Abstract
Objectives:
Florbetapir F 18 PET can image amyloid-β (Aβ) aggregates in the brains of living subjects. We prospectively evaluated the prognostic utility of detecting Aβ pathology using florbetapir PET in subjects at risk for progressive cognitive decline.
Methods:
A total of 151 subjects who previously participated in a multicenter florbetapir PET imaging study were recruited for longitudinal assessment. Subjects included 51 with recently diagnosed mild cognitive impairment (MCI), 69 cognitively normal controls (CN), and 31 with clinically diagnosed Alzheimer disease dementia (AD). PET images were visually scored as positive (Aβ+) or negative (Aβ−) for pathologic levels of β-amyloid aggregation, blind to diagnostic classification. Cerebral to cerebellar standardized uptake value ratios (SUVr) were determined from the baseline PET images. Subjects were followed for 18 months to evaluate changes in cognition and diagnostic status. Analysis of covariance and correlation analyses were conducted to evaluate the association between baseline PET amyloid status and subsequent cognitive decline.
Results:
In both MCI and CN, baseline Aβ+ scans were associated with greater clinical worsening on the Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-Cog (p < 0.01) and Clinical Dementia Rating–sum of boxes (CDR-SB) (p < 0.02). In MCI Aβ+ scans were also associated with greater decline in memory, Digit Symbol Substitution (DSS), and Mini-Mental State Examination (MMSE) (p < 0.05). In MCI, higher baseline SUVr similarly correlated with greater subsequent decline on the ADAS-Cog (p < 0.01), CDR-SB (p < 0.03), a memory measure, DSS, and MMSE (p < 0.05). Aβ+ MCI tended to convert to AD dementia at a higher rate than Aβ− subjects (p < 0.10).
Conclusions:
Florbetapir PET may help identify individuals at increased risk for progressive cognitive decline.
Clinical studies indicate that 10%–15% of subjects with mild cognitive impairment (MCI) progress annually to dementia.1,2 However, there is considerable variability in progression rates, reflecting the underlying heterogeneity of this population. A reliable biomarker that could identify subjects at greatest risk for future cognitive decline might enhance the clinical evaluation of MCI subjects and accelerate the testing of preventive strategies.1,3
Although the cause of Alzheimer disease (AD) is not definitively known, accumulation of amyloid-β (Aβ) fibrils in the form of amyloid plaques is a neuropathologic requirement for definitive diagnosis.4 AD genetic mutations as well as apolipoprotein ϵ4 carrier status lead to increased formation of amyloid plaques.5
PET tracers that bind to the aggregated Aβ peptides offer promise to directly assess fibrillar amyloid pathology both visually and quantitatively.6,7 Prior studies have found that 11C-labeled Pittsburgh compound B (11C-PiB)-positive normal subjects and subjects with MCI are more likely to show faster cognitive deterioration than PIB-negative subjects.3,8–16 However, the short half-life of 11C (20 minutes) limits its use as a routine clinical test. Since 18F has a half-life of 110 minutes, 18F-labeled tracers are better suited for general clinical use.17 Florbetapir F 18 (18F-AV-45) is a PET ligand with high affinity and specificity to Aβ.18 A multicenter clinical-histopathologic validation study found a significant correlation between majority visual ratings of florbetapir PET in living subjects and autopsy-measured Aβ pathology.19 Other F 18 amyloid PET tracers are also being developed.20,21 One limitation of current F 18 amyloid PET tracers has been the lack of longitudinal data. The present study was designed to examine whether florbetapir PET can predict subsequent cognitive decline in older at-risk subjects.
METHODS
AV45-A11 (NCT00857506) is a prospective, multicenter, observational study, sponsored by Avid Radiopharmaceuticals (a subsidiary of Eli Lilly), that is being conducted at 21 US clinical sites. It is a longitudinal extension of a cross-sectional phase 2 florbetapir PET study (AV45-A05; NCT00702143), whose results are being reported separately.
Subjects.
Fifty-one subjects with MCI, 69 clinically normal cognitively healthy controls (CN), and 31 subjects clinically diagnosed with AD dementia who had previously received a florbetapir PET scan in the cross-sectional study agreed to participate in this longitudinal observational study. Patients with AD dementia met National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association22 criteria for probable AD and had Mini-Mental State Examination (MMSE) scores ≤24. MCI subjects were presenting for an initial evaluation, or had received a diagnosis of MCI within the past year. They were ≥50 years of age, had a complaint of memory or cognitive impairment corroborated by an informant, had a Clinical Dementia Rating (CDR) scale global rating of 0.5, and MMSE >24; no episodic memory cutoff was required. The CN subjects were ≥50 years of age, assessed clinically as cognitively normal, and had a CDR global of 0 and an MMSE of 29 or 30. CN subjects were recruited approximately equally across age deciles (50–59, 60–69, 70–79, and ≥80 years of age). At screening, all subjects underwent a detailed medical history, physical and neurologic examinations, clinical interview, and laboratory evaluations. An MRI performed at screening or within 6 months prior to enrollment ruled out significant CNS lesions. Subjects were excluded if they had other relevant neuropsychiatric diseases, received anti-amyloid investigational drugs, were unable to complete psychometric testing, or had contraindications to PET.
