Abstract
The timing for dialysis initiationis still debated. The aim of this study was to compare mortality rates, using a propensity-score approach, in dialysis patients with early or late starts. From January 2000 to June 2009, incident adult patients (n = 836) starting dialysis for end-stage renal disease (ESRD) were enrolled. The patients were assigned to either an early- or late-start group depending on the initiation time of the dialysis. After propensity-score-basedmatching, 450 patients remained. At the initiation of dialysis, the mean estimated glomerular filtration rate (eGFR) was 11.1 mL/min/1.73 m2 in the early-start group compared with 6.1 mL/min/1.73 m2 in the late-start group. There were no significant differences in survival between the patients in the early- and late-start groups (Log rank tests P = 0.172). A higher overall mortality risk was observed in the early-start group than in the late-start group for the patients aged ≥ 70 yr (hazard ratio [HR]: 3.29; P = 0.048) and/or who had albumin levels ≥ 3.5 g/dL (HR: 2.53; P = 0.046). The survival of the ESRD patients was comparable between the patients in the early and late-start groups. The time to initiate dialysis should be determined based on clinical findings as well as the eGFR.
Keywords: End Stage Renal Disease, Glomerular Filtration Rate, Renal Dialysis, Mortality, Peritoneal Dialysis
INTRODUCTION
Despite the worldwide use of long-term dialysis, there remains a lack of consensus on the timing of dialysis initiation. Most guidelines recommended an earlier start if there are uremia-related symptoms or malnutrition.
Although many studies have investigated the optimal timing of dialysis initiation, the differences in mortality between early- and late-start dialysis are still debated. There are a number of cohort or case-control studies suggesting that early initiation of dialysis affects morbidity, mortality, the capacity for employment, and the quality of life. The mean estimated glomerular filtration rate (eGFR) at initiation for US Renal Data System (USRDS) patients was higher in patients who initiated dialysis in 2007 than in those who began in 1997 (10.8 mL/min/1.73 m2 vs 8.1 mL/min/1.73 m2) (1). However, in a recent randomized controlled trial, the early initiation of dialysis in patients with chronic kidney disease was not associated with an improvement in survival or clinical outcomes (2). Moreover, the largest retrospective analysis of the USRDS data has suggested that late initiation of dialysis is associated with a reduced risk of mortality (3).
Mortality comparisons of the timing of dialysis initiation have been conducted in many Western countries. East Asian countries, such as Taiwan (4), Japan (5) and Korea (6), have some of the highest incidence and prevalence rates of end-stage renal disease (ESRD). However, there are few publications on this subject for Asian populations.The aim of this study was to compare the mortality in dialysis patients with early or late starts, as measured by the renal function at the start of dialysis, using a propensity-score (PS) approach to compensate for confounding biases.
MATERIALS AND METHODS
Study population
From January 2000 to June 2009, we enrolled incident patients who werestarting dialysis for ESRD at the Gachon University Gil Hospital, Korea. Adult patients (≥ 18 yr old) on hemodialysis (HD) and peritoneal dialysis (PD) were included. Patients were excluded if they had recovered renal function.
All patient medical records were reviewed retrospectively, collecting data on age, gender, height, weight, cause of ESRD, laboratory data (hemoglobin, blood urea nitrogen, creatinine, calcium, phosphorous, albumin, cholesterol, C-reactive protein, pro-B-type natriuretic peptide and troponin I), and comorbid conditions at the time of initiation of dialysis. Comorbid conditions were assessed using the modified Charlson comorbidity index (MCCI) (7). The eGFR was calculated from serum creatinine measured at the time of dialysis initiation using the Modification of Diet in Renal Disease formula with an ethnic factor for the Korean population: eGFR (mL/min/1.73 m2) = 186 × serum creatinine (mg/dL)-1.154 × age (years)-0.203 × 0.742 (if female) × 1.09 (Korean ethnic factor) (8, 9).
