Abstract
Background
Vitamin E is widely known to be one of the reactive oxygen species (ROS) scavengers and a drug that can easily be obtained, and it has been shown to attenuate the pain responses induced by various causes in animal pain models. Thus, this experiment was conducted to assess the antinociceptive effects of vitamin E by comparing intraperitoneal and intrathecal injections in rats subjected to the formalin test.
Methods
After the intraperitoneal and intrathecal injections of vitamin E were carried out, respectively (IP: 500 mg/kg, 1 g/kg, and 2 g/kg, IT: 3 mg/kg, 10 mg/kg, and 30 mg/kg), the formalin test was perfumed. As soon as 5% formalin was injected into left hind paw, the number of flinches induced by pain was measured at 5-minute intervals for 1 hour.
Results
Formalin injected into the left hind paw induced biphasic nociceptive behavior in all animals. Intraperitoneal injection of vitamin E diminished the nociceptive behavior in a dose-dependent manner during the early and late phase. Intrathecal vitamin E diminished nociceptive behavior dose dependently during the late phase but showed no significant difference in the early phase.
Conclusions
Vitamin E attenuated acute nociception when it was injected systemically, while both systemic and intrathecal injection produced analgesia in a rat model of formalin-induced hyperalgesia.
Keywords: formalin test, intraperitoneal, intrathecal, vitamin E
INTRODUCTION
Chronic pain is an abnormal state that occurs from various causes and factors, and a complex pathophysiologic mechanism is known to be involved. In the presentation of such symptoms, peripheral sensitization and central sensitization caused by continuous nociceptive stimuli play an important role, and many factors are known to be involved; these include excitatory neurotransmitters such as substance P or glutamate, N-methyl-D-aspartate (NMDA) receptors, and non-NMDA receptors such as α-amino-3-hydroxy-5-methylisoxazole-4-propanoic acid (AMPA) receptor [1,2]. Recently, reactive oxygen species (ROS) have been reported to be involved in the occurrence of chronic pain, so the antinociceptive effect of ROS scavengers is receiving attention.
ROS are metabolites of normal cell metabolism, and their increase, due to excessive production or a decline of endogenous inhibitory factors, can impair the function of normal cells and cause cell damage [3]. This increase in ROS is not only related to neurodegenerative diseases such as Alzheimer's disease or Parkinson's disease [4,5], but is also reported to be related to the pathophysiology of chronic pain, such as neuropathic pain or inflammatory pain. In one example, a neuropathic pain model induced by spinal nerve ligation showed an increase in ROS in the dorsal horn of the spinal cord [6], and an ROS scavenger such as phenyl-N-tert-butylnitrone (PBN) showed the effect of suppressing mechanical allodynia from spinal nerve ligation in both intraperitoneal and intrathecal injection [3]. In addition, superoxide dismutase mimetic not only reduced hyperalgesia caused by formalin injected in the hind leg of a mouse, but also appeared to suppress secretion of cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, and the enlargement of edema [1].
Vitamin E, which is a relatively easy ROS scavenger to obtain, has also proved its antinociceptive effect in numerous animal experiment studies. In precedent studies, the systemic administration of vitamin E reduced both acute pain and hyperalgesia induced by formalin [7] and suppressed the mechanical allodynia and hyperalgesia shown in alcoholic neuropathy or diabetic neuropathy models, as well as the increased secretion of cytokine in the sciatic nerve [8,9]. Also, in the spinal nerve ligation model, vitamin E reduced dosage-dependent mechanical allodynia in intrathecal injection as well as intraperitoneal injection [10]. Therefore, the antinociceptive effect of ROS scavengers, including vitamin E, is thought to arise from the mechanism that suppresses anti-inflammatory effect and central sensitization and is anticipated to be effective in controlling acute pain and chronic pain.
This research performed intraperitoneal and intrathecal injections of vitamin E in a formalin test, showing the biphasic pain response due to acute pain and hyperalgesia, to investigate the effect of each phase and dose relationship. Through this method, differences in the effect on acute pain and hyperalgesia according to the administration method of vitamin E were investigated.
MATERIALS AND METHODS
All animal experiments related to this research obtained approval from the animal experiment committee of the School of Medicine at our university and were performed according to its regulations. Three or fewer male Sprague-Dawley mice of 6-7 weeks (230-260 g) were placed in a plastic cage with clean sawdust, and they were allowed to ingest water and food freely. The light in the breeding room was naturally controlled at 12-hour intervals, and consistent temperature (within 23℃) and humidity (within 50%) were maintained.
