Abstract
The hepatocyte growth factor (HGF/SF) receptor, Met, regulates mitogenesis, motility, and morphogenesis in a cell type-dependent fashion. Activation of Met via autocrine, paracrine, or mutational mechanisms can lead to tumorigenesis and metastasis and numerous studies have linked inappropriate expression of this ligand-receptor pair to most types of human solid tumors. To prepare mAbs to human HGF/SF, mice were immunized with native and denatured preparations of the ligand. Recloned mAbs were tested in vitro for blocking activity against scattering and branching morphogenesis. Our results show that no single mAb was capable of neutralizing the in vitro activity of HGF/SF, and that the ligand possesses a minimum of three epitopes that must be blocked to prevent Met tyrosine kinase activation. In vivo, the neutralizing mAb combination inhibited s.c. growth in athymic nu/nu mice of tumors dependent on an autocrine Met-HGF/SF loop. Importantly, growth of human glioblastoma multiforme xenografts expressing Met and HGF/SF were markedly reduced in the presence of HGF/SF-neutralizing mAbs. These results suggest interrupting autocrine and/or paracrine Met-HGF/SF signaling in tumors dependent on this pathway is a possible intervention strategy.
Hepatocyte growth factor/scatter factor (HGF/SF) is a multifunctional heterodimeric polypeptide produced by mesenchymal cells, and an effector of cells expressing the Met tyrosine kinase receptor (1, 2). HGF/SF has been shown to mediate the growth and scattering of various cell types, to mediate epithelial mesenchymal transition (3) and the formation of tubules and lumens (4, 5), and to promote angiogenesis (6, 7). In vivo, this ligand-receptor pair is believed to play a role in neural induction (8), liver regeneration, wound healing (9, 10), and normal embryological development. Both Met and HGF/SF knockout mice are embryonic lethal and show developmental defects in the placenta, fetal liver, limb/muscle formation, etc. (11, 12).
In addition to the role of HGF/SF-Met signaling in development and homeostasis, this ligand receptor has also been shown to be uniquely involved in most human solid tumors (13) and to participate in tumor development, invasion, and metastasis (14). Met was originally isolated as the product of the human oncogene, tpr-met, which encodes an altered Met protein possessing constitutive, ligand-independent tyrosine kinase activity and transforming ability (15). Acting through Met, HGF/SF plays multifunctional roles on different target cells by autocrine or paracrine mechanisms, and HGF/SF-transgenic mice develop a remarkably broad array of histologically distinct tumors of both mesenchymal and epithelial origin (16). Met activation has also been shown to markedly enhance the metastatic spread of cancer stemming from its stimulatory influence of processes such as angiogenesis (6, 7), cell motility (17), and cell surface protease regulation (18, 19). HGF/SF has been shown to induce invasiveness in vitro in various types of tumor cells (18, 20, 21). Neovascularization is also essential for the growth of solid tumors, and tumor cells are thought to secrete angiogenic factors that promote neovascularization. HGF/SF is angiogenic and along with basic fibroblast growth factor has been shown to stimulate blood vessel formation directly or mediate angiogenesis through paracrine induction of vascular endothelial growth factor (VEGF) (22). HGF/SF also displays antiapoptotic activity (23–25) and is reported to be involved in other diseases such as cardiovascular disease and Alzheimer's disease (26, 27).
Malignant gliomas are extremely aggressive solid tumors, and their poor prognosis is linked to their ability to induce vascular proliferation and to invade surrounding brain. Both Met and HGF/SF are expressed in glioblastoma multiforme (GBM) (28), and ectopic expression of HGF/SF in GBM enhances both tumor growth and angiogenesis in vivo (29). Moreover, HGF/SF biological activity inhibited by a chimeric U1 small nuclear RNA/ribozyme (30) or by the NK2 antagonist of HGF/SF interferes with GBM growth (31).
A rabbit polyclonal Ab can neutralize HGF/SF (28, 32), but there is no single mAb able to significantly inhibit all of the biological activities of HGF/SF. Here, we describe the generation of neutralizing mAbs to HGF/SF. We have determined that at low IgG concentration, a minimum of three mAbs used in combination are required to inhibit the Met-HGF/SF signaling pathway in vitro. Importantly, growth in athymic nu/nu mice of a human GBM tumor xenograft autocrine for Met and HGF/SF is markedly inhibited with the neutralizing combination of mAbs.
Materials and Methods
Cell Lines.
Madin–Darby canine kidney (MDCK) cells were cultured in DMEM supplemented with 5% FBS. S-114 cells (NIH 3T3 cells transformed with human HGF/SF and Met; ref. 33) were grown in DMEM containing 8% of calf serum. SK-LMS-1 human leiomyosarcoma cell line (19) was maintained in DMEM containing 10% FBS. C-127 cells transformed with human HGF/SF and mouse Met (20), and U-118 cells (a human glioma cell line autocrine for endogenous HGF/SF and Met; ref. 28), were maintained in DMEM supplemented with 10% FBS. All cell lines were cultured at 37°C with 5% CO2.
