Abstract
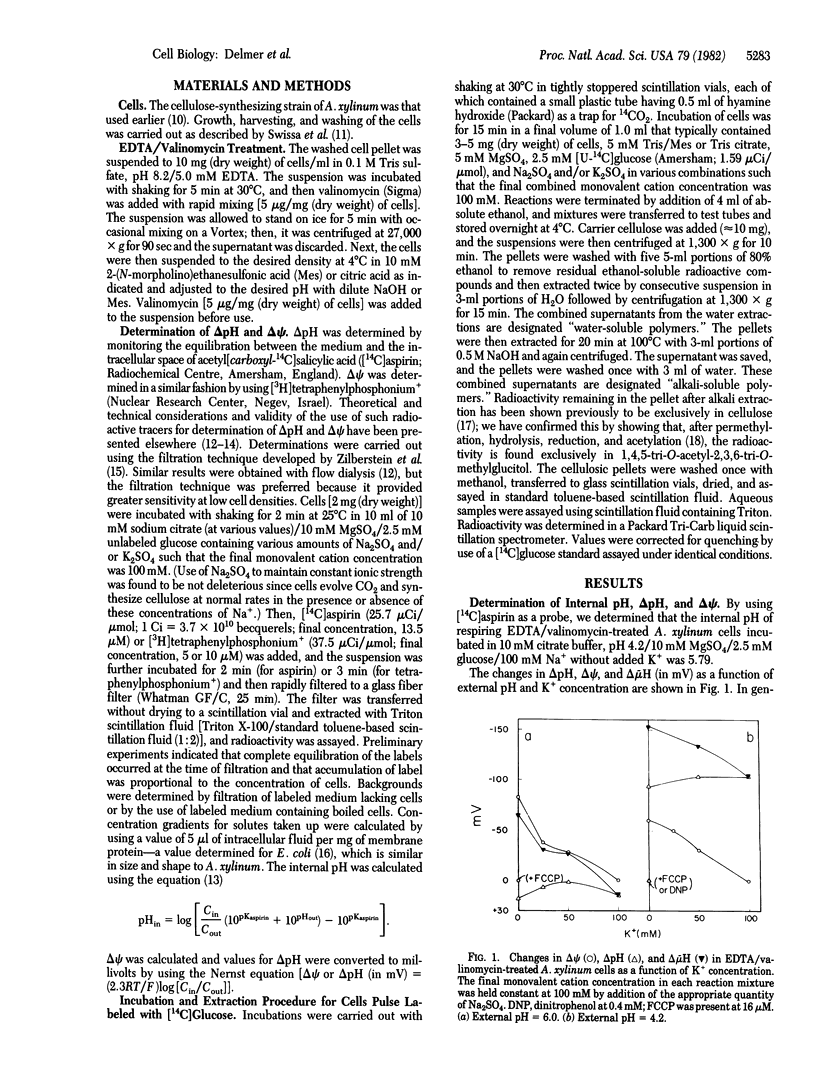
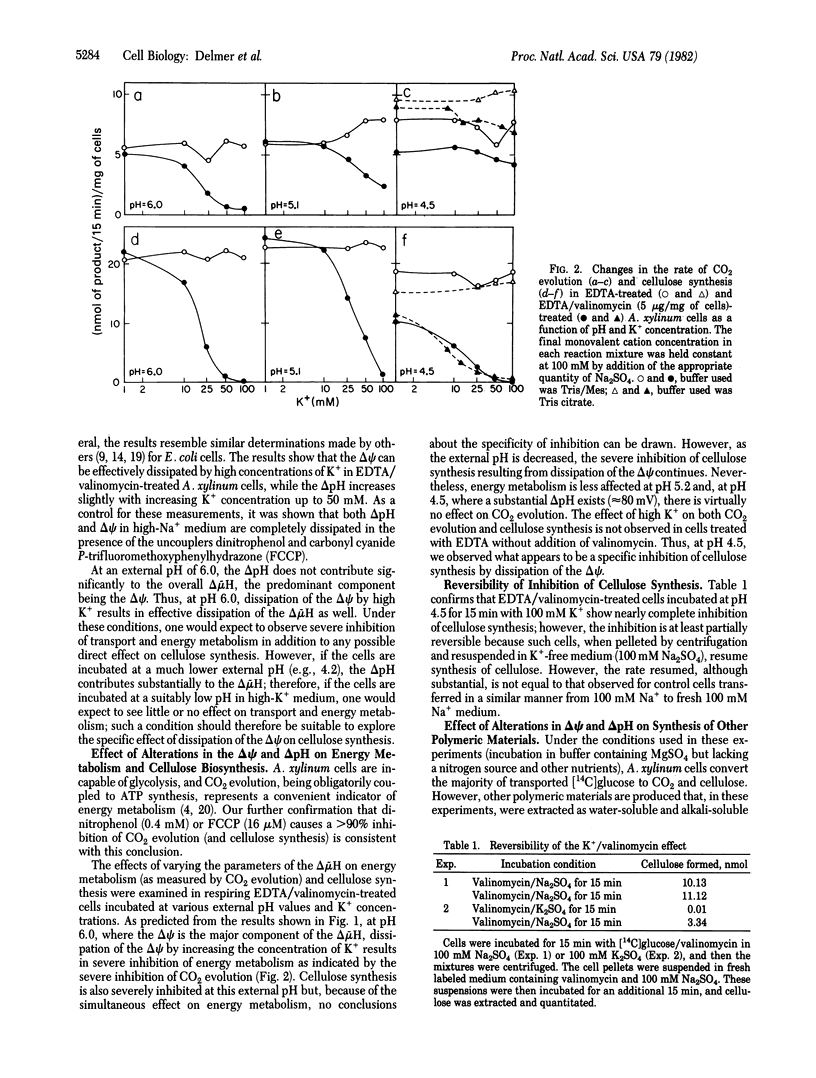
The marked lability in cell-free preparations of the enzyme system involved in cellulose biosynthesis in most organisms studied led us to investigate factors responsible for loss of activity on cellular disruption. Previous studies have led to the suggestion that the existence of a transmembrane electrical potential (ΔΨ) may be one factor responsible for maintaining an active system in intact cells. In this report, we show that dissipation of the ΔΨ in metabolizing cells of Acetobacter xylinum results in severe inhibition of cellulose synthesis. The effect can be reversed by restoration of the ΔΨ. Inhibition of cellulose biosynthesis by dissipation of the ΔΨ can be observed under conditions in which no substantial impairment of energy metabolism occurs—i.e., under conditions in which a transmembrane pH gradient is of sufficient magnitude to maintain an adequate overall protonmotive force across the membrane. The inhibition of cellulose biosynthesis is specifically related to changes in the ΔΨ, since the process can proceed normally in the absence of the pH gradient. These results support the suggestion that loss of the ΔΨ on cellular disruption may be one of the factors responsible for the low capacity for cellulose synthesis in isolated membrane preparations and also raise the possibility that modulation of the ΔΨ could be one means of regulating the rate of cellulose synthesis in vivo.
Keywords: protonmotive force, plasma membrane, glucan, polysaccharide biosynthesis
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar A., Rottem S., Razin S. Disposition of membrane proteins as affected by changes in the electrochemical gradient across Mycoplasma membranes. Biochem Biophys Res Commun. 1978 Sep 29;84(2):306–312. doi: 10.1016/0006-291x(78)90171-7. [DOI] [PubMed] [Google Scholar]
- Bevers E. M., Leblanc G., Le Grimellec C., Op den Kamp J. A., van Deenen L. L. Disposition of phosphatidylglycerol in metabolizing cells of Acholeplasma laidlawii. FEBS Lett. 1978 Mar 1;87(1):49–51. doi: 10.1016/0014-5793(78)80130-6. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Jr, Willison J. H., Richardson C. L. Cellulose biosynthesis in Acetobacter xylinum: visualization of the site of synthesis and direct measurement of the in vivo process. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4565–4569. doi: 10.1073/pnas.73.12.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C., Delmer D. P. Protection of cellulose synthesis in detached cotton fibers by polyethylene glycol. Plant Physiol. 