Abstract
Occult breast cancer is a type of breast cancer without any symptoms on the breasts or any abnormalities upon radiologic examination such as mammography. In males, there are few cases of breast cancer, the rate of diagnosis of occult breast cancer is very low, and little is known about this disease. We experienced two cases of occult breast cancers manifesting as axillary lymph node metastasis in men. They had a palpable lesion on axillary area several years ago and had not seen a doctor about it. As such there was no abnormality on evaluations for cancer except for axillary lymph node showing signs of carcinoma (primary or metastatic) on biopsy and estrogen receptor-positive and progesterone receptor-positive on immunohistochemistry. The patients were diagnosed with occult breast cancer, and treatments were performed. Herein, we report the rare cases of occult breast cancers in men.
Keywords: Axilla, Breast neoplasms, Male, Neoplasm metastasis, Occult primary neoplasms
INTRODUCTION
Male breast cancer, a very rare form of cancer in males, accounts for less than 1% of all breast cancers worldwide and less than 0.6% in Korea [1,2]. Most of the male breast cancers, which form on the subareolar area, appear as a non-painful and firm subareolar mass. Most of the symptoms occur on the breast, and include nipple discharge, nipple retraction, ulceration of the nipple or skin, and, in rare cases, Paget disease [3]. In this study, cases are reported of a type of male occult breast cancer that exhibited axillary lymph node metastasis without any associated symptoms on the breasts.
CASE REPORT
Case 1
The patient, a 59-year-old male, discovered a palpable mass on the right axillary area 10 years ago, but he did not consult a doctor for treatment. Two years ago, positron emission tomography (PET) for medical check-up revealed a lesion with increased uptake on the right axillary area of his breast. The patient had not followed up the lesion until a month ago, when he underwent an excisional biopsy at another hospital due to an increase in the size of the mass. The biopsy, followed by histology, showed signs of carcinoma (primary or metastatic), and the patient visited our hospital for further evaluation. The patient had no history of disease other than an appendectomy 40 years ago. He had a 21-pack-years smoking history, and his father had died of gastric cancer. A mildly tender palpable node was detected during a physical examination, and no symptom was found on the breast. A pigmented nevi of less than 1 cm size was found on the left auricular area and the right anterior chest of the patient. A review of the results of the histopathology of the patient from the other hospital revealed signs of metastatic carcinoma, thought to have originated from the breast. In addition, the patient's immunohistochemistry showed the following signs: estrogen receptor (+, Allred score 7), progesterone receptor (+, Allred score 6), c-erbB-2 (1+), focally positive BRST-2, and S-100 (-). Only the right axillary metastatic lymph node was found without any suspicious lesion on the breast and any accessory or ectopic breast in the mammography, breast ultrasonography (US), breast magnetic resonance imaging (MRI), and PET that were performed in our hospital (Figure 1). No sign of malignancy was observed in the chest-computed tomography (CT), ostiomeatal unit CT, abdominal US, esophagogastroduodenoscopy (EGD), and colonoscopy. All the tumor markers, such as serum AFP, CEA, PSA, CA 19-9, and CA 15-3, were within the normal ranges. A biopsy followed by histopathology was performed on the pigmented nevi that were found on the left auricular area and the right anterior chest. Signs of an intradermal nevus were found, but there were no signs of skin cancer such as melanoma. Based on all these test results, the patient was diagnosed with occult breast cancer, and a right breast skin-sparing mastectomy with axillary lymph node dissection was performed. During the surgery, no abnormality was found on the breast, but two to three 1 cm sized lymph nodes were found at the axillary level I, which were suspected to have been metastatic. The histopathology after the surgery revealed only a fibroadipose tissue without a malignant lesion on the breast and an accessory breast tissue. Two of the 29 dissected axillary lymph nodes showed signs of metastasis (Figure 2). The patient was discharged without any complication after the surgery, and subsequently underwent adjuvant chemotherapy (doxorubicin and cyclophosphamide, 4 cycles+docetaxel, 4 cycles). The patient is currently on tamoxifen (20 mg/day) and is being followed up in outpatient visits without any signs of recurrence.
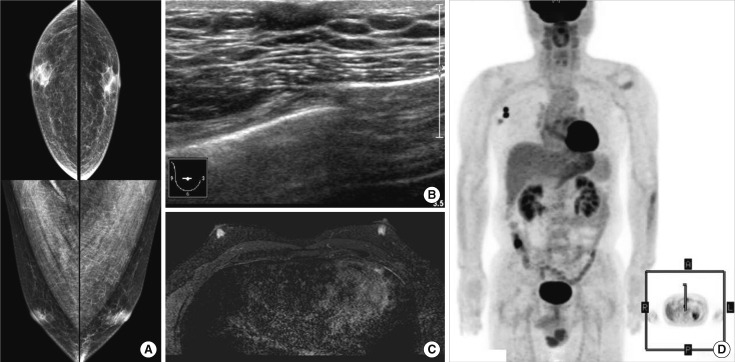
Figure 1.