Neutralizing mAb Production.
HGF/SF was prepared from S-114 cells and mouse mAbs against the ligand were produced by injecting BALB/c mice i.p. with purified native and denatured (boiling in SDS sample buffer) HGF/SF protein in complete Freund's adjuvant, followed by four additional injections in incomplete Freund's adjuvant. After 1 mo, a final HGF/SF injection was given i.p. and i.v. without adjuvant. Polyclonal antisera from immunized mice were tested for neutralizing activity of HGF/SF in the MDCK cell scatter assay. Spleen cells were fused with P3X63AF8/653 myeloma cells using standard techniques 3 days after the final injection. Rabbit polyclonal Ab to HGF/SF was used as positive control (28).
ELISA Screening.
Hybridoma cells were screened for reactivity to HGF/SF by ELISA using 96-well plates coated with 2.5 μg/ml HGF/SF in coating buffer (0.2 M Na2CO3/NaHCO3, pH 9.6; 50 μl per well) overnight at 4°C. The plates were then blocked with PBS containing 1% BSA (200 μl/well) overnight at 4°C. Fifty microliters of hybridoma supernatant were added to wells for 1.5 h at room temperature (RT). Plates were washed twice in washing buffer (PBS with 0.05% Tween 20), and alkaline phosphatase-coupled goat anti-mouse IgG (Sigma) was added (50 μl/well) at 1:3,000 dilution for 1.5 h at RT. After washing four times in washing buffer, phosphatase substrate CP-nitrophenyl phosphate (Kirkegaard & Perry Laboratories) was added for 30 min, and absorbance was measured at 405 nm. Hybridomas with strong reactivity with HGF/SF (OD value >0.5, negative controls <0.02) were recloned twice, and reactivity was again confirmed by ELISA.
HGF/SF Neutralization in the MDCK Scatter Assay.
Recloned hybridomas supernatants, either individually or in pools, were screened for neutralizing activity to HGF/SF using the MDCK cell scatter assay (34). Briefly, MDCK cells were plated at 7.5 × 104 cells per 100 μl per well with or without HGF (5 ng/well) in DMEM with 5% FBS. Three hundred microliters of supernatants (either individually or as pools), at 2-fold serial dilutions, were then added to 96-well plates. A rabbit polyclonal neutralizing antiserum (1 μl/well; ref. 28) was included as control. Following overnight incubation at 37°C, cells were then stained with 0.5% crystal violet in 50% ethanol (vol/vol) for 10 min at RT, and scattering was viewed using a light microscope. The IgGs were purified from the hybridoma cell lines showing the strongest neutralizing activity by using protein G columns and were adjusted to a final concentration of 2 mg/ml. The purified mAbs were assayed for neutralizing activity in the MDCK scatter assay.
Branching Morphogenesis Assay.
Branching morphogenesis assay using SK-LMS-1 cells was conducted as described (19). Briefly, cell suspensions were mixed with an equal volume of GFR-Matrigel (Becton Dickinson), plated at 5 × 104 cells per 125 μl per well in a 96-well culture plate, and incubated for 30 min at 37°C. HGF/SF, with or without neutralizing mAbs, was added along with DMEM containing 10% FBS on top of the gel. After 72–96 h of incubation at 37°C, representative wells were photographed at ×400 magnification.
HGF/SF Immunoprecipitation and Western Blotting.
S-114 cells were grown in serum-free medium for 48 h. The supernatant containing HGF/SF was collected and precleared with normal rabbit serum and protein G-Sepharose (Sigma). One milliliter of precleared supernatant was then immunoprecipitated with 1 μg of HGF/SF mAb and 20 μl of protein G-Sepharose, followed by immunoblotting as described (28). Briefly, the immune complexes were washed, and the proteins were separated by 10% SDS/PAGE gel and transferred onto poly(vinylidene difluoride) membrane (Bio-Rad). The membrane was blocked with PBS containing 1% BSA (Sigma) at 4°C overnight, incubated with rabbit polyclonal anti-HGF/SF at 1:4,000 dilution for 1.5 h at RT, washed in PBS with 0.05% Tween 20, and reacted with goat anti-rabbit IgG alkaline phosphatase conjugate (Sigma) at 1:10, 000 dilution for an additional 1.5 h at RT. Following the same washing, the proteins were detected with chemiluminescent substrate as suggested by the manufacturer (Bio-Rad).
mAb Inhibition of Tumor Growth in Athymic Nude Mice.
Animal experiments were performed using female athymic nude (nu/nu) mice at 6 weeks of age. mAb combinations (e.g., mAbs A-1, -5, and -7) prepared against native HGF/SF were compared with nonneutralizing mAbs prepared against denatured HGF/SF (e.g., mAbs 7-2 and -3).