Participants were followed from the initiation of dialysis until the end of the study (December 31, 2010) or death (determined from the Korea National Statistical Office). Patients on dialysis during the follow-up period were censored on the date of renal transplantation or loss to follow-up.
Statistical analysis
We used PS matching to increase the comparability between the early- and late-start groups by reducing the selection bias and controlling for potential confounding factors. The estimated PS for being assigned to the early-start group versus the late-start group at the time of initiation of dialysis was calculated for each patient using multiple logistic regression models with the following covariates: age, gender, dialysis modality, medical history (diabetes, coronary artery disease, and peripheral artery disease), and laboratory tests (serum levels of hemoglobin, albumin, calcium, and phosphorus). We matched one early-start patient to one late-start patient based on a greedy 8-1 digit matching algorithm. This algorithm attempted to match early-started and late-started subjects on the first 8 digits of the propensity score. The early-start subjects that did not match were then matched to late-start subjects on 7 digits of the propensity score. We processed through the algorithm sequentially to the 1-digit match on the propensity score. The patients with no corresponding match were excluded. PS matching was performed using SAS 9.1.3.
Continuous variables are presented as means ± SD, and categorical variables are presented as frequencies (percentage). Categorical variables were compared using the chi-squared test, and continuous variables were compared using Student's t-test and the Mann-Whitney U-test when appropriate. Mortality was determined using the Kaplan-Meier method and compared using the log-rank test in the matched-pair cohort. A Cox regression analysis was used to assess the association between mortality and the timing of the initiation of dialysis. The Cox regression model stratified on matched pairs was used in the matched cohort. Associations are presentedas hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs). All analyses except PS matching were performed with SPSS version 12.0 (SPSS, Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Ethics statement