Mammography (A), breast ultrasound (B), and magnetic resonance imaging scans (C) do not show abnormalities in the breast. Positron emission tomography (D) shows malignant lymphadenopathy in right axilla without other suspicious malignant lesion.
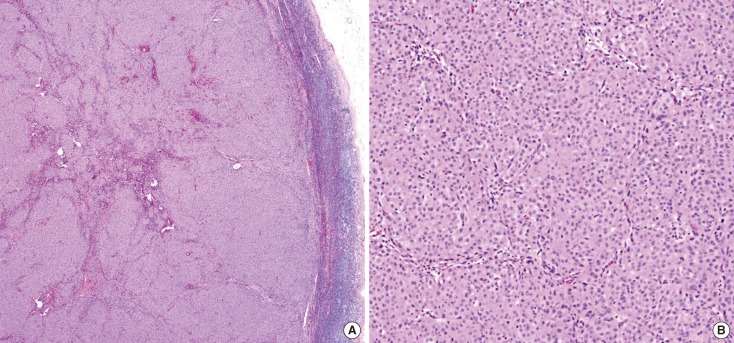
Figure 2.
Case 1. Histopathologic examination of the lymph node specimen. It identify these as metastatic carcinoma in H&E stain (A, ×50; B, ×100).
Case 2
A 45-year-old patient, who had discovered that he had a left axillary palpable lesion 2 years ago and had not seen a doctor about it, visited our hospital for further evaluation after an abnormality was found in his left axillary excisional biopsy 2 weeks before the visit. The patient had no history of cancer or other diseases, and had no family member who had been diagnosed with cancer. The patient was a non-smoker and was only a social drinker. In a physical examination, no abnormality other than a wound from the excisional biopsy was found in the left axilla, and no mass or suspicious skin change was detected on the breast. A review of the results of the histopathological examination performed at another hospital revealed a sign of adenocarcinoma, and the immunohistochemistry showed the following signs: TTF-1 (-), CK20 (-), and CEA (+). In the mammography, breast US, and breast MRI exams, no suspicious lesions on the breast or signs of lymphadenopathy on the axilla were observed. In addition, there was no evidence of accessory breast or ectopic breast on the axilla. The PET, chest CT, abdominal CT, and EGD exams, which were performed to determine the origin of the carcinoma, did not reveal any malignant lesion. In the right thyroid lobe, the thyroid US revealed a 0.5 cm-big hypoechoic nodular lesion that was suspected to have been malignant, but the fine needle aspiration cytology confirmed that the lesion was not malignant. In addition, all the tumor markers, such as serum CEA, PSA, CA 19-9, CA 15-3, and calcitonin, were within normal ranges. Based on all these results, the patient was diagnosed with occult breast cancer. Left axillary lymph node dissection (levels I and II) was performed on the patient, but not mastectomy. The post-surgery pathology revealed that four of the 26 dissected lymph nodes had signs of metastatic adenocarcinoma, and the immunohistochemistry showed the following results: estrogen receptor (+, Allred score 7), progesterone receptor (+, Allred score 7), c-erbB-2 (-), Ki-67 (1+), CK5/6 (-), epidermal growth factor receptor (-), and BRST-2 (+) (Figure 3). The patient was discharged without any complications after the surgery and subsequently underwent adjuvant radiation therapy on his remaining breast and adjuvant chemotherapy (doxorubicin and cyclophosphamide, 4 cycles+docetaxel, 4 cycles). He is currently on tamoxifen after adjuvant chemotherapy, and is being followed up in outpatient visits without any signs of recurrence.
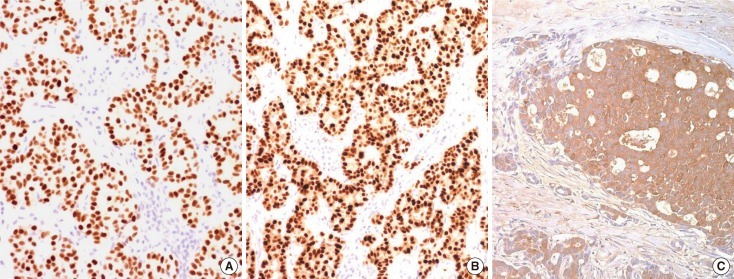
Figure 3.