C-127 cells expressing human HGF/SF and mouse Met (35) were trypsinized, washed twice, and resuspended in PBS prior to injection. Mice were divided into five groups of five mice per group, and each mouse was injected s.c. with the cell suspension (2 × 105 cells per 0.1 ml per mouse). The first day after cell injection, the animals were administered Abs (0.2 mg per 100 μl per animal). Group one animals were injected s.c. with mAbs A-1, -5, and -7. Group 2 animals were injected i.p. with the same mAb pool. Groups 3 and 4 were injected with a combination of mAbs 7-2, -3, and -4, either s.c. or i.p., respectively. Group 5 animals received C-127 tumor cells without Abs. The Ab injections were repeated every day for 20 days, and tumor size was measured twice a week. The final data were collected when the control group was killed due to tumor size.
Xenografts of the U-118 human GBM tumor cell line, expressing HGF/SF and Met (28), were produced by injecting 5 × 105 cells s.c. into seven mice per group. As in the C-127 studies above, neutralizing mAb combination A-1, -5, and -7 and nonneutralizing mAb combination 7-2 and -3 were injected twice a week (0.2 mg per 100 μl/animal) for 10 weeks after cell injection. An additional experiment was performed using the U-118 cell line to test the ability of the mAbs to interfere with the growth of established tumors. At 30 days after s.c. injection (5 × 105 cells per mouse), mice were divided into five groups (10 mice per group) with an average tumor size about 100 mm3. Neutralizing (A-1, -5, and -7) and nonneutralizing (7-2 and -3) mAb combinations were injected either s.c. (intratumor) or i.p. every 2 days (0.2 mg per 100 μl per mouse) for 10 weeks. Tumor size was measured twice a week, and animals were killed when the health of the animal was severely affected by the size of the tumor.
Results
Production of mAbs to HGF/SF.
We raised mAbs to both native and denatured forms of human HGF/SF. Sera from immunized mice were tested for neutralizing activity in the MDCK scatter assay. We found that only the sera from mice immunized with native HGF/SF inhibited scattering. After fusion, single clones of hybridoma cells positive by ELISA reactivity with HGF/SF were selected. mAbs prepared against native and denatured HGF/SF were tested individually for neutralizing activity in the MDCK scatter assay. No single mAb displayed neutralizing activity (data not shown). Because the sera from the mice immunized with native HGF/SF routinely displayed neutralizing activity (data not shown), we pooled mAb culture supernatants to test for neutralization activity against HGF/SF. One group of 10 pooled mAbs (A-1 to -10) showed strong neutralizing activity (Table 1).
Table 1.
In vitro neutralizing activity by mAbs to HGF/SF
| mAb combination* | mAbs: Human HGF/SF (molar ratio)
|
|
|---|---|---|
| MDCK cells scattering† | Branching morphogenesis‡ | |
| Pool A (10 mAbs) | 80:1 | ND |
| Pool B (10 mAbs) | Neg. | ND |
| Pool C (11 mAbs) | Neg. | ND |
| A-1, -4, -5, -7, and -10 | 24:1 | 10:1 |
| A-1, -4, -5, and -7 | 20:1 | 10:1 |
| A-1, -4, -5, and -10 | 20:1 | 10:1 |
| A-1, -4, -7, and -10 | 20:1 | 10:1 |
| A-1, -5, -7, and -10 | 20:1 | 10:1 |
| A-1, -4, and -5 | Neg. | Neg. |
| A-1, -4, and -7 | 60:1 | 40:1 |
| A-1, -4, and -10 | 60:1 | 20:1 |
| A-1, -5, and -7 | 30:1 | 10:1 |
| A-1, -5, and -10 | 30:1 | 10:1 |
| A-1, -7, and -10 | 240:1 | Neg. |
| A-4, -5, and -7 | Neg. | 40:1 |
| A-4, -5, and -10 | Neg. | 20:1 |
| A-4, -7, and -10 | Neg. | 40:1 |
| A-5, -7, and -10 | Neg. | 40:1 |
ND, not done; Neg., negative.
mAb isotyping: A-1, -4, -5, and -10 are IgG1/κ; A-7 is IgG2b/κ.
mAbs A-2, -3, -6, -8, and -9 did not contribute to neutralization.
Scatter assay: all mAbs were adjusted to a final concentration of 2.0 mg/ml in PBS, 2.5 μl of each mAb pool were added to the first well, and 2-fold diluted to well 12. The end point is the mAb concentration that prevents scattering at the highest dilution. HGF/SF is 20 ng/ml, 250 μl per well.
Branching morphogenesis assay; HGF/SF is 250 ng/ml, 200 μl per well. mAbs were 0.2, 0.4, 0.8, 1.6, and 3.2 μg per 200 μl; results were read and photographed after a 96-h incubation.
