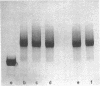
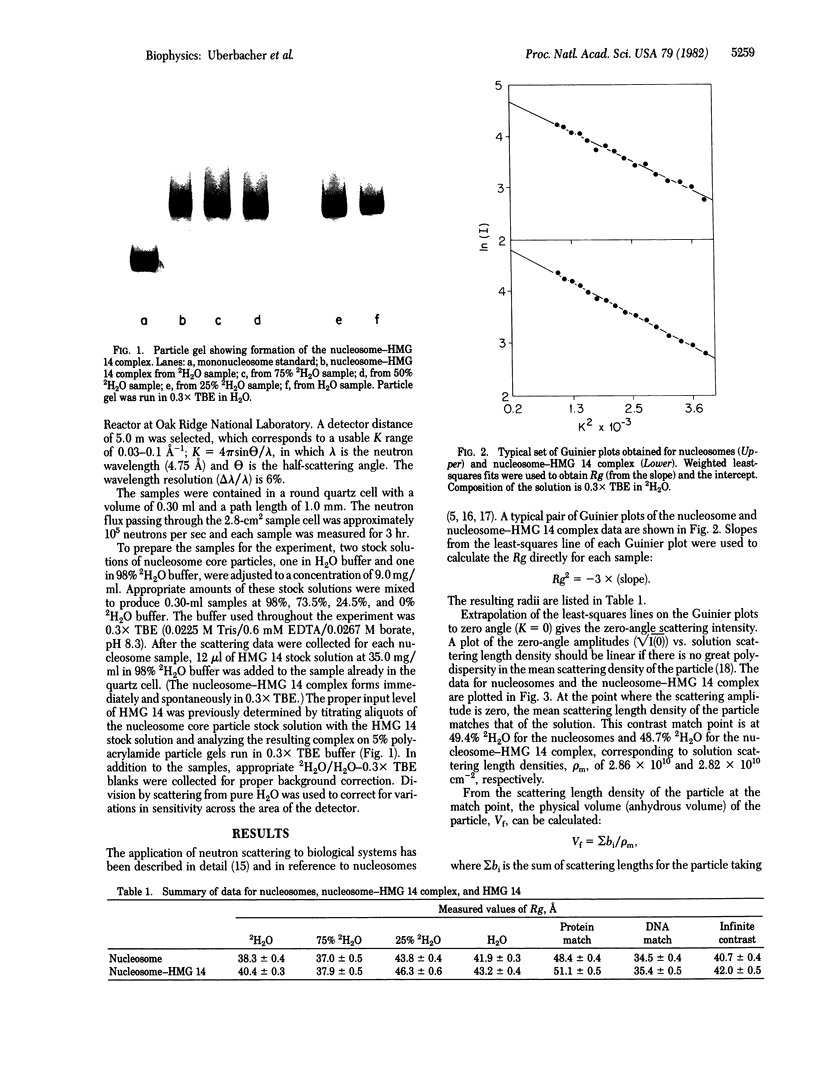
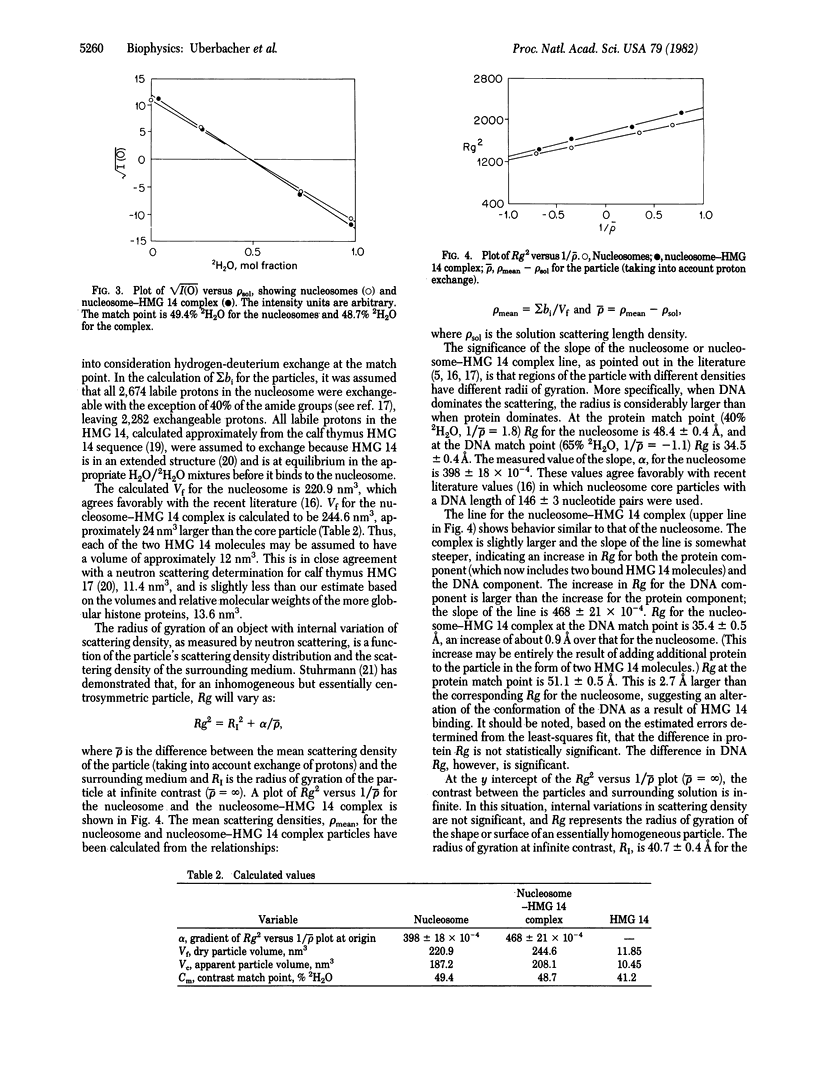
Abstract
Considerable evidence relates the nonhistone proteins high mobility group (HMG) 14 and HMG 17 with the structure of active or potentially active chromatin. In this study, bulk nucleosome core particles prepared from chicken erythrocytes and the complex formed by binding two HMG 14 molecules per nucleosome core were studied by use of small-angle neutron scattering techniques. By varying the H2O/2H2O ratio, and hence the contrast between the solvent and the particles, it was possible to determine the radius of gyration of the protein and of the DNA independently and as a function of HMG 14 binding. The results show an increase of 0.9 +/- 0.6 A (mean +/- SEM) in the protein radius of gyration and of 2.7 +/- 0.6 A in the DNA radius of gyration upon binding of HMG 14 to the nucleosome. These changes are considered in the light of several postulated modes for the unfolding or perturbation of the nucleosome structure. Modeling calculations demonstrate that the observed changes in radius of gyration for the DNA and for the protein are too small to be consistent with an overall unfolding or opening of the core particle upon HMG 14 binding. However, the observed changes are consistent with several models that involve only minor changes in the structure. It is postulated that the differences observed may be an indication of the type of conformational change occurring in active nucleosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie B. D., Kneale G. G., Crane-Robinson C., Bradbury E. M., Goodwin G. H., Walker J. M., Johns E. W. Studies on the conformational properties of the high-mobility-group chromosomal protein HMG 17 and its interaction with DNA. Eur J Biochem. 1978 Mar;84(1):173–177. doi: 10.1111/j.1432-1033.1978.tb12154.x. [DOI] [PubMed] [Google Scholar]
- Albanese I., Weintraub H. Electrophoretic separation of a class of nucleosomes enriched in HMG 14 and 17 and actively transcribed globin genes. Nucleic Acids Res. 1980 Jun 25;8(12):2787–2805. doi: 10.1093/nar/8.12.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley G. A., Finch J. T., Lewit-Bentley A. Neutron diffraction studies on crystals of nucleosome cores using contrast variation. J Mol Biol. 1981 Feb 5;145(4):771–784. doi: 10.1016/0022-2836(81)90314-4. [DOI] [PubMed] [Google Scholar]
- Braddock G. W., Baldwin J. P., Bradbury E. M. Neutron-scattering studies of the structure of chromatin core particles in solution. Biopolymers. 1981 Feb;20(2):327–343. doi: 10.1002/bip.1981.360200206. [DOI] [PubMed] [Google Scholar]
