Abstract
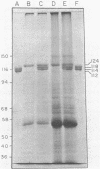
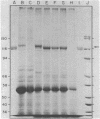
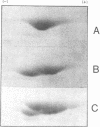
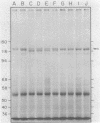
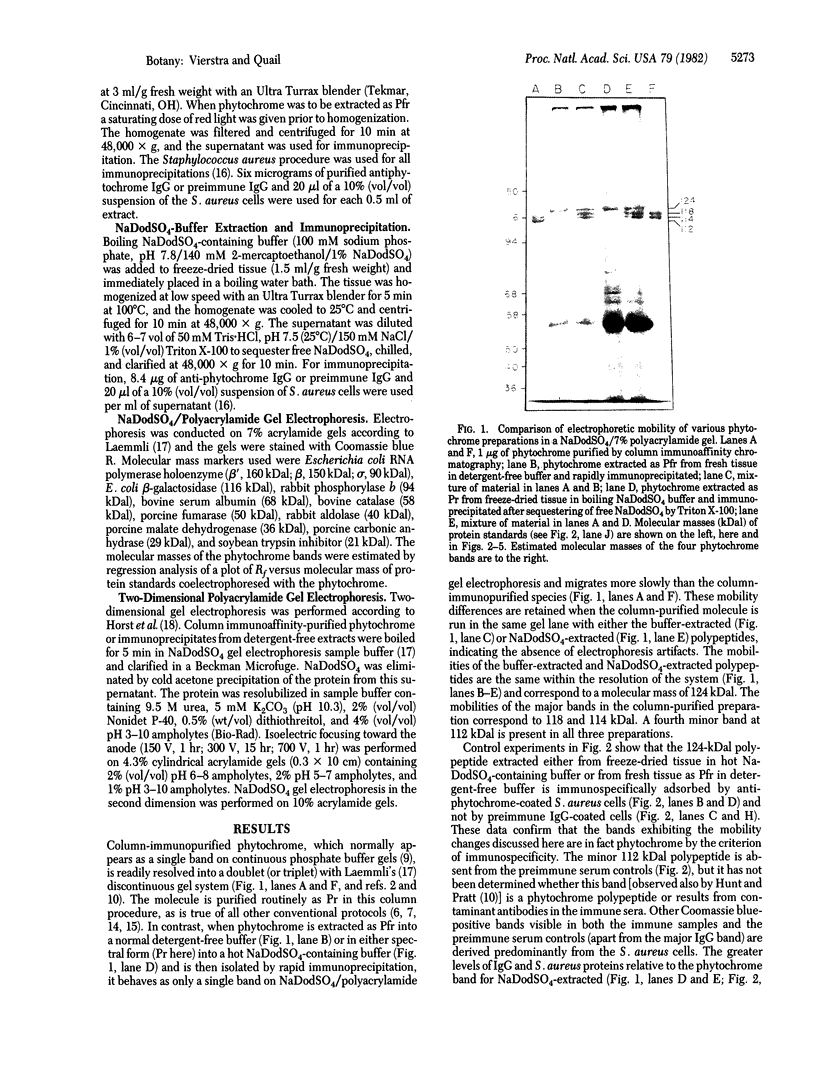
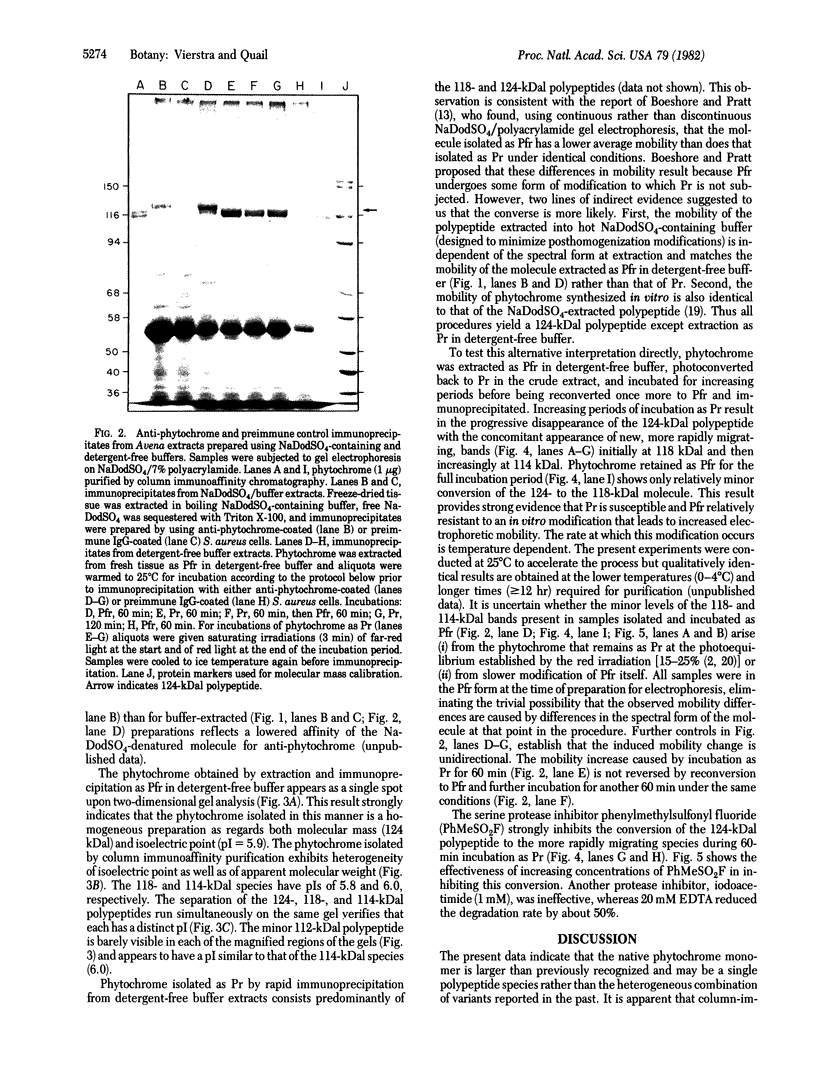
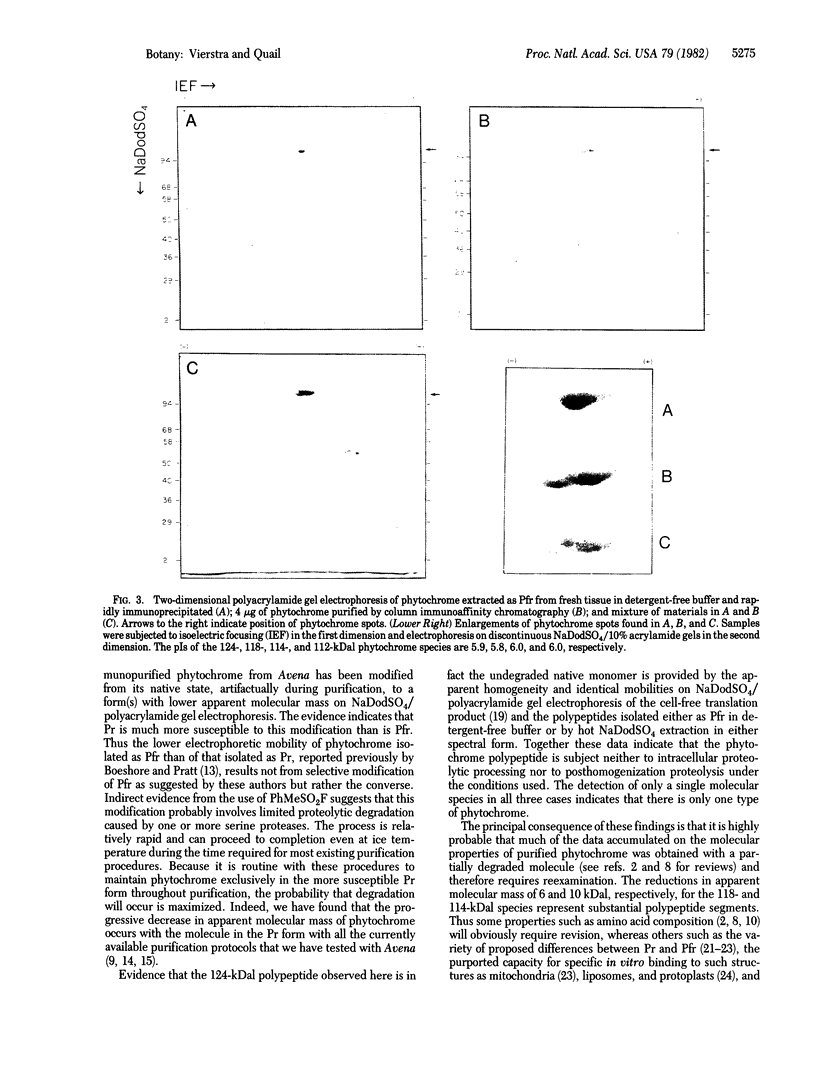
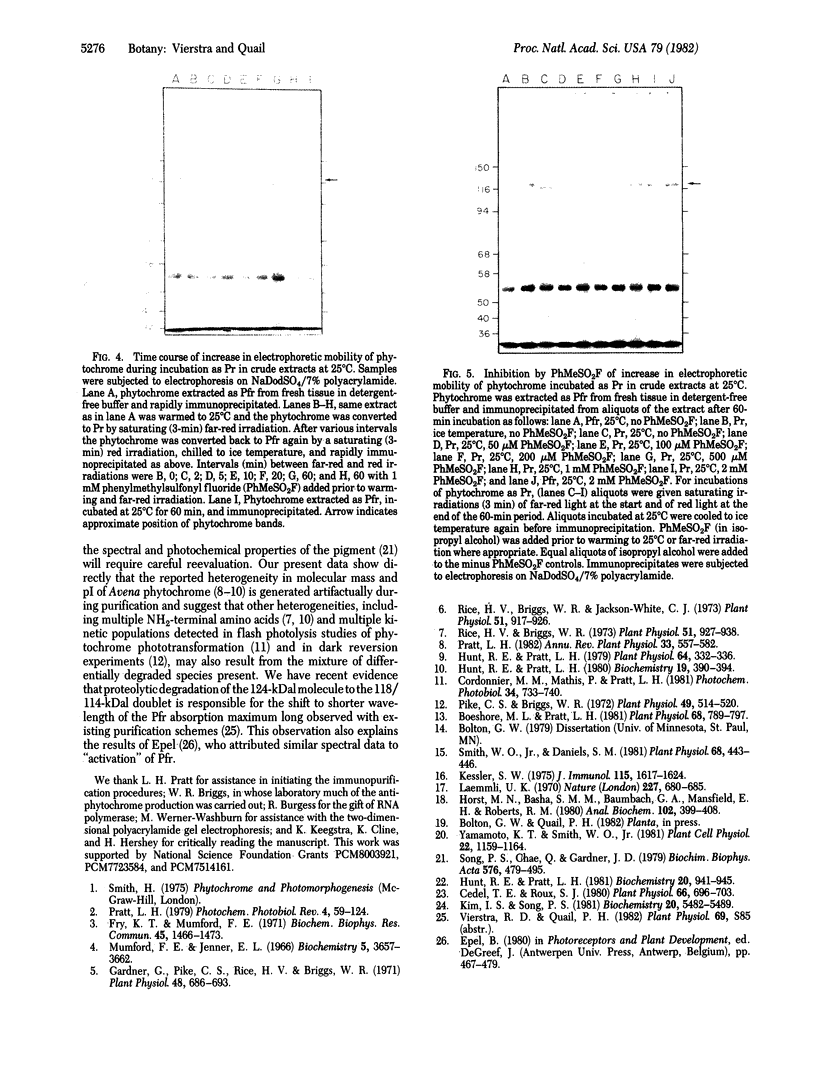
Phytochrome purified from Avena as the red-absorbing form, Pr, by an established immunoaffinity column procedure is heterogeneous. Two major polypeptides and one minor polypeptide with apparent molecular masses of 118, 114, and 112 kilodaltons (kDal), respectively, are observed on NaDodSO4/polyacrylamide gel electrophoresis. In contrast, only a single band of 124 kDal is obtained when phytochrome is rapidly immunoprecipitated after extraction either (i) as the far-red absorbing form, Pfr, in detergent-free buffer or (ii) in either spectral form in a 100°C NaDodSO4-containing buffer. On two-dimensional gel electrophoresis the three column-purified species have pIs of 5.8, 6.0, and 6.0, whereas 124-kDal phytochrome is a single spot with a pI of 5.9. Incubation as Pr in extracts causes progressive conversion of the 124-kDal polypeptide to the 118- and 114-kDal species. This process is inhibited by phenylmethylsulfonyl fluoride, suggesting that Pr is susceptible and Pfr resistant to limited proteolysis during extraction. These data, and the fact that the cell-free translation product of phytochrome mRNA is also 124 kDal [Bolton, G. W. & Quail, P. H. (1982) Planta, in press], indicate that the native monomer from Avena is a single species of 124 kDal. Thus the heterogeneous preparations of slightly lower molecular weight (“large” or “120-kilodalton” phytochrome) previously extensively characterized appear to have consisted of a mixture of partially degraded molecules that have undergone limited proteolysis during purification as Pr, as is established practice. A reexamination of the molecular properties of phytochrome appears necessary.