1980 Nov;66(5):911–916. doi: 10.1104/pp.66.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Heiniger U., Kulow C. UDP-glucose: Glucan Synthetase in Developing Cotton Fibers: I. Kinetic and Physiological Properties. Plant Physiol. 1977 Apr;59(4):713–718. doi: 10.1104/pp.59.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- HESTRIN S., SCHRAMM M. Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem J. 1954 Oct;58(2):345–352. doi: 10.1042/bj0580345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Van Brunt J. Circulation of H+ and K+ across the plasma membrane is not obligatory for bacterial growth. Science. 1977 Jul 22;197(4301):372–373. doi: 10.1126/science.69317. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Crofts A. R. The initial stages of photophosphorylation. Studies using excitation by saturating, short flashes of light. Biochim Biophys Acta. 1978 Apr 11;502(1):87–102. doi: 10.1016/0005-2728(78)90134-2. [DOI] [PubMed] [Google Scholar]
- Harris D. A., von Tscharner V., Radda G. K. The ATPase inhibitor protein in oxidative phosphorylation. The rate-limiting factor to phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1979 Oct 10;548(1):72–84. doi: 10.1016/0005-2728(79)90188-9. [DOI] [PubMed] [Google Scholar]
- Jolliffe L. K., Doyle R. J., Streips U. N. The energized membrane and cellular autolysis in Bacillus subtilis. Cell. 1981 Sep;25(3):753–763. doi: 10.1016/0092-8674(81)90183-5. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Robertson D. E., Kaback H. R. Mechanism of lactose translocation in membrane vesicles from Escherichia coli. 2. Effect of imposed delata psi, delta pH, and Delta mu H+. Biochemistry. 1979 Aug 21;18(17):3697–3704. doi: 10.1021/bi00584a010. [DOI] [PubMed] [Google Scholar]
- Komor E., Weber H., Tanner W. Greatly decreased susceptibility of nonmetabolizing cells towards detergents. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1814–1818. doi: 10.1073/pnas.76.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Heller K. B., Jasaitis A. A., Wilson T. H., Goldberg E. B. A membrane potential threshold for phage T4 DNA injection. Biochem Biophys Res Commun. 1980 Mar 28;93(2):625–630. doi: 10.1016/0006-291x(80)91124-9. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K., Silverman M. P. Gating effects in Halobacterium halobium membrane transport. J Biol Chem. 1979 Jun 10;254(11):4750–4755. [PubMed] [Google Scholar]