- Cary P. D., Moss T., Bradbury E. M. High-resolution proton-magnetic-resonance studies of chromatin core particles. Eur J Biochem. 1978 Sep 1;89(2):475–482. doi: 10.1111/j.1432-1033.1978.tb12551.x. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Lutter L. C., Rhodes D., Brown R. S., Rushton B., Levitt M., Klug A. Structure of nucleosome core particles of chromatin. Nature. 1977 Sep 1;269(5623):29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Johns E. W. An improved large scale fractionation of high mobility group non-histone chromatin proteins. Biochim Biophys Acta. 1975 Oct 20;405(2):280–291. doi: 10.1016/0005-2795(75)90094-x. [DOI] [PubMed] [Google Scholar]
- Hjelm R. P., Kneale G. G., Sauau P., Baldwin J. P., Bradbury E. M., Ibel K. Small angle neutron scattering studies of chromatin subunits in solution. Cell. 1977 Jan;10(1):139–151. doi: 10.1016/0092-8674(77)90148-9. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Klug A., Rhodes D., Smith J., Finch J. T., Thomas J. O. A low resolution structure for the histone core of the nucleosome. Nature. 1980 Oct 9;287(5782):509–516. doi: 10.1038/287509a0. [DOI] [PubMed] [Google Scholar]
- Lutter L. C. Kinetic analysis of deoxyribonuclease I cleavages in the nucleosome core: evidence for a DNA superhelix. J Mol Biol. 1978 Sep 15;124(2):391–420. doi: 10.1016/0022-2836(78)90306-6. [DOI] [PubMed] [Google Scholar]
- Mardian J. K., Paton A. E., Bunick G. J., Olins D. E. Nucleosome cores have two specific binding sites for nonhistone chromosomal proteins HMG 14 and HMG 17. Science. 1980 Sep 26;209(4464):1534–1536. doi: 10.1126/science.7433974. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Rau D. C., Felsenfeld G. The high mobility group proteins HMG 14 and 17, do not prevent the formation of chromatin higher order structure. Nucleic Acids Res. 1982 Mar 25;10(6):2007–2016. doi: 10.1093/nar/10.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbani A., Goodwin G. H., Johns E. W. High mobility group non-histone chromosomal proteins from chicken erythrocytes. Biochem Biophys Res Commun. 1978 Mar 30;81(2):351–358. doi: 10.1016/0006-291x(78)91540-1. [DOI] [PubMed] [Google Scholar]
- Sandeen G., Wood W. I., Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980 Sep 11;8(17):3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. M., Goodwin G. H., Johns E. W. The primary structure of the nucleosome-associated chromosomal protein HMG 14. FEBS Lett. 1979 Apr 15;100(2):394–398. doi: 10.1016/0014-5793(79)80378-6. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. T. Properties of active nucleosomes as revealed by HMG 14 and 17 chromatography. Nucleic Acids Res. 1982 Mar 25;10(6):2017–2042. doi: 10.1093/nar/10.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]