Baseline assessments.
All subjects underwent a clinical diagnostic interview and cognitive/functional battery including the CDR, MMSE, Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS-Cog; 11-item version), Wechsler Logical Memory (immediate and delayed recall), Digit-Symbol Substitution, Category Verbal Fluency (animals and vegetables), Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale (ADCS-ADL), and Geriatric Depression Scale (GDS). APOE genotyping was performed as an optional procedure, in a double-blind fashion.
Florbetapir PET scan.
PET scanners at all sites were qualified using a Hoffman brain phantom. Subjects underwent PET amyloid imaging, as part of AV45-A05, during a 10-minute acquisition (acquired in 2 × 5 minutes frames), 50 minutes after IV injection of 10 mCi (370 MBq) of florbetapir F 18. Study site PET scanners included ECAT HR+ (Siemens), Discovery LS PET/CT (General Electric), Biograph PET/CT (Siemens), and Advance PET (GE) models. Images were reconstructed using an iterative reconstruction algorithm (4 iterations, 16 subsets), with a post reconstruction Gaussian filter of 5 mm.
Three nuclear medicine physicians, blinded to clinical data, independently reviewed all PET images at an imaging core lab (ICON Medical Imaging, Warrington, PA) and rated each on both a semiquantitative (0–4) and a binary qualitative scale (amyloid positive: Aβ+ or amyloid negative: Aβ−) based on the pattern of tracer uptake in gray matter cortical areas, as previously described.19 The majority of the 3 binary ratings was used for analyses in this report. Details of PET methods, visual rater training, and reliability are being reported separately (appendix e-1 on the Neurology® Web site at www.neurology.org). Two of 3 readers agreed with the majority in more than 96% of cases and the third reader agreed in 74%. The κ statistic for all 3 raters was 0.58 (binary scale).
Cerebral-to-whole-cerebellar florbetapir standard uptake value ratios (SUVr) were also calculated, as previously described.19,23 The 2 frames of each image were averaged and normalized to a florbetapir template in Talairach space using SPM-2. No partial volume correction was performed. Previously defined volumes of interest (VOI) representing gray matter regions known to be vulnerable to amyloid deposition in frontal, temporal, and parietal cortices, anterior cingulate, posterior cingulate, and precuneus were used to extract counts for each region. SUVr were calculated using whole cerebellum as the reference region. The average of the SUVr across the 6 cortical target regions was used for analysis.
Follow-up assessments.
Subjects completing the initial PET scan were eligible to participate in the follow-up protocol. The primary objective of the protocol is to determine whether florbetapir F 18 PET predicts progressive cognitive impairment. The current report describes changes in cognitive performance that were found at the 18-month interim timepoint; a final assessment will be obtained at 36 months. Each subject/informant was contacted at 6-month intervals for a brief phone screen and status update. Subjects returned to the clinic 18 months after the original PET scan to undergo a diagnostic interview and cognitive test battery. Upon completion of the diagnostic evaluation, subjects were classified as CN, MCI, AD, or non-AD dementia. Clinical diagnoses were determined blind to the florbetapir F 18 scan results.
Standard protocol approvals, registrations, and patient consents.
This study (NCT00857506) was approved by the institutional review boards at all participating sites and all subjects or their appropriate representatives provided informed consent.
Statistical analysis.
All statistical tests were 2-sided unless otherwise noted and performed at the 0.05 significance level. Differences between diagnostic groups and Aβ status (by binary ratings) on baseline continuous characteristics were assessed with 2-sample t tests; categorical variables were assessed with χ2 tests. Analysis of covariance (ANCOVA) models, adjusting for baseline age and cognitive test score, evaluated differences between Aβ+ and Aβ− subjects with respect to change on cognitive measures between baseline and month 18. To further evaluate the robustness of these findings, correlational analyses were conducted between the baseline SUVr and subsequent change on the cognitive and functional measures adjusting for baseline test performance and age. To evaluate factors other than amyloid classification, additional stepwise regression models were performed in which amyloid classification, baseline score, age, education, and APOE ϵ4 carrier status were considered. Variables were entered in a forward fashion and retained in the model if p < 0.15. For all analyses, last observation carried forward (LOCF) imputation was used if subjects had a postbaseline cognitive assessment; if a subject discontinued the study prior to the scheduled 18-month cognitive follow-up testing and had cognitive testing at the time of discontinuation, these test scores were carried forward to the 18-month timepoint. Analyses used SAS Windows (version 9 or later). The analyses in this report are considered exploratory and the reported p values are unadjusted for multiple comparisons. p Values <0.05 are considered significant. Since in clinical practice, florbetapir scans will likely be assessed for the presence or absence of amyloid, we considered the ANCOVA model that compares the differences between Aβ+ and Aβ− of primary importance, and utilized the Benjamani-Hochberg procedure24 post hoc to control the false discovery rate for this set of comparisons.