Case 2. Histopathologic examination of the lymph node specimen (immunohistochemical staining, ×200). It shows positive expression of estrogen receptor (A), progesterone receptor (B), and BRST-2 (C) in immunohistochemistry stain.
DISCUSSION
Occult breast cancer is a type of breast cancer without any symptoms in the breasts (no cancer lesion is found in the breasts in a physical examination or radiologic examination such as mammography). The frequency of diagnosis of occult breast cancer is decreasing with the increase in the quality of mammography, breast US, and breast MRI. It has been reported that about 0.2% to 0.9% of breast cancer cases involve occult breast cancer in females [4-6]. In males, however, among whom there are few cases of breast cancer, the rate of diagnosis of occult breast cancer is very low, and little is known about this disease. Because occult breast cancer does not usually show any symptoms on the breasts, it is often discovered from symptoms of lymph node metastasis in the axillary area, supraclavicular fossa, or infraclavicular fossa [7]. The two male patients in this study were also diagnosed after multiple tests due to the presence of a mass in their axillary area, without any symptoms on their breasts. Unlike in other cases of an occult breast cancer, wherein the breast US, breast MRI, or final pathology after the mastectomy reveals malignant lesions on the breasts, no malignant lesion was detected on the breasts in these two cases. So far, this type of occult breast cancer has not been reported in Korea.
When only metastatic lesions are found without an original lesion, they are defined as carcinoma of unknown primary (CUP). CUP accounts for 3% to 5% of solid tumors. When CUP is found in the form of metastasis in an axillary lymph node, it is difficult to determine if occult breast cancer caused the axillary metastatic lymph nodes, even though axillary lymph node metastasis is known to be highly relevant to breast cancer. In addition to breast cancer, various other malignancies, including lymphoma, melanoma, lung carcinoma, thyroid cancer, and digestive tract cancers (such as pancreatic cancer, stomach cancer, and colon cancer), can metastasize to axillary lymph nodes [8]. For proper treatment, these malignant diseases must be ruled out and an accurate diagnosis should be made via PET, chest CT, abdomen CT, upper and lower gastrointestinal endoscopy, and multiple tumor markers.
It is well-known that breast cancer and hormonal receptors (estrogen and progesterone) are related, and the existence of hormonal receptors is used to assess the effectiveness of adjuvant hormonal therapy as a treatment method and to evaluate the prognosis. It has also been reported that the assessment of hormonal receptors via immunohistochemistry (IHC) helps in the diagnosis of occult breast cancer, where no abnormality is detected in tests for other malignant cancers that can metastasize to axillary lymph nodes [9]. In male breast cancer, it is known that estrogen receptors (ER) are expressed in more than 90% of cases and that progesterone receptors (PR) are expressed in more than 80% of cases [10]. Therefore, the presence of both ER and PR plays a crucial role in determining if the primary disease behind the axillary metastatic lymph node is breast cancer. The presence of ER and PR does not confirm breast cancer, however, and other cancers that can metastasize to axillary lymph nodes should be ruled out. In addition, even if both ER and PR are negative, breast cancer cannot be eliminated as a possible cause. In a case like this, it is helpful to check the other IHC results (such as c-erbB-2, proliferating cell nuclear antigen, multidrug-resistance protein, p53, Bcl-2 and nm23 protein) for diagnosis of breast cancer [11].
A standard surgical treatment for male breast cancer is modified radical mastectomy. As a form of adjuvant treatment, chemotherapy is known to improve the survival rate of patients with metastatic lymph nodes, and tamoxifen therapy has also been reported to improve their survival rate [10]. Data collected from male breast cancer patients suggest that adjuvant radiation therapy, used to treat female post-surgery patients, could be effective for male patients, even though no randomized clinical study has been conducted to prove the effectiveness of adjuvant radiation therapy for male breast cancer [10,12]. According to a study that analyzed different stages of breast cancer, there was no gender difference in the prognosis. Therefore, male breast cancer is treated in the same way as female breast cancer [13]. Until date, however, there has been no report, on the treatment methods for male occult breast cancer. It has been suggested that occult breast cancer with axillary metastasis should be treated in the same way as breast cancer with a comparable nodal stage is treated [6]. According to a report that analyzed 24 studies on axillary nodal metastasis from CUP, 321 of 446 patients (72%) who underwent mastectomy showed pathological signs of occult breast cancer, which suggests that mastectomy is an effective diagnostic and therapeutic modality [14]. On the contrary, other studies reported that there was no difference in the survival rates of patients, who underwent mastectomy and patients, who preserved their breasts, which suggests that mastectomy is not necessary. These studies suggest that breast preservation therapy including adjuvant radiation is an effective treatment [4,5,15].