To further characterize the mAb A-1 to -10 pool, culture supernatants were produced individually, and mAbs were purified on a protein G column. Various combinations of mAbs were tested to determine which members of the pool contributed to neutralizing activity. We found that combinations of any of two mAbs of A-1 to -10 did not neutralize MDCK scattering, even when mAbs were used at molar ratios 1,000 times greater than HGF/SF (data not shown). However, when three or more mAbs were combined, seven different combinations were identified with neutralizing activity at molar ratios of <30-fold (Table 1). Combinations of four mAbs, which include A-1, and any three of mAbs A-4, -5, -7, or -10, had the highest neutralizing activity. However, combinations of mAb A-1, plus either A-4 or -5 and -7 or -10, also efficiently neutralized HGF/SF-mediated MDCK scatter activity with mAbs A-1, -5, and -7 and/or A-1, -5, and -10 showing the greatest activity (Table 1 and Fig. 1). The mAb series generated against denatured HGF/SF (7-2, -3, and -4) alone or in combination did not inhibit HGF-induced scattering (Fig. 1D and data not shown).
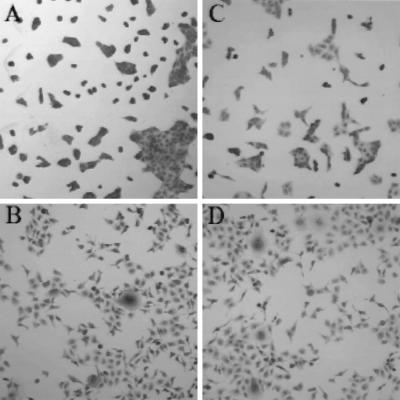
Figure 1.
Neutralization of HGF/SF-mediated MDCK cells scattering. (A) Control without any treatment. (B) Human HGF/SF. (C) Human HGF/SF plus mAbs A-1, -5, and -7. (D) Human HGF/SF plus mAbs 7-2, -3, and -4.
Branching Morphogenesis.
We tested the neutralizing activity of the mAbs in the HGF/SF-mediated branching morphogenesis assay using SK-LMS-1 cells. The mAb combinations A-1, -5, and -7 and A-1, -5, and -10 displayed the greatest inhibitory activity (Table 1). However, A-1 is not required for blocking HGF/SF-induced branching morphogenesis and combinations of mAbs A-4, -5, and -10 showed the highest neutralizing activity. These results suggest that a component(s) provided by the basement membrane Matrigel or the SK-LMS-1 cells abrogates the requirement for mAb A-1. The mAbs against denatured HGF 7-2 and -3 showed no neutralizing activity (data not shown).
Immunoprecipitation and Western Blot Analyses of mAbs to HGF/SF.
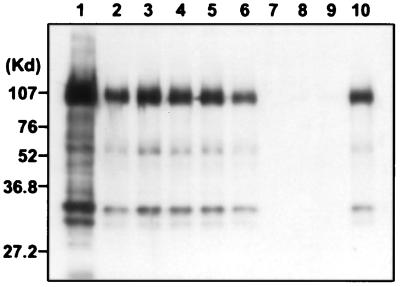
The neutralizing mAbs are also very effective for immunoprecipitating native HGF/SF from cell supernatants, whereas the mAbs raised against denatured HGF/SF are not (mAbs 7-2, -3, or -4; Fig. 2). The protein migrating at ≈90 kDa is uncleaved HGF/SF, and the smaller proteins represent the α, β-activated cleavage product. By contrast, the neutralizing mAbs do not recognize denatured HGF/SF on Western blot, whereas mAbs 7-2, -3, or -4 work well (data not shown).
Figure 2.
Immunoprecipitation of HGF/SF from S-114 cell culture supernatant by mAbs. Lane 1, positive control, purified human HGF/SF; lanes 2–6, mAbs A-1, -4, -5, -7, and -10; lanes 7–9, mAbs 7-2, -3, and -4; and lane 10, positive serum from mouse immunized with native HGF/SF. The molecular weight standards are shown on the Left.
Antitumor Activity of HGF/SF-Neutralizing mAbs.