RESULTS
Subject disposition.
Of the 151 subjects (69 CN, 51 MCI, 31 AD) who entered this longitudinal observational study (figure e-1), 97% of CN, 90% of MCI, and 87% of AD completed 18 months of follow up. The most common reasons for termination were withdrawal of consent (n = 7) and loss to follow-up (n = 3). The proportion of study completers did not differ by visual ratings of amyloid status in the AD, MCI, or CN groups (p = 0.74, p = 0.89, p = 0.55, respectively).
Baseline florbetapir PET amyloid positivity by diagnosis.
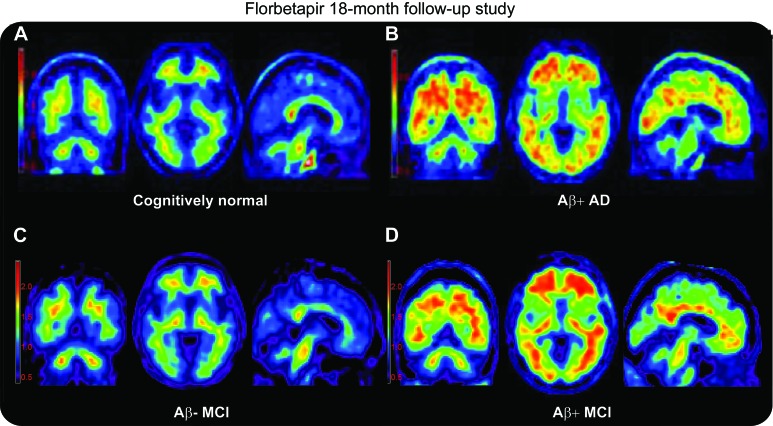
Figure 1 depicts illustrative amyloid-positive (Aβ+) and -negative (Aβ−) PET scans. At entry, 37% (19/51) of subjects with MCI, 14% (10/69) of subjects with CN, and 68% (21/31) of subjects with AD dementia were rated as PET Aβ+ (p < 0.0001) by the majority read.
Figure 1. Illustrative florbetapir PET scans in controls, patients with mild cognitive impairment (MCI), and patients with Alzheimer disease (AD).
Representative florbetapir PET images for an amyloid-negative (Aβ−) cognitively normal subject (A), an amyloid-positive (Aβ+) patient with AD (B), an Aβ− patient with MCI (C), and an Aβ+ patient with MCI who converted to dementia during the course of this study (D). Aβ+ was determined per the majority of 3 raters. Color scale is shown in SUVr units. Standardized uptake value ratio (SUVr) was calculated as described in the text.
Baseline demographic characteristics and cognitive performance.
As expected, there were differences among diagnostic groups in cognitive and functional variables, and subjects with AD dementia were also slightly older (table e-1). Educational level, height, weight, gender, and race distribution did not differ significantly among groups. Aβ+ subjects classified by visual ratings tended to be older and have worse cognitive performance at baseline than Aβ− subjects (table 1). Because of these potential differences, we adjusted for baseline score and age in longitudinal statistical models evaluating change by amyloid status.
Table 1.
Baseline demographics, SUVr, and functional performance by the presence or absence of amyloid on florbetapir F 18 PET as determined by binary visual ratingsa

Abbreviations: Aβ = amyloid-β; AD = Alzheimer disease; ADAS-Cog = Alzheimer's Disease Assessment Scale–Cognitive subscale; CDR-SB = Clinical Dementia Rating–Sum of Boxes; DSS = Digit Symbol Substitution; MMSE = Mini-Mental State Examination; SUVr = standardized uptake value ratio; WMS = Wechsler Memory Scale.
Values provided are means (SD) unless otherwise noted.
Differences with p values <0.05.
Florbetapir PET and change from baseline to 18 months on cognitive assessments.
MCI subjects rated Aβ+ showed a significantly greater mean worsening than Aβ− rated subjects on almost all cognitive assessments (table 2 and figure 2). Among CN subjects, Aβ+ classification predicted greater worsening on the ADAS-Cog and CDR–Sum of Boxes (CDR-SB) (p < 0.01). Among subjects with clinically diagnosed AD dementia, Aβ+ classification predicted greater decline on the verbal fluency test for animals (p < 0.01) and a trend toward greater decline on the MMSE and DSS (p < 0.10). In stepwise regression analyses among subjects with MCI, APOE ϵ4 carrier status and florbetapir PET amyloid classification were never retained together in the same model (table e-2). The association between amyloid positivity and greater decline was retained in the model for the ADAS (p = 0.0061), DSS (p = 0.0001), MMSE (p = 0.0133), CDR-SB (p = 0.1060), and Verbal Fluency (Animals) (p = 0.1413), whereas APOE was retained in the model for Verbal Fluency (vegetables) and Wechsler immediate and delayed recall. When florbetapir PET amyloid classification and APOE ϵ4 carrier status were forced into statistical models together, neither significantly predicted cognitive decline, with the exception of amyloid classification for DSS and APOE ϵ4 carrier status for delayed recall.