This study was approved by the institutional review board of Gil Hospital Gachon University (IRB No. GIRBA2347). Informed consent was waived by the board.
RESULTS
We enrolled 836 subjects in the study, and defined the initiation of dialysis as late-start if the eGFR was < 7.74 mL/min/1.73 m2 (the median eGFR at initiation of dialysis in the 836 subjects). After we applied the propensity method adjusted for the variables, 450 patients (225 in each group) remained.
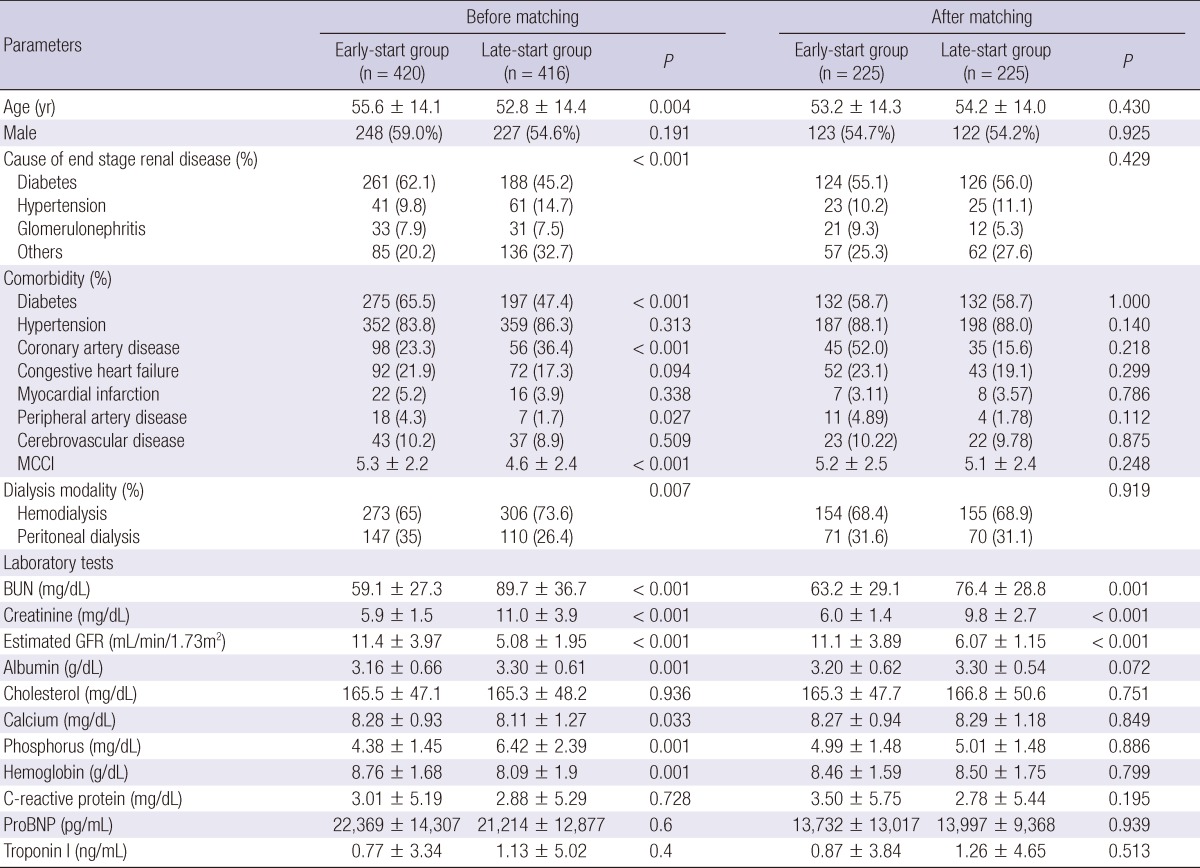
The clinical characteristics of the patients at the initiation of dialysis are presented in Table 1. In the entire cohort, the patients in the early-start group were older and had a higher prevalence of diabetes and PD, higher levels of MCCI and lower levels of albumin. After PS matching, the mean age was 53.7 yr, and 54.4% were male. Diabetes was the most common causes of ESRD (55.6%) at the initiation of dialysis. The mean follow-up duration was 41.9 months for the early-start group and 44.2 months for the late-start group. At the start of dialysis, the mean eGFR was 11.1 ± 3.9 mL/min/1.73 m2 in the early-start group compared with 6.1 ± 1.2 mL/min/1.73 m2 in the late-start group. PD was the initial method of dialysis in 70 patients (31.1%) in the early-start group and 71 (31.6%) in the late-start group. HD was the initial method of dialysis in 155 and 154 patients in the early-start and late-start groups, respectively. By the end of the study, 12 PD patients had shifted to HD, and 13 patients had undergone kidney transplantation in the early-start group. Twelve PD patients had shifted to HD, and 10 had undergone kidney transplantation in the late-start group. There were no differences in age, gender, causes of ESRD, hypertension, diabetes, and laboratory tests (hemoglobin and albumin) between the two groups.
Table 1.
Baseline characteristics of matched patients
MCCI, modified Charlson comorbidity index; BUN, blood urea nitrogen; proBNP, pro-B-Type natriuretic peptide.
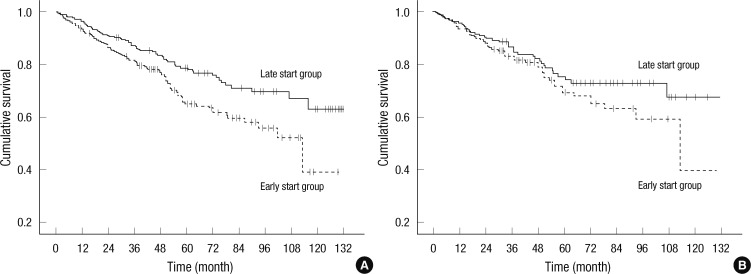
A Kaplan-Meier actuarial survival curve was suggestive of an incrementally increased survival time for patients initiating dialysis later in the entire cohort (Log rank tests P = 0.002) (Fig. 1A). However, there was no significant difference in survival between the patients in the late-start and early-start groups in the PS-matched cohort (Log rank tests P = 0.172) (Fig. 1B).
Fig. 1.