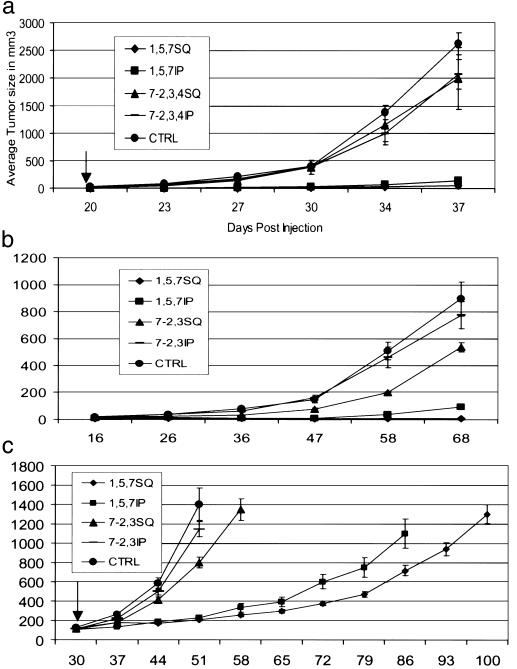
We have previously demonstrated that cells with autocrine Met-HGF/SF signaling are tumorigenetic and metastastic in nude mice (14, 18, 20). We have characterized several human tumor types that display autocrine HGF/SF-Met signaling, such as human osteosarcomas (32) and GBM (28). To determine whether the neutralizing mAb to human HGF/SF has any effect on tumors in vivo, we performed animal experiments with C-127 mouse cells created to express mouse Met and human HGF/SF in an autocrine fashion (20). These cells (2 × 105) when injected s.c. formed tumors in athymic nude mice in 2–3 weeks. Animals injected with C-127 tumor cells were also injected with pools of either mAbs A-1, -5, and -7, or 7-2, -3, and -4, either intratumorally or i.p. every day for 20 days. The experiment was terminated at 37 days after C-127 cell injection. Dramatically, the animals treated with the combination of mAbs A-1, -5, and -7 showed 90% inhibition of tumor growth with either s.c. or i.p. mAb injection compared with the controls (Fig. 3a).
Figure 3.
(a) Inhibition of C127 tumor growth by neutralizing mAb to HGF/SF. C-127 tumor cells were injected s.c. into athymic nude mice in PBS on day 0. Anti-HGF/SF mAbs A-1, -5, and -7 or mAb 7-2, -3, and -4 were administered either s.c. intratumor or i.p. every day for 20 days. One group of animals did not receive Ab. The values are an average of the size of five tumors in mm3. (b) Inhibition of U-118 glioblastoma tumor growth by neutralizing mAb to HGF/SF. U-118 human glioblastoma tumor cells were injected s.c. into athymic nude mice. On day 1, anti-HGF/SF mAbs A-1, -5, and -7 or mAbs 7-2 and -3 were administered either s.c. intratumor or i.p. twice a week for 10 weeks. One group of animals did not receive Ab. The values are an average size of six to seven tumors in mm3. (c) Tumor regression experiment using U-118 GBM cells. GBM cells were s.c. injected to athymic nude mice. After 30 days, animals were divided to five groups with average tumor size about 100 mm3. mAbs A-1, -5, and -7 or 7-2 and -3 were administered either s.c. intratumor or i.p. every 2 days until 10 weeks. One group of mice did not receive Ab. The values are an average size of eight to nine tumors in mm3.
Using the same procedure described above, we tested the mAb A-1, -5, and -7 combination in vivo in athymic nu/nu mice with the U-118 GBM tumor cell xenograft. Human GBM cell lines coexpress HGF/SF and Met, which is postulated to contribute to tumorigenesis. In this experiment, mAbs were injected twice in 1 week for approximately 7 weeks. Intratumor injection of the neutralizing mAb combination significantly inhibited tumor growth, whereas i.p. injection was less effective. Nevertheless, the average tumor size in the i.p. group was reduced compared with the controls (Fig. 3b). We also tested the mAb combination 30 days after the U-118 GBM tumor growth was initiated. A significant delay in tumor growth was observed in animals receiving mAb A-1, -5, and -7 beginning on day 30 and treated every other day for up to 70 days. Although tumor regression did not occur, tumor growth was delayed (Fig. 3c).
Discussion
Multiple biological activities of HGF/SF are associated with its unique structure. The ligand is composed of an α-chain containing the N-terminal domain and four kringle domains (NK4) covalently disulfide linked to a serine protease-like β-chain C-terminal domain. The N-terminal hairpin domain of HGF/SF, in combination with the first kringle domain (NK1), is able to bind the Met receptor and mediate partial activities even in the absence of the second kringle domain (NK2). NK1 shows agonistic or antagonistic activities depending on the assay system (36), whereas NK2 induces scattering, but not mitogenic activity (37). The α subunit of HGF/SF (NK4) also binds to Met, but abrogates the mitogenic, motogenic, and morphogenic activities of HGF/SF (38). The biological function of the β subunit of HGF/SF has not been well characterized. Although it does not bind to the receptor alone, it has been shown that this subunit is crucial for the optimum activation of Met receptor induced by HGF/SF (39). Therefore, the N-terminal and the kringle domains of the α-chain and the β-chain are all required for the full spectrum of biological activity of HGF/SF (40).