Table 2.
Change from baseline to 18 months by florbetapir PET amyloid status and correlation with SUVra
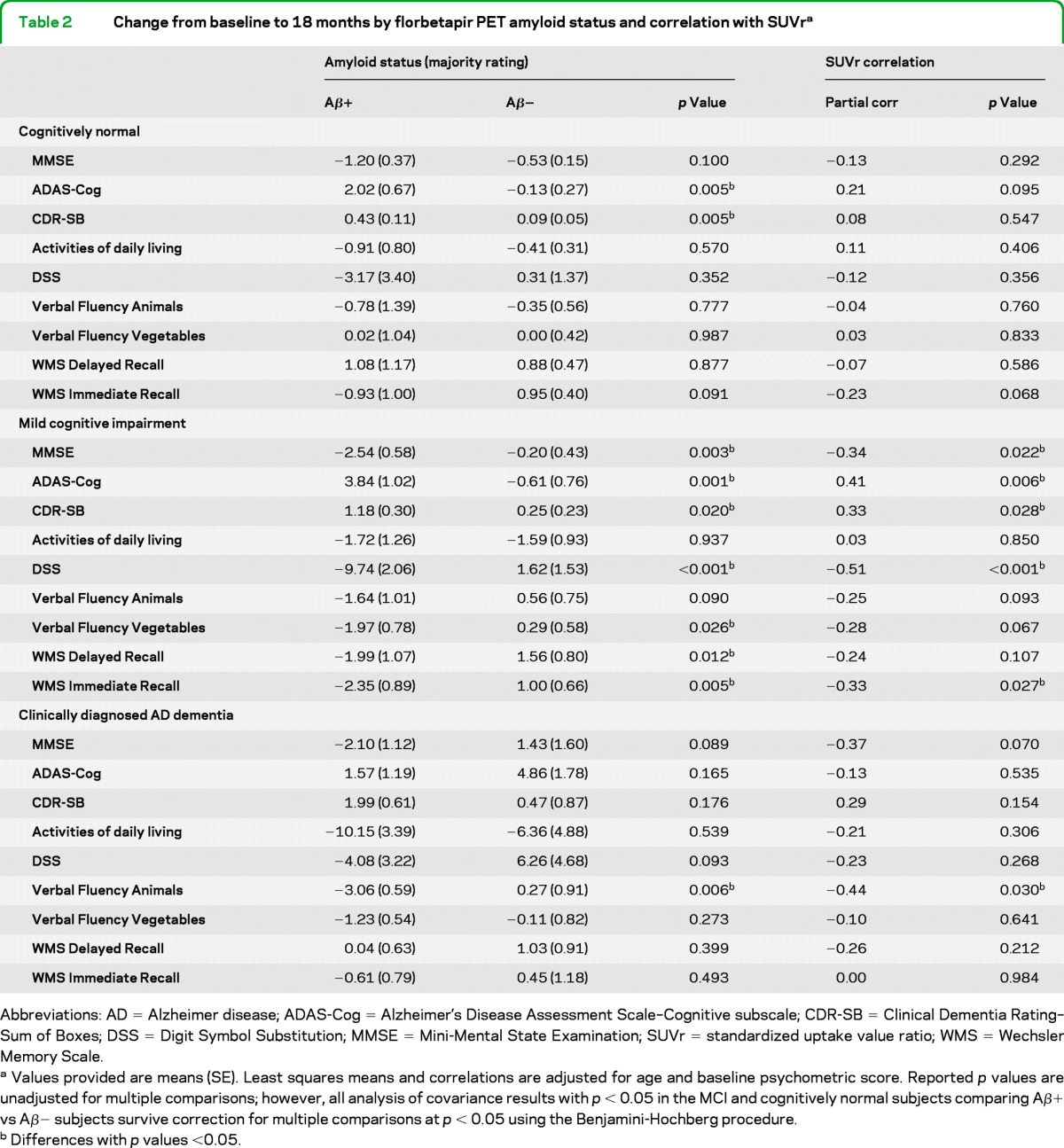
Abbreviations: AD = Alzheimer disease; ADAS-Cog = Alzheimer's Disease Assessment Scale–Cognitive subscale; CDR-SB = Clinical Dementia Rating–Sum of Boxes; DSS = Digit Symbol Substitution; MMSE = Mini-Mental State Examination; SUVr = standardized uptake value ratio; WMS = Wechsler Memory Scale.