Kaplan-Meier survival curves at the initiation of dialysis. (A) Survival of the late-start group is increased in the entire cohort (Log rank tests P = 0.002). (B) There is no significant difference in survival between the patients in the late-start and early-start groups in the propensity score matched cohort (Log rank tests P = 0.172).
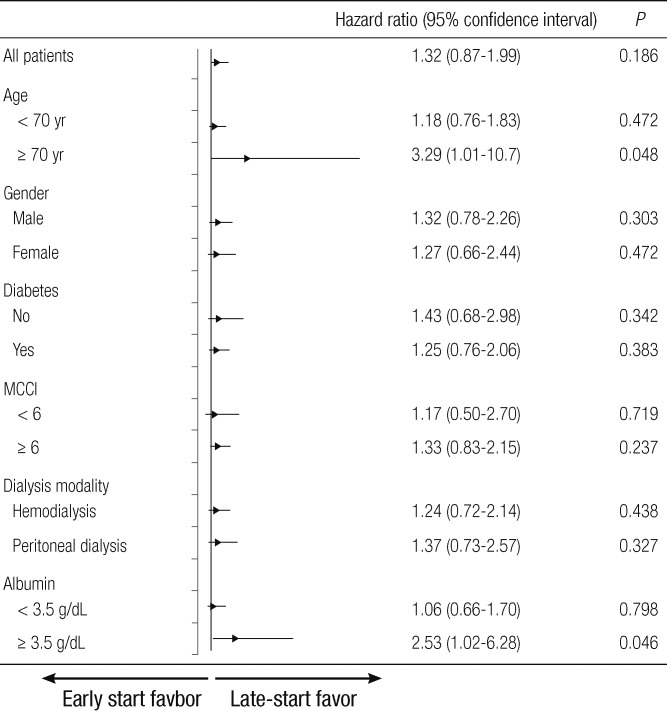
Fig. 2 shows the mortality HRs within the subgroups in the PS matched cohort. Overall survival was similar for the early-start and late-start groups (HR: 1.32; 95% CI: 0.87-1.99, P = 0.186). A higher overall mortality risk was observed in the early-start group relative to the late-start group in those aged ≥ 70 yr compared with those aged < 70 yr. Compared with the patients aged < 70 yr (HR: 1.18; 95% CI: 0.76-1.83, P = 0.472), the late-start patients aged ≥ 70 yr had a significantly lower risk of death compared with the early-start group aged ≥ 70 yr (HR: 3.29; 95% CI: 1.01-10.7, P = 0.048). A higher overall mortality risk was observed in the early-start group relative to the late-start group in patients with albumin levels ≥ 3.5 g/dL compared with < 3.5 g/dL. The greater mortality risk in the early-start group with albumin levels < 3.5 g/dL relative to the late-start group with albumin levels < 3.5 g/dL (HR: 1.06; 95% CI: 0.66-1.70, P = 0.798) was less pronounced than the relationship between the two groups with albumin levels ≥ 3.5 g/dL (HR: 2.53; 95% CI: 1.02-6.28, P = 0.046).
Fig. 2.
Adjusted HR of the initiation of dialysis for mortality using a Cox proportional analysis in the propensity-score matched cohort.
DISCUSSION
We found no significant difference in survival between patients in the early-start and late-start groups using a PS approach. In our entire cohort, the patients who initiated dialysis later had increased survival times. However, the patients in the early-start group were older and had a higher prevalence of diabetes, higher levels of MCCI and lower levels of albumin. To balance the prognostically important baseline characteristics, we matched early-start and late-start patients based on PS at the time of dialysis initiation. PS was able to control for the measured confounding factors, allowing us to reduce the distortions originating from the selection bias. The use of a propensity stratification based on PS has been shown to reduce or eliminate the imbalances in the distribution of baseline covariables across treatment groups in nonrandomized studies (10, 11). However, the use of PS does notmitigate unmeasured confounders because the calculation of PS relies on explicitly measured factors alone.