It has been shown that HGF/SF-induced biological activities can be inhibited with rabbit polyclonal anti-HGF/SF Ab requiring serum concentrations 500- to 1,000-fold greater than HGF/SF (28, 41, 42). By contrast, we are able to achieve HGF/SF neutralization with mAb combinations (e.g., A-1, -5, and -7) at molar ratios 20- to 30-fold greater than HGF/SF. Moreover, our results suggest that only certain mAbs that bind to specific epitopes of HGF/SF can inhibit biological activity, and that blocking three or more of the epitopes is required to inhibit HGF/SF activity. What are the characteristics of such Abs? Interestingly, none of the mAbs A-1, -4, -5, or -7 react with sulfhydryl detergent-denatured HGF/SF after Western blot analyses, indicating that the epitopes are complex in configuration. Moreover, only two mAbs, A-4 and A-5, share epitopes (M. Fivash, M. Medaglia, and R.J.F., unpublished data). We cannot exclude that a single mAb with neutralizing activity may yet be discovered. However, among the hundreds of mAbs derived from fusions from animals with HGF/SF-neutralizing serum, no single mAb displayed neutralizing activity. Although we have not determined the optimal concentration of each mAb in the combination, both our in vitro and in vivo results showed strong HGF/SF-neutralizing activity with equal molar concentrations of mAb A-1, -5, and -7.
mAb A-1 recognition site is located within the NK2 domain (data not shown). Even though recognition sites for other mAbs are yet to be mapped, our results show a minimum of two additional domains of HGF/SF must be important. mAbs A-7 and A-10 recognize unique epitopes outside of the NK2 domain (data not shown). Although we have not determined whether mAb binding to the β subunit is required for neutralizing HGF/SF, our results show that multiple ligand binding surfaces must be blocked to completely inhibit receptor activation. When any one epitope is vacant, receptor activation can take place and result in the induction of a partial, if not full, spectrum of biological effects. One possible interpretation is that ligand binding to any one site on the receptor causes a distortion in the remaining ligand epitope recognition sites displacing the mAb associated with the other sites. These results are consistent with the partial ligand activities observed with NK1 and NK2 (36, 43). Interestingly, the three-dimensional structure of fibroblast growth factor with its receptor reveals two ligand affinity sites and one heparin binding site. Heparin bridges the ligand to the receptor through adjacent affinity sites (44). Given the fact that HGF/SF also has high affinity to heparin (45) and that heparin is required for Met binding and signaling (46), the requirement for the three mAbs to neutralize HGF/SF may indicate that this ligand also has three binding sites, two to the Met receptor and one to heparin.
Neovascularization is a key process in the growth of solid tumors, and this process is regulated by angiogenic factors. Several growth factors have been identified that are involved in tumor angiogenesis such as basic fibroblast growth factor, VEGF, and HGF/SF (41, 42, 47). Studies have indicated that these growth factors can have synergistic angiogenic effects. It has been shown that HGF/SF has maximum potency for angiogenesis in the presence of basic fibroblast growth factor and VEGF (42). Progress has been made to use neutralizing Abs of these growth factors to block their angiogenic effect. Neutralizing mAbs to basic fibroblast growth factor and VEGF are able to inhibit tumor growth in vivo in animal models (48, 49), and anti-VEGF Abs are in Phase I clinical trials (50). However, there has been no report on using neutralizing mAb to human HGF/SF to study its potential antitumor effect. In this study, we demonstrate that HGF/SF-Met signaling-mediated tumor growth in vivo can be inhibited by administering neutralizing mAb combinations to HGF/SF. Moreover, the result of mAb-mediated inhibition of GBM growth in nude mice strongly suggests that xenograft growth of this tumor depends on autocrine Met-HGF/SF signaling.
The multiple biological functions of HGF/SF can be neutralized by addition of a combination of specific mAbs. In vivo, these mAbs are able to inhibit the growth of tumors dependent on HGF/SF-Met signaling in an autocrine and presumably paracrine fashion. These results suggest that interrupting autocrine and paracrine HGF/SF signaling may be a useful intervention strategy for treating a variety of human solid tumors.
Acknowledgments
We thank Maxine V. Medaglia and Dr. Matthew Fivash for sharing their unpublished results from Ab interference analysis using surface plasmon resonance spectroscopy. We also acknowledge Eric A. Hudson, Dr. James H. Resau, and Dr. Han-Mo Koo for technical assistance in the preliminary study, and Lynn Ritsema and Michelle Reed for administrative assistance. Finally, we thank Drs. Arthur Frankel, Itzhak Goldberg, J. Eliot Rosen, Jeff Rubin, and Joseph Schlessinger for critical review of this manuscript.