Values provided are means (SE). Least squares means and correlations are adjusted for age and baseline psychometric score. Reported p values are unadjusted for multiple comparisons; however, all analysis of covariance results with p < 0.05 in the MCI and cognitively normal subjects comparing Aβ+ vs Aβ− subjects survive correction for multiple comparisons at p < 0.05 using the Benjamini-Hochberg procedure.
Differences with p values <0.05.
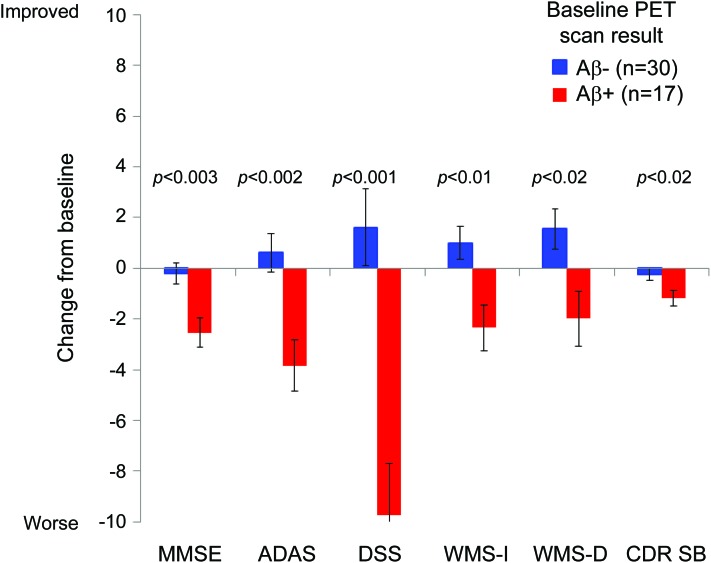
Figure 2. Change from baseline on cognitive assessment measures in subjects with mild cognitive impairment (MCI).
Least squares mean change (SE) in test scores from baseline to month 18. Note that in this graph the direction of change on the Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS) and Clinical Dementia Rating–Sum of Boxes (CDR SB) are reversed to show worsening scores as a negative change consistent with other measures (graph based on MCI data from table 2). DSS = Digit Symbol Substitution; MMSE = Mini-Mental State Examination; WMS = Wechsler Memory Scale.
SUVr correlation analysis.
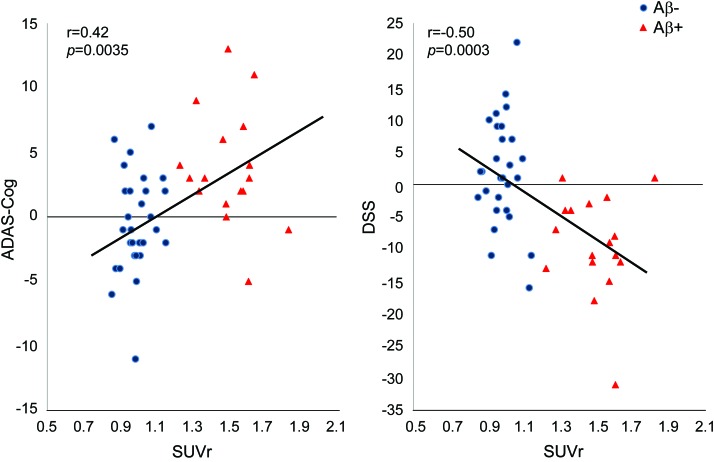
Similar to the visual rating analysis, in patients with MCI, higher baseline florbetapir SUVr correlated with greater subsequent decline on the ADAS-Cog, as well as measures of memory, executive function, and the MMSE (table 2; figure 3).
Figure 3. Correlation between baseline florbetapir standardized uptake value ratio (SUVr) (averaged across 6 brain regions) and change from baseline to 18 months on the Alzheimer's Disease Assessment Scale–Cognitive subscale (ADAS) and Digit Symbol Substitution (DSS) in subjects with mild cognitive impairment.
The correlations are not adjusted for other variables. Correlations adjusted for age and baseline cognitive score remained significant (table 2). Correlations conducted separately within Aβ+ and Aβ− groups were not statistically significant, indicating that the presence of Aβ may be a more important predictor of cognitive decline than the exact amount of Aβ present.
Florbetapir PET ratings, change in diagnosis, and CDR global ratings.