Most reported studies have argued either for an early or a late initiation of dialysis. The ideal method for comparing the difference is a randomized controlled trial. However, conducting a randomized controlled trial on the optimal timing of the initiation of dialysis is difficult because the decision to initiate dialysis depends not only on objective numerical criteria but also on subjective clinical factors. Our results were consistent with the Initiating Dialysis Early and Late (IDEAL) study (2), whose findings differ from those of some previously published observational cohort and case-control studies, which showed that the early initiation of dialysis was associated with improved survival (12, 13) or with potentially poorer survival times (3, 14, 15). Hakim and Lazarus (16) advocated an early initiation strategy because it results in a significantly better outcome, partly by improving nutrition-related comorbidity. In contrast, patients who initiate dialysis at high eGFR levels usually have a greater comorbidity burden (15) and are less able to tolerate uremic symptoms, which may be the reason why the early initiation of dialysis does not have a favorable effect on survival. An early start may cause increased cumulative exposure to additional hazards (3). These hazards include clinical or subclinical bloodstream and peritoneal infections; heightened inflammation; exposure to antibiotic-resistant organisms or bacterial fragments; protein or blood loss; hemodynamic effects including possible accelerated loss of remaining renal function; dialysis access complications; exposure to heparin; and the risks of higher doses of erythropoietin required because of its reduced potency when administered intravenously.
There have been very few studies comparing mortality rates for the timing of dialysis initiation in Asian populations. Oh et al. (17) showed that all cause mortality and technical failure were not different between early and late starters of PD. This finding is consistent with our results, although our study included patients with HD and PD. However, Tang et al. (18) reported that PD patients who electively started chronic dialysis when their GFR reached ≤ 10 mL/min had a better 1-yr survival rate than did those who initially refused and only started dialysis once they developed a uremic emergency. In contrast, Shiao et al. (19) found in a retrospective cohort of 275 PD patients that the late start of PD (as defined by the initiation of dialysis at eGFR < 5 mL/min) was associated with better survival and a reduced risk for all-cause hospitalization. Hwang et al. (20) analyzed the Taiwan Dialysis Registry data between 2001 and 2004 to evaluate the impact of different levels of GFR on mortality after the initiation of chronic dialysis. They found that lower eGFR at the initiation of dialysis is associated with lower mortality.
We have shown that there was a higher overall mortality risk in the early-start group relative to the late-start group in those patients with albumin levels ≥ 3.5 g/dL and/or who are aged ≥ 70 yr. Rosansky et al. (14) reported that an early start is still harmful, especially in a healthier subset of patients with serum albumin levels of 3.5 g/dL or higher. A possible reason for the significantly poorer outcomes is the competitive risk of harm from unnecessary dialysis in patients who are not at high risk of death from other causes. In contrast, patients with high comorbidity and low serum albumin levels may have a greater risk of death, independent of HD. It is difficult to justify an early start of dialysis based on eGFR measurements alone, especially in the frail subset of elderly patients with high comorbidity (21). The elderly patients had low serum creatinine levels due to sarcopenia and therefore had higher eGFR levels. They also had severe disease and more uremic symptoms despite a higher eGFR.
This study has some limitations. First, the GFR was estimated and not measured. The eGFR may overestimate the true GFR in patients with advanced kidney failure, low muscle mass, and low creatinine generation (22). Second, survival bias is a potential issue, because only those who survived to the initiation of dialysis were analyzed, eliminating those who could have started dialysis early but died before initiation. Third, the lead-time bias should be taken into account in the interpretation of the results. This bias may result in a falsely prolonged survival simply because patients are started at an earlier stage of the disease. The observational nature of this study requires cautious interpretation of our results and future clinical trials are warranted to confirm the clinical indications for the initiation of dialysis.
In conclusion, early-start groups had no survival benefit in our study when using a PS approach. Physicians should not determine the timing for initiating dialysis based solely on eGFR in a stable and symptom-free patient. Dialysis may be delayed until more traditional clinical indications for dialysis are present.
Footnotes
This study was supported by intramural grant of Gachon University Gil Hospital and grant No. 2011-0008605 from the National Research Foundation of Korea.