Abbreviations
- HGF/SF
hepatocyte growth factor
- VEGF
vascular endothelial growth factor
- GBM glioblastona multiforme
MDCK, Madin–Darby canine kidney
- RT
room temperature
References
- 1.Bottaro D P, Rubin J S, Faletto D L, Chan A M, Kmiecik T E, Vande Woude G F, Aaronson S A. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 2.Rubin J S, Bottaro D P, Aaronson S A. Biochim Biophys Acta. 1993;1155:357–371. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 3.Tsarfaty I, Rong S, Resau J H, Shen R, Pedro Pinto D S, Vande Woude G F. Science. 1994;263:98–101. doi: 10.1126/science.7505952. [DOI] [PubMed] [Google Scholar]
- 4.Montesano R, Matsumoto K, Nakamura T, Orci L. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 5.Tsarfaty I, Resau J H, Rulong S, Keydar I, Faletto D L, Vande Woude G F. Science. 1992;257:1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- 6.Rosen E M, Grant D S, Kleinman H K, Goldberg I D, Bhargava M M, Nickoloff B J, Kinsella J L, Polverini P. Symp Soc Exp Biol. 1993;47:227–234. [PubMed] [Google Scholar]
- 7.Silvagno F, Follenzi A, Arese M, Prat M, Giraudo E, Gaudino G, Camussi G, Comoglio P M, Bussolino F. Arterioscler Thromb Vasc Biol. 1995;11:1857–1865. doi: 10.1161/01.atv.15.11.1857. [DOI] [PubMed] [Google Scholar]
- 8.Streit A, Stern C D, Thery C, Ireland G W, Aparicio S, Sharpe M J, Gherardi E. Development (Cambridge, UK) 1995;121:813–824. doi: 10.1242/dev.121.3.813. [DOI] [PubMed] [Google Scholar]
- 9.Burr A W, Toole K, Chapman C, Hines J E, Burt A D. J Pathol. 1998;185:298–302. doi: 10.1002/(SICI)1096-9896(199807)185:3<298::AID-PATH88>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 10.Nusrat A, Parkos C A, Bacarra A E, Godowski P J, Delp-Archer C, Rosen E M, Madara J L. J Clin Invest. 1994;93:2056–2065. doi: 10.1172/JCI117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Nature (London) 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- 12.Birchmeier C, Gherardi E. Trends Cell Biol. 1998;8:404–10. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 13.Cherrington J M, Strawn L M, Shawver L K. Adv Cancer Res. 2000;79:1–38. doi: 10.1016/s0065-230x(00)79001-4. [DOI] [PubMed] [Google Scholar]
- 14.Rong S, Bodescot M, Blair D, Dunn J, Nakamura T, Mizuno K, Park M, Chan A, Aaronson S, Vande Woude G F. Mol Cell Biol. 1992;12:5152–5158. doi: 10.1128/mcb.12.11.5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper C S, Park M, Blair D G, Tainsky M A, Huebner K, Croce C M, Vande Woude G F. Nature (London) 1984;311:29–23. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 16.Takayama H, LaRochelle W J, Sharp R, Otsuka T, Kriebel P, Anver M, Aaronson S A, Merlino G. Proc Natl Acad Sci USA. 1997;94:701–706. doi: 10.1073/pnas.94.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gherardi E, Sharpe M, Lane K, Sirulnik A, Stoker M. Symp Soc Exp Biol. 1993;47:163–181. [PubMed] [Google Scholar]
- 18.Rong S, Segal S, Anver M, Resau J H, Vande Woude GF. Proc Natl Acad Sci USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffers M, Rong S, Vande Woude G F. Mol Cell Biol. 1996;16:1115–1125. doi: 10.1128/mcb.16.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeffers M, Rong S, Anver M, Vande Woude G F. Oncogene. 1996;13:853–861. [PubMed] [Google Scholar]
- 21.Scarping S, Stoppacciaro A, Colarossi C, Cancellario F, Marzullo A, Marchesi M, Biffoni M, Comoglio P M, Prat M, Ruco L P. J Pathol. 1999;189:570–575. doi: 10.1002/(SICI)1096-9896(199912)189:4<570::AID-PATH470>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner J M. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 23.Fan S, Wang J A, Yuan R Q, Rockwell S, Andres J, Zlatapolskiy A, Goldberg I D, Rosen E M. Oncogene. 1998;17:131–141. doi: 10.1038/sj.onc.1201943. [DOI] [PubMed] [Google Scholar]
- 24.Xiao G H, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude G F, Testa J R. Proc Natl Acad Sci USA. 2001;98:247–252. doi: 10.1073/pnas.011532898. . (First Published December 26, 2000; 10.1073/pnas.011532898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowers D C, Fan S, Walter K A, Abounader R, Williams J A, Rosen E M, Laterra J. Cancer Res. 2000;60:4277–4283. [PubMed] [Google Scholar]
