triceps surae and the Achilles tendon. However, only rarely does tendon overgrowth compensation allow obtaining a normal length of the whole muscle–tendon unit. For that reason, surgical lengthening procedures, mainly of the triceps surae, are required in order to correct congenital clubfoot deformity. In group 1 patients, percutaneous tenotomy might have contributed to the relative overlengthening of the Achilles tendon, but its length kept increasing in group 2 and reached its maximum in group 3, several years after percutaneous tenotomy. Moreover, this muscle–tendon length discrepancy was already described in foetuses and stillborns with clubfoot before any treatment had been done [2, 3, 6–8].
In conclusion, our study shows that, in UCCF, all three muscle compartments of the affected leg are thinner and shorter than those of the normal leg. The difference between the normal and the clubfoot leg compartments is more or less constant until 4–6 years of age, with the exception of the postero-medial muscular compartment, in which the difference rises to nine-fold in group 3 patients in comparison to groups 1 and 2, showing a marked impairment of muscle growth in that age range. This finding might explain the tendency to relapse of congenital clubfoot during this period of time, regardless of the conservative and surgical procedures performed to either prevent or manage recurrence of the deformity [11].
Very few studies have been done so far on the mechanisms regulating muscle–tendon unit growth [12–15]. An increase in muscular thickness and length expresses the amount of muscle growth [12–14]. In normal conditions, physical activity stimulates radial muscle growth, whereas stretching stimulates longitudinal muscle growth, as in limb-lengthening procedures [11, 13–16]. Growth factors regulate both processes by promoting the formation of new sarcomeres by satellite cells present in the myotendinous junction [12, 16]. Further studies are in process in our university hospital to investigate muscle growth mechanisms in congenital clubfoot.
Conflict of interest
None.
References
- 1.Ippolito E, De Maio F, Mancini F, Bellini D, Orefice A. Leg muscle atrophy in idiopathic congenital clubfoot: is it primitive or acquired? J Child Orthop. 2009;3:171–178. doi: 10.1007/s11832-009-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechtol CO, Mossman HW. Clubfoot; an embryological study of associated muscle abnormalities. J Bone Joint Surg Am. 1950;32:827–838. [PubMed] [Google Scholar]
- 3.Flinchum D. Pathological anatomy in talipes equinovarus. J Bone Joint Surg Am. 1953;35-A:111–114. [PubMed] [Google Scholar]
- 4.Ippolito E, Ponseti IV. Congenital club foot in the human fetus. A histological study. J Bone Joint Surg Am. 1980;62:8–22. [PubMed] [Google Scholar]
- 5.Irani RN, Sherman MS. The pathological anatomy of clubfoot. J Bone Joint Surg Am. 1963;45:45–52. [Google Scholar]
- 6.Schlicht D. The pathological anatomy of talipes equino-varus. Aust N Z J Surg. 1963;33:1–11. doi: 10.1111/j.1445-2197.1963.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 7.Waisbrod H. Congenital club foot. An anatomical study. J Bone Joint Surg Br. 1973;55:796–801. [PubMed] [Google Scholar]
- 8.Wiley AM. Club foot: an anatomical and experimental study of muscle growth. J Bone Joint Surg Br. 1959;41:821–835. [Google Scholar]
- 9.Ponseti IV. Treatment of congenital club foot. J Bone Joint Surg Am. 1992;74:448–454. [PubMed] [Google Scholar]
- 10.Pirani S, Outerbridge HK, Sawatzky B, Stothers K (1999) A reliable method of clinically evaluating a virgin clubfoot evaluation. In: Proceedings of the 21st SICOT World Congress, Sydney, Australia, April 1999
- 11.Masrouha KZ, Morcuende JA. Relapse after tibialis anterior tendon transfer in idiopathic clubfoot treated by the Ponseti method. J Pediatr Orthop. 2012;32(1):81–84. doi: 10.1097/BPO.0b013e31823db19d. [DOI] [PubMed] [Google Scholar]
- 12.Caiozzo VJ, Utkan A, Chou R, Khalafi A, Chandra H, Baker M, Rourke B, Adams G, Baldwin K, Green S. Effects of distraction on muscle length: mechanisms involved in sarcomerogenesis. Clin Orthop Relat Res. 2002;403(Suppl):S133–S145. doi: 10.1097/00003086-200210001-00016. [DOI] [PubMed] [Google Scholar]
- 13.Goldspink G, Williams P, Simpson H. Gene expression in response to muscle stretch. Clin Orthop Relat Res. 2002;403(Suppl):S146–S152. doi: 10.1097/00003086-200210001-00017. [DOI] [PubMed] [Google Scholar]
- 14.Caiozzo VJ, Green S. Breakout session 3: issues related to muscle growth, atrophy, and tissue engineering. Clin Orthop Relat Res. 2002;403(Suppl):S252–S261. doi: 10.1097/00003086-200210001-00029. [DOI] [PubMed] [Google Scholar]
- 15.Snow CJ, Goody M, Kelly MW, Oster EC, Jones R, Khalil A, Henry CA. Time-lapse analysis and mathematical characterization elucidate novel mechanisms underlying muscle morphogenesis. PLoS Genet. 2008;4(10):e1000219. doi: 10.1371/journal.pgen.1000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shisha T, Kiss S, Pap K, Simpson H, Szöke G. Relative ability of young and mature muscles to respond to limb lengthening. J Bone Joint Surg Br. 2006;88(12):1666–1669. doi: 10.1302/0301-620X.88B12.17850. [DOI] [PubMed] [Google Scholar]