Eight subjects with MCI progressed to AD dementia during follow-up, while 7 subjects with MCI reverted to CN status. Subjects with MCI rated Aβ+ tended to have a higher conversion rate to AD dementia (5/17 Aβ+ subjects with MCI [29.4%] vs 3/29 rated Aβ− [10.3%]; p = 0.0996). Proportionately fewer Aβ+ (1/17 [5.9%]) vs Aβ− (6/29 [20.7%]) subjects with MCI converted to CN status, although not significantly (p = 0.1772). A higher proportion of Aβ+ subjects with MCI and CN subjects worsened on their CDR global score by at least 0.5 points, i.e., 23.5% and 30%, respectively, as compared to 6.7% and 5.5% for Aβ− subjects with MCI and CN subjects (odds ratio = 5.5, 95% confidence interval 1.49–20.0; p < 0.0052).
DISCUSSION
This multicenter, longitudinal study of florbetapir F 18 amyloid imaging confirms and extends results from prior, mostly single-site, studies with 11C-PiB8,9–11,13,14,25,26 showing that both cognitively normal subjects and subjects with MCI with higher levels of cortical Aβ on PET are at higher risk for future cognitive progression than individuals with lower levels of amyloid after controlling for age and baseline cognitive performance. These findings support the potential utility of florbetapir PET as a predictive biomarker of cognitive decline in at-risk subjects. While additional longer-term confirmatory studies are necessary, this study also provides preliminary support to the proposed research concepts of preclinical AD (in cognitively healthy subjects)27 and MCI of the Alzheimer type.2,28
The present findings also suggest that amyloid PET may have predictive value in MCI for developing AD dementia. Over the course of the 18-month follow-up period, 29% of Aβ+ vs 10% of Aβ− subjects with MCI converted to AD dementia (20%/year vs 7%/year, respectively). Rates of conversion from PiB Aβ+ MCI to AD dementia have varied in prior studies depending on MCI sampling criteria, duration of follow-up, and PET measurement used. Reported conversion rates include 82% over 3 years,9 50% over 2 years,10 67% over 20 months,12 and 38% over 21 months.14
The somewhat lower conversion rates in this study among Aβ+ individuals need to be interpreted in the context of the sample size and follow-up duration as well as the fact that subjects with MCI in this study were less impaired at baseline compared to subjects with MCI in ADNI. For example, the mean 11-item ADAS-Cog baseline total for 397 subjects with MCI in ADNI-1was 11.5 (www.adni-info.org) compared to 9.4 in our MCI sample. This is likely due to use in this study of different entry criteria and use of more private practice sites that recruit from the community via advertisement (the latter may also account for the lower prevalence of amyloid-positive scans in patients with clinically diagnosed AD dementia, despite the demonstrated sensitivity and specificity of florbetapir for detecting Aβ pathology at autopsy).19 Thus, the subjects with MCI in this study may be at an earlier stage of disease, analogous to those presenting with mild symptoms in a community setting, and for this reason, may require more than 18 months of follow-up to demonstrate a higher conversion rate to AD dementia. The present study has been amended to extend follow-up to 36 months to further test the effect of baseline amyloid status on rates of conversion from MCI to AD dementia at 36 months.
In contrast to conversion rates, performance on psychometric tests provides an objective measure of cognitive performance, with increased power due to the continuous nature of the data. Subjects with MCI who had a greater amyloid burden showed a statistically significant greater mean worsening of scores over time on psychometric assessments. In very mildly impaired patients with MCI, where conversion to dementia is a relatively distant outcome, the ability to assess risk of cognitive decline (e.g., from mild to more severe MCI) could be clinically useful for making ongoing care recommendations. Such data could also be useful to power secondary prevention clinical trials since the mean decline in MCI Aβ+ group on the ADAS-Cog over 18 months was 4.45 points greater than that in Aβ− subjects (+3.84 decline vs −0.61 improvement).
CN subjects classified as Aβ+ worsened significantly more than Aβ− CN subjects on the ADAS-Cog and the CDR-SB but not on other assessments. The lack of significant differences seen on the other cognitive instruments and the relatively slower rate of deterioration in the Aβ+ CN subjects compared to Aβ+ MCI subjects (table 2) are not unexpected. It has been hypothesized that the gap between the first appearance of amyloid plaques of sufficient density to be detected on a PET scan and onset of dementia may be as long as 10 years.1,27,28 Studies of normal subjects that have shown an association between Aβ levels as assessed by 11C-PIB and decline on specific cognitive measures have been longer-term, largely retrospective, observations (e.g., 4–10 years).13,15,16 It is likely that a much larger sample and follow-up period longer than the 18 months will be required to test for greater declines in the Aβ+ CN subjects. Thus, the present data are insufficient to predict whether, or when, cognitive deterioration will occur in individual Aβ+ CN subjects.