- 26.Morishita R, Aoki M, Nakamura S, Matsushita H, Tomita N, Hayashi S, Moriguchi A, Matsumoto K, Nakamura T, Higaki J, Ogihara T. J Atheroscler Thromb. 1997;4:12–19. doi: 10.5551/jat1994.4.12. [DOI] [PubMed] [Google Scholar]
- 27.Fenton H, Finch P W, Rubin J S, Rosenberg J M, Taylor W G, Kuo-Leblanc V, Rodriguez-Wolf M, Baird A, Schipper H M, Stopa E G. Brain Res. 1998;779:262–270. doi: 10.1016/s0006-8993(97)00958-x. [DOI] [PubMed] [Google Scholar]
- 28.Koochekpour S, Jeffers M, Rulong S, Taylor G, Klineberg E, Hudson E A, Resau J H, Vande Woude G F. Cancer Res. 1997;57:5391–5398. [PubMed] [Google Scholar]
- 29.Laterra J, Nam M, Rosen E, Rao J S, Lamszus K, Goldberg I D, Johnston P. Lab Invest. 1997;76:565–577. [PubMed] [Google Scholar]
- 30.Abounader R, Ranganathan S, Lal B, Fielding K, Book A, Dietz H, Burger P, Laterra J. J Natl Cancer Inst. 1999;91:1548–1556. doi: 10.1093/jnci/91.18.1548. [DOI] [PubMed] [Google Scholar]
- 31.Guerin C, Luddy C, Abounader R, Lal B, Laterra J. Biochem Biophys Res Commun. 2000;273:287–293. doi: 10.1006/bbrc.2000.2935. [DOI] [PubMed] [Google Scholar]
- 32.Rong S, Jeffers M, Resau J H, Tsarfaty I, Oskarsson M, Vande Woude G F. Cancer Res. 1993;53:5355–5360. [PubMed] [Google Scholar]
- 33.Rong S, Oskarsson M, Faletto D, Tsarfaty I, Resau J H, Nakamura T, Rosen E, Hopkins R F, III, Vande Woude GF. Cell Growth Differ. 1993;4:563–569. [PubMed] [Google Scholar]
- 34.Jeffers M, Fiscella M, Webb C P, Anver M, Koochekpour S, Vande Woude G F. Proc Natl Acad Sci USA. 1998;95:14417–14422. doi: 10.1073/pnas.95.24.14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeffers M, Schmidt L, Nakaigawa N, Webb C, Weirich G, Kishida T, Zbar B, Vande Woude G F. Proc Natl Acad Sci USA. 1997;94:11445–11450. doi: 10.1073/pnas.94.21.11445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cioce V, Csaky K G, Chan A M L, Bottaro D P, Taylor W G, Jensen R, Aaronson S A, Rubin J S. J Biol Chem. 1996;271:13110–13115. doi: 10.1074/jbc.271.22.13110. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann G, Naldini L, Weidner K M, Sachs M, Vigna E, Comoglio P M, Birchmeier W. Proc Natl Acad Sci USA. 1992;89:11574–11578. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. FEBS Lett. 1997;22:1–6. doi: 10.1016/s0014-5793(97)01475-0. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto K, Kataoka H, Date K, Nakamura T. J Biol Chem. 1998;273:22913–22920. doi: 10.1074/jbc.273.36.22913. [DOI] [PubMed] [Google Scholar]
- 40.Gherardi E, Sharpe M, Lane K. EXS. 1998;65:31–48. [PubMed] [Google Scholar]
- 41.Okada M, Matsumori A, Ono K, Miyamoto T, Takahashi M, Sasayama S. Biochem Biophys Res Commun. 1999;255:80–87. doi: 10.1006/bbrc.1999.0150. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt N, Westphal M, Hagel C, Ergun S, Stavrou D, Rosen E, Lamszus K. Int J Cancer. 1999;84:10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Schwall R H, Chang L Y, Godowski P J, Kahn D W, Hillan K J, Bauer K D, Zioncheck T F. J Cell Biol. 1996;133:709–718. doi: 10.1083/jcb.133.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlessinger J, Plotnikov A N, Ibrahimi O A, Eliseenkova A V, Yeh B K, Yayon A, Linhardt R J, Mohammadi M. Mol Cell. 2000;6:743–750. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 45.Zou H, Casas-Finet J R, Heath Coasts R, Kaufman J D, Stahl S J, Winfield P T, Rubin J S, Bottaro D P, Byrd R A. Biochemistry. 1999;38:14793–14802. doi: 10.1021/bi9908641. [DOI] [PubMed] [Google Scholar]
- 46.Sakata H, Stahl S J, Taylor W G, Rosenberg J M, Sakaguchi K, Wingfield P T, Rubin J S. J Biol Chem. 1997;272:9457–9463. doi: 10.1074/jbc.272.14.9457. [DOI] [PubMed] [Google Scholar]
- 47.Leung D W, Cachianes G, Kuang W J, Goeddel D V, Ferrara N. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 48.Coppola G, Atlas-White M, Katsahambas S, Bertolini J, Hearn M T, Underwood J R. Anticancer Res. 1997;17:2033–2040. [PubMed] [Google Scholar]
- 49.Asano M, Yukita A, Matsumoto T, Kondo S, Suzuki H. Cancer Res. 1995;55:5296–5301. [PubMed] [Google Scholar]
- 50.Gorden M S, Margolin K, Talpaz M, Sledge G W, Jr, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]