- Lelkes P. I. Potential dependent riigidity changes in lipid membrane vesicles. Biochem Biophys Res Commun. 1979 Sep 27;90(2):656–662. doi: 10.1016/0006-291x(79)91285-3. [DOI] [PubMed] [Google Scholar]
- Luqmani Y. A., Bradford H. F., Birdsall N. J., Hulme E. C. Depolarisation-induced changes in muscarinic cholinergic receptors in synaptosomes. Nature. 1979 Feb 8;277(5696):481–483. doi: 10.1038/277481a0. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Kaczorowski G. J., Garcia M. L., Kaback H. R. Active transport in membrane vesicles from Escherichia coli: the electrochemical proton gradient alters the distribution of the lac carrier between two different kinetic states. Biochemistry. 1980 Dec 9;19(25):5692–5702. doi: 10.1021/bi00566a005. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- SCHRAMM M., HESTRIN S. Factors affecting production of cellulose at the air/liquid interface of a culture of Acetobacter xylinum. J Gen Microbiol. 1954 Aug;11(1):123–129. doi: 10.1099/00221287-11-1-123. [DOI] [PubMed] [Google Scholar]
- Santos E., Kaback H. R. Involvement of the proton electrochemical gradient in genetic transformation in Escherichia coli. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1153–1160. doi: 10.1016/0006-291x(81)90739-7. [DOI] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein in planar membranes: clusters of channels in a native environment and their functional reassembly. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2302–2306. doi: 10.1073/pnas.78.4.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swissa M., Aloni Y., Weinhouse H., Benizman M. Intermediatry steps in Acetobacter xylinum cellulose synthesis: studies with whole cells and cell-free preparations of the wild type and a celluloseless mutant. J Bacteriol. 1980 Sep;143(3):1142–1150. doi: 10.1128/jb.143.3.1142-1150.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse H., Benziman M. Regulation of hexose phosphate metabolism in Acetobacter xylinum. Biochem J. 1974 Mar;138(3):537–542. doi: 10.1042/bj1380537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaar K. Visualization of pores (export sites) correlated with cellulose production in the envelope of the gram-negative bacterium Acetobacter xylinum. J Cell Biol. 1979 Mar;80(3):773–777. doi: 10.1083/jcb.80.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Agmon V., Schuldiner S., Padan E. The sodium/proton antiporter is part of the pH homeostasis mechanism in Escherichia coli. J Biol Chem. 1982 Apr 10;257(7):3687–3691. [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]