Several other caveats should be considered when evaluating the present results. First, a positive scan was defined by the majority read of 3 nuclear medicine physicians. Although agreement with the majority read was >95% for 2 of 3 readers, it was only moderate (74%) for the third. Thus, the prognostic value of the majority read may be greater than that obtained by some individual readers in a clinical setting. Conversely, the imaging component of this study was conducted as part of a Phase II trial, prior to the evaluation of florbetapir imaging in patients followed to autopsy.19 The availability of these autopsy-verified cases for use in development and testing of read criteria and training methods has led to improved inter-rater reliability. Importantly, the majority read classifications with this improved training produced high agreement with the current study majority read classifications, indicating that the current majority reads are reliable (appendix e-1). While majority reads serve a specific purpose in a research setting, in routine clinical practice florbetapir scans are intended to be read by individual nuclear medicine physicians after they undergo online or in-person case-based training. Such training has already begun and available data (appendix e-1; reference 29) indicate that trained individual physician raters can read florbetapir PET scans with acceptable accuracy; however, further experience in practice settings is needed to confirm these initial findings.
Second, corrections were not planned for multiple comparisons across the cognitive assessments. Nevertheless, all the ANCOVA findings reported as significant (p < 0.05) in the MCI and CN groups survive correction for multiple comparisons by the Benjamini-Hochberg24 procedure.
Our study did not collect other biomarker data and cannot assess the relative utility of PET vs other biomarkers. PET data from ongoing studies, such as ADNI3 and anti-amyloid drug trials,30 may help clarify some of these issues. Too few subjects with MCI were taking AD medications to assess the potential effect of these medications on cognitive decline.
Our findings and other reports suggest that amyloid PET tracers may have promise for indicating risk of subsequent cognitive decline in patients with MCI and CN older adults. Longitudinal PET and cognitive data may help clarify its prognostic role in the clinical setting, its ability to improve confidence in the recently proposed diagnoses of dementia and MCI due to AD, and for subject enrichment of therapeutic trials in the early clinical and preclinical stages of AD.
Supplementary Material
GLOSSARY
- Aβ
amyloid-β
- AD
Alzheimer disease
- ADAS-Cog
Alzheimer's Disease Assessment Scale–Cognitive subscale
- ADCS-ADL
Alzheimer's Disease Cooperative Study–Activities of Daily Living Scale
- ANCOVA
analysis of covariance
- CDR-SB
Clinical Dementia Rating–Sum of Boxes
- CN
cognitively normal
- DSS
Digit Symbol Substitution
- GDS
Geriatric Depression Scale
- LOCF
last observation carried forward
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- PiB
Pittsburgh compound B
- SUVr
standardized uptake value ratio
- VOI
volume of interest.
Footnotes
Editorial, page 1628
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
P.M.D. served as the primary author of the manuscript, advised on study design, assisted with data collection, drafted the initial version of the manuscript, and was involved in data interpretation and critical revisions. R.A.S. contributed to data collection and manuscript revisions. R.E.C. contributed to subject recruitment and participated in manuscript writing. K.A.J. collected data and revised the manuscript. E.M.R. contributed to the study design, interpretation of the findings, and reviewing the manuscript. M.D.D. performed the statistical analyses. M.G. contributed to the data analysis, interpretation, and manuscript revisions. M.N.S. contributed to data collection, manuscript review, and study design. C.H.S. contributed to data collection and reviewing the manuscript. A.F. contributed to data collection and editing the manuscript. A.C. contributed to study design, data review, and interpretation. C.M.C. contributed to study design and data interpretation. A.D.J. contributed to study design, reviewing the literature, drafting figures, and drafting the manuscript. M.A.M. contributed to administrative support, funding, data analysis, and manuscript review. D.M.S. contributed to study design, data review, and manuscript writing. M.J.P. contributed to study design, conduct, data analysis and interpretation, and review of the manuscript.