References
- 1.O'Hare AM, Choi AI, Boscardin WJ, Clinton WL, Zawadzki I, Hebert PL, Kurella Tamura M, Taylor L, Larson EB. Trends in timing of initiation of chronic dialysis in the United States. Arch Intern Med. 2011;171:1663–1669. doi: 10.1001/archinternmed.2011.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 3.Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, Ragasa R, Goldfarb-Rumyantzev AS. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5:1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang WC, Hwang SJ. Incidence, prevalence and mortality trends of dialysis end-stage renal disease in Taiwan from 1990 to 2001: the impact of national health insurance. Nephrol Dial Transplant. 2008;23:3977–3982. doi: 10.1093/ndt/gfn406. [DOI] [PubMed] [Google Scholar]
- 5.Nakai S, Suzuki K, Masakane I, Wada A, Itami N, Ogata S, Kimata N, Shigematsu T, Shinoda T, Syouji T, et al. Overview of regular dialysis treatment in Japan (as of 31 December 2008) Ther Apher Dial. 2010;14:505–540. doi: 10.1111/j.1744-9987.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 6.Jin DC. Current status of dialysis therapy in Korea. Korean J Intern Med. 2011;26:123–131. doi: 10.3904/kjim.2011.26.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis. 2003;42:125–132. doi: 10.1016/s0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Cha RH, Lim YH, Kim H, Song KH, Gu N, Yu KS, Lim CS, Han JS, Kim S, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25:1616–1625. doi: 10.3346/jkms.2010.25.11.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 12.Bonomini V, Feletti C, Scolari MP, Stefoni S. Benefits of early initiation of dialysis. Kidney Int Suppl. 1985;17:S57–S59. [PubMed] [Google Scholar]
- 13.Tattersall J, Greenwood R, Farrington K. Urea kinetics and when to commence dialysis. Am J Nephrol. 1995;15:283–289. doi: 10.1159/000168850. [DOI] [PubMed] [Google Scholar]
- 14.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171:396–403. doi: 10.1001/archinternmed.2010.415. [DOI] [PubMed] [Google Scholar]
- 15.Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, Stengel B. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77:700–707. doi: 10.1038/ki.2010.14. [DOI] [PubMed] [Google Scholar]
- 16.Hakim RM, Lazarus JM. Initiation of dialysis. J Am Soc Nephrol. 1995;6:1319–1328. doi: 10.1681/ASN.V651319. [DOI] [PubMed] [Google Scholar]
- 17.Oh KH, Hwang YH, Cho JH, Kim M, Ju KD, Joo KW, Kim DK, Kim YS, Ahn C, Oh YK. Outcome of early initiation of peritoneal dialysis in patients with end-stage renal failure. J Korean Med Sci. 2012;27:170–176. doi: 10.3346/jkms.2012.27.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang SC, Ho YW, Tang AW, Cheng YY, Chiu FH, Lo WK, Lai KN. Delaying initiation of dialysis till symptomatic uraemia: is it too late? Nephrol Dial Transplant. 2007;22:1926–1932. doi: 10.1093/ndt/gfm109. [DOI] [PubMed] [Google Scholar]
- 19.Shiao CC, Huang JW, Chien KL, Chuang HF, Chen YM, Wu KD. Early initiation of dialysis and late implantation of catheters adversely affect outcomes of patients on chronic peritoneal dialysis. Perit Dial Int. 2008;28:73–81. [PubMed] [Google Scholar]
- 20.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010;25:2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 21.Rosansky S, Glassock RJ, Clark WF. Early start of dialysis: a critical review. Clin J Am Soc Nephrol. 2011;6:1222–1228. doi: 10.2215/CJN.09301010. [DOI] [PubMed] [Google Scholar]
- 22.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Pappas LM, Cheung AK. Creatinine production, nutrition, and glomerular filtration rate estimation. J Am Soc Nephrol. 2003;14:1000–1005. doi: 10.1097/01.asn.0000057856.88335.dd. [DOI] [PubMed] [Google Scholar]