The assessment of hormonal receptors and human epidermal receptors (HER2) via IHC is useful for differential diagnosis of breast cancer when a lesion is observed as a mass in the axillary area and is determined to be metastatic, but only when a primary lesion could not be confirmed. In the two cases reported in this study, one case was treated with mastectomy, and the other case was treated with adjuvant radiation therapy. Because little is known about surgery on occult breast cancer, it is suggested that the surgical method be chosen for each patient. If the breast preservation method is chosen, patients should be treated with adjuvant radiation therapy. In addition, it is thought that adjuvant chemotherapy and adjuvant hormonal therapies, which are used for breast cancer with node metastasis, can effectively treat occult breast cancer.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Sasco AJ, Lowenfels AB, Pasker-de Jong P. Review article: epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer. 1993;53:538–549. doi: 10.1002/ijc.2910530403. [DOI] [PubMed] [Google Scholar]
- 2.The Korean Breast Cancer Society. Survival analysis of Korean breast cancer patients diagnosed between 1993 and 2002 in Korea: a nationwide study of the cancer registry. J Breast Cancer. 2006;9:214–229. [Google Scholar]
- 3.Donegan WL, Redlich PN. Breast cancer in men. Surg Clin North Am. 1996;76:343–363. doi: 10.1016/s0039-6109(05)70443-6. [DOI] [PubMed] [Google Scholar]
- 4.Vilcoq JR, Calle R, Ferme F, Veith F. Conservative treatment of axillary adenopathy due to probable subclinical breast cancer. Arch Surg. 1982;117:1136–1138. doi: 10.1001/archsurg.1982.01380330004002. [DOI] [PubMed] [Google Scholar]
- 5.Baron PL, Moore MP, Kinne DW, Candela FC, Osborne MP, Petrek JA. Occult breast cancer presenting with axillary metastases. Updated management. Arch Surg. 1990;125:210–214. doi: 10.1001/archsurg.1990.01410140088014. [DOI] [PubMed] [Google Scholar]
- 6.Ellerbroek N, Holmes F, Singletary E, Evans H, Oswald M, McNeese M. Treatment of patients with isolated axillary nodal metastases from an occult primary carcinoma consistent with breast origin. Cancer. 1990;66:1461–1467. doi: 10.1002/1097-0142(19901001)66:7<1461::aid-cncr2820660704>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Burga AM, Fadare O, Lininger RA, Tavassoli FA. Invasive carcinomas of the male breast: a morphologic study of the distribution of histologic subtypes and metastatic patterns in 778 cases. Virchows Arch. 2006;449:507–512. doi: 10.1007/s00428-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copeland EM, McBride CM. Axillary metastases from unknown primary sites. Ann Surg. 1973;178:25–27. doi: 10.1097/00000658-197307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan PH, Sng IT. Male breast cancer: a retrospective study with immunohistochemical analysis of hormone receptor expression. Pathology. 1997;29:2–6. doi: 10.1080/00313029700169444. [DOI] [PubMed] [Google Scholar]
- 10.Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005;10:471–479. doi: 10.1634/theoncologist.10-7-471. [DOI] [PubMed] [Google Scholar]
- 11.Gu GL, Wang SL, Wei XM, Ren L, Zou FX. Axillary metastasis as the first manifestation of male breast cancer: a case report. Cases J. 2008;1:285. doi: 10.1186/1757-1626-1-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakravarthy A, Kim CR. Post-mastectomy radiation in male breast cancer. Radiother Oncol. 2002;65:99–103. doi: 10.1016/s0167-8140(02)00210-4. [DOI] [PubMed] [Google Scholar]
- 13.Borgen PI, Senie RT, McKinnon WM, Rosen PP. Carcinoma of the male breast: analysis of prognosis compared with matched female patients. Ann Surg Oncol. 1997;4:385–388. doi: 10.1007/BF02305550. [DOI] [PubMed] [Google Scholar]
- 14.Pentheroudakis G, Lazaridis G, Pavlidis N. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): a systematic review of published evidence. Breast Cancer Res Treat. 2010;119:1–11. doi: 10.1007/s10549-009-0554-3. [DOI] [PubMed] [Google Scholar]
- 15.Merson M, Andreola S, Galimberti V, Bufalino R, Marchini S, Veronesi U. Breast carcinoma presenting as axillary metastases without evidence of a primary tumor. Cancer. 1992;70:504–508. doi: 10.1002/1097-0142(19920715)70:2<504::aid-cncr2820700221>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]