DISCLOSURE
P.M. Doraiswamy has received research grants through Duke University from NIA, NIMH, NINDS, NHLBI, University of California (ADCS), Northern California Research Institute (ADNI), Avid/Lilly, Elan, Bristol-Myers, Ono, Sanofi, Novartis, Medivation, and Neuronetrix in the recent past. He has received advisory or speaking fees from Avid/Lilly, Medivation, BristolMyers, Accera, Sonexa, Schering, TauRx, Baxter, Neuroptix, Bayer, Neuronetrix, Otsuka, AstraZeneca, Lundbeck, and Edwards Hospital. He owns stock in Sonexa, Clarimedix, and Adverse Events Inc, whose products are not discussed in this manuscript. R.A. Sperling has served as a site investigator for Avid, BMS, Elan, Janssen, Pfizer, and Wyeth and as a consultant to Bayer, BMS, Elan, Eisai, Janssen, Pfizer, Roche, and Wyeth, and as an unpaid consultant to Avid. She has received speaking honoraria from Prizer, Janssen, Eli Lilly, and Bayer. R.E. Coleman was a site PI for the clinical study and served on an advisory board to Avid and Lilly. K.A. Johnson was a co-investigator in the trial and has consulted for GE Healthcare, Bayer-Schering, Pfizer, Elan/Janssen, and Seimens. He has received research support from Avid/Lilly, Bristol-Myers-Squib, Janssen (JanssenAI), and Pfizer. E.M. Reiman has served as a scientific advisor to Sygnis, AstraZeneca, Bayer, Eisai, Elan, Eli Lilly, GlaxoSmithKline, Intellect, Link Medicine, Novartis, Siemens, and Takeda. He has had research contracts with NIA the Arizona Dept. of Health Services, AstraZeneca, and Avid. M.D. Davis has received training support from NIDDK grant 2T32DK060455, is an employee at Theorem Clinical Research, and performed statistical analyses under a contract from Avid. M. Grundman has served as a consultant to Acumen, Adamas, ALSP, Avid, Biogen Idec, Elan, Helicon, Intellect Neurosciences, Janssen Alzheimer Immunotherapy, J&J, Lilly, Neurophage, and Teva and on advisory boards for Helicon, Nutricia North America, and Bristol Myers Squibb. He owns stock in Elan and formerly held Avid stock options. M.N. Sabbagh has served in a consulting or advisory capacity for Lilly, Amerisciences, Takeda, Eisai, Pfizer, and GSK and has received royalties from Wiley and Amerisciences. He has received contracts and grants from Celgene, Ceregene, Bayer, Baxter, BMS, Lilly, Pfizer, Wyeth, Janssen, Elan, Avid, Genentech, and Eisai. C.H. Sadowsky has served on speaker bureaus for Novartis, Forest, and Accera and as a consultant to Lilly. A.S. Fleisher has served as a consultant to Lilly and Avid and received grant funding from Avid. A. Carpenter is an employee of Avid, a division of Eli Lilly, and formerly held Avid stock or options. C.M. Clark, deceased, was an employee at Avid; disclosures are not included for this author. A.D. Joshi, M.A. Mintun, D.M. Skovronsky, and M.J. Pontecorvo are employees of Avid, a division of Eli Lilly, and formerly held Avid stock or options. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Aisen PS, Andrieu S, Sampaio C, et al. Report of the task force on designing clinical trials in early (predementia) AD. Neurology 2011;76:280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiner MW, Aisen PS, Jack CR, Jr, et al. Alzheimer's Disease Neuroimaging Initiative: progress report and future plans. Alzheimer Dement 2010;6:202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging 1997;18:S1–S2. [PubMed] [Google Scholar]
- 5. Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 2009;106:6820–6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 7. Nordberg A, Rinne JO, Kadir A, Långström B. The use of PET in Alzheimer disease. Nat Rev Neurol 2010;6:78–87. [DOI] [PubMed] [Google Scholar]
- 8. Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009;66:1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PiB PET study. Neurology 2009;73:754–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jack CR, Jr, Wiste HJ, Vemuri P, et al. , Alzheimer's Disease Neuroimaging Initiative. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain 2010;133:3336–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koivunen J, Scheinin N, Virta JR, et al. Amyloid PET imaging in patients with mild cognitive impairment: a 2-year follow-up study. Neurology 2011;76:1085–1090. [DOI] [PubMed] [Google Scholar]
- 12. Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol 2011;69:181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Villemagne VL, Pike KE, Darby D, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia 2008;46:1688–1697. [DOI] [PubMed] [Google Scholar]
- 14. Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol 2009;65:557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B. Arch Neurol 2009;66:1476–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-β measured by [11C]PiB. Neurology 2010;74:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pontecorvo M, Mintun M. PET amyloid imaging as a tool for early diagnosis and identifying patients at risk for progression to Alzheimer's disease. Alzheimers Res Ther 2011;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi SR, Golding G, Zhuang Z, et al. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med 2009;50:1887–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clark CM, Schneider JA, Bedell BJ, et al. , AV45-A07 Study Group. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011;305:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barthel H, Gertz HJ, Dresel S, et al. , for the Florbetaben Study Group. Cerebral amyloid-β PET with florbetaben (18)F in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 2011;10:424–435. [DOI] [PubMed] [Google Scholar]
- 21. Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid beta in Alzheimer's disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol 2008;7:129–135. [DOI] [PubMed] [Google Scholar]
- 22. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group on Alzheimer's disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 23. Fleisher AS, Chen K, Liu X, et al. Using positron emission tomography and florbetapir F 18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol Epub 2011 Jul 11. [DOI] [PubMed] [Google Scholar]
- 24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. JR Stat Soc B 1995;57:289–300. [Google Scholar]
- 25. Rowe CC, Ellis KA, Rimajovae M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010;31:1275–1283. [DOI] [PubMed] [Google Scholar]
- 26. Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's Disease Neuroimaging Initiative: The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimer Dement 2010;6:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amyvid (florbetapir F 18 injection) [product insert]. Indianapolis: Eli Lilly & Co; April 2012. [Google Scholar]
- 30. Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 2010;9:363–372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.