Keywords: hot NaDodSO4 extraction, protease inhibitors, solid-phase immunoprecipitation, one- and two-dimensional gel electrophoresis
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeshore M. L., Pratt L. H. Characterization of a molecular modification of phytochrome that is associated with its conversion to the far-red-absorbing form. Plant Physiol. 1981 Oct;68(4):789–797. doi: 10.1104/pp.68.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedel T. E. Further characterization of the in vitro binding of phytochrome to a membrane fraction enriched for mitochondria. Plant Physiol. 1980 Oct;66(4):696–703. doi: 10.1104/pp.66.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry K. T., Mumford F. E. Isolation and partial characterization of a chromophore-peptide fragment from pepsin digests of phytochrome. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1466–1473. doi: 10.1016/0006-291x(71)90185-9. [DOI] [PubMed] [Google Scholar]
- Gardner G., Pike C. S., Rice H. V., Briggs W. R. "Disaggregation" of phytochrome in vitro-a consequence of proteolysis. Plant Physiol. 1971 Dec;48(6):686–693. doi: 10.1104/pp.48.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst M. N., Basha S. M., Baumbach G. A., Mansfield E. H., Roberts R. M. Alkaline urea solubilization, two-dimensional electrophoresis and lectin staining of mammalian cell plasma membrane and plant seed proteins. Anal Biochem. 1980 Mar 1;102(2):399–408. doi: 10.1016/0003-2697(80)90174-8. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Partial characterization of undegraded oat phytochrome. Biochemistry. 1980 Jan 22;19(2):390–394. doi: 10.1021/bi00543a022. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Physicochemical differences between the red- and the far-red-absorbing forms of phytochrome. Biochemistry. 1981 Feb 17;20(4):941–945. doi: 10.1021/bi00507a046. [DOI] [PubMed] [Google Scholar]
- Hunt R. E., Pratt L. H. Phytochrome immunoaffinity purification. Plant Physiol. 1979 Aug;64(2):332–336. doi: 10.1104/pp.64.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kim I. S., Song P. S. Binding of phytochrome to liposomes and protoplasts. Biochemistry. 1981 Sep 15;20(19):5482–5489. doi: 10.1021/bi00522a021. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mumford F. E., Jenner E. L. Purification and characterization of phytochrome from oat seedlings. Biochemistry. 1966 Nov;5(11):3657–3662. doi: 10.1021/bi00875a039. [DOI] [PubMed] [Google Scholar]
- Pike C. S., Briggs W. R. The dark reactions of rye phytochrome in vivo and in vitro. Plant Physiol. 1972 Apr;49(4):514–520. doi: 10.1104/pp.49.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R., Jackson-White C. J. Purification of oat and rye phytochrome. Plant Physiol. 1973 May;51(5):917–926. doi: 10.1104/pp.51.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice H. V., Briggs W. R. Partial characterization of oat and rye phytochrome. Plant Physiol. 1973 May;51(5):927–938. doi: 10.1104/pp.51.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. O., Daniels S. M. Purification of Phytochrome by Affinity Chromatography on Agarose-Immobilized Cibacron Blue 3GA. Plant Physiol. 1981 Aug;68(2):443–446. doi: 10.1104/pp.68.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P. S., Chae Q., Gardner J. D. Spectroscopic properties and chromophore conformations of the photomorphogenic receptor: phytochrome. Biochim Biophys Acta. 1979 Feb 26;576(2):479–495. doi: 10.1016/0005-2795(79)90423-9. [DOI] [PubMed] [Google Scholar]