The mice to be used in the experiment were randomly assigned to either the intraperitoneal group or the intrathecal group. The single dosage of vitamin E was decided on the basis of the toxic dietary level (about 16-64 g/kg), the dosage used without toxic effect in a bird (5 g/kg), and the dosage clinically used in adult patient (2-5 g/day for 60 kg adult) as referred by Kim et al. [10]. The intraperitoneal group was divided into an olive oil control group (Sigma Chemical Co., St. Louis, MO, USA; intraperitoneal injection of 3 ml) and a vitamin E group (DL-α-Tocopherol acetate; Sigma Chemical Co., St. Louis, MO, USA; 500 mg/kg, 1 g/kg, and 2 g/kg). The intrathecal group was also divided into an olive oil control group (intrathecal injection of 50 µl) and a vitamin E group (3 mg/kg, 10 mg/kg, and 30 mg/kg). Altogether, the intraperitoneal groups and intrathecal groups comprised 8 groups total. The number of mice used in each group was 8. Intraperitoneal injection was performed by dissolving vitamin E in olive oil and titrating to 3 ml dosages each, using a 21-gauge needle after checking aspiration of gas or blood in the right lower abdomen of the mice. The intrathecal injection of vitamin E was performed only on mice that showed normal motor function after mounting an intrathecal catheter in the lumbar area approximately 5-7 days before performing the formalin test. As with the intraperitoneal injection, vitamin E was dissolved in olive oil and dose titration was performed. The total administration dosage was set at 50 µl, and after insertion, olive oil 10 µl was additionally administered into the catheter for the remaining amount inside the catheter.
The surgical procedures for mounting the lumbar intrathecal catheter were as follows. Anesthesia was induced using enflurane, and, after confirming that there was no response to stimuli and that spontaneous respiration was maintained, an incision was performed on the L5-6 area. Surgical vision was obtained with a microscope, and the L6 spinous process was cut to expand the L5-6 space. Through this space a 20-gauge guide needle was inserted until the dura matter was penetrated. Then the polyethylene catheter (PE-10 tube, INTRAMEDIC, Becton Dickinson and Company, Franklin Lakes, NJ, USA) was inserted into the lumbar enlargement area through the guide needle. Here, the insertion of the catheter into the intrathecal area was confirmed through the sudden movement of the tail. The catheter, once mounted in the intrathecal area, was attached to the nuchal area through subcutaneous tunneling from the lumbar area, where an incision was performed to the nuchal area, and the outer end was blocked. Then the incised area was sutured to complete the surgical procedure, and only mice that showed normal motor function after completely recovering from anesthesia were chosen for further observation for approximately 5-7 days.
Before performing the formalin test, the mice selected as experimental subjects were placed in a semi-circular cage 20 minutes before administering medication to allow them to adapt to the changed environment. Then, to determine whether sedation or anesthesia that could affect the pain response occurred from administration of vitamin E, the posture and righting reflex were checked after administering the chemicals [3,11]. The posture was evaluated using the following five-point scale: 0=normal posture, rearing and grooming; 1=moderate atonia and ataxia; 2=weight support, but severe ataxia; 3=muscle tone but no weight support and only small purposive movement; 4=flaccid atonia, fully immobilized with no attempts at movement. Righting reflex was evaluated using the following five-point scale: 0=the rat struggles when placed on its side, followed by rapid forceful righting; 1=moderate resistance when the rat is placed on its side, with rapid but not forceful righting; 2=no resistance to the rat being placed on its side, with labored but ultimately successful righting; 3=unsuccessful righting; 4=no movements.
The formalin test was performed 1 hour after intraperitoneal injection of vitamin E and 20 minutes after the intrathecal injection. The test was conducted according to the following method. Using a 25-gauge needle on the left hind leg, 5% formalin solution 50 µl was inserted into the subcutaneous tissue. Then the flinching of the leg, showing a pain response, was recorded for 60 minutes at 5-minute intervals. In the intrathecal group, the pain response was recorded for 60 minutes, and then 2% lidocaine 50 µl was inserted into the catheter to observe whether there was lower limb paralysis. Only the mice that showed lower limb paralysis were used in the statistical analysis. All mice were used only once in the formalin experiment and were euthanized after the experiment through exposure to an excess quantity of enflurane.
All values were expressed in mean ± standard error of the mean (SEM). SPSS (version 18.0) was used for statistical analysis, and repeated measure ANOVA and Dunnett's post hoc test were performed to compare the pain response of the control group and vitamin E group. A time-effect curve and dose-response curve were used to compare the change in pain response in each phase according to intraperitoneal and intrathecal injection of vitamin E. When generating the dose-response curve, the response according to vitamin E dosage was defined as the total sum of flinching that appeared in each phase. SigmaPlot11® software (SYSTAT Software Inc, Chicago, IL) was used to create the graph. All values were considered statistically significant only when the P value was less than 0.05.
RESULTS
In the evaluation of posture and righting reflex according to each injection method and each dosage of vitamin E during the formalin test, all mice recorded a score of 0, and there were no reductions in pain response due to sedation or anesthesia.
Flinching observed after subcutaneous injection of formalin continued from directly after injection for up to 5 minutes in all intraperitoneal groups and intrathecal groups. Flinching showed a biphasic aspect, decreasing from 5 minutes until 10 minutes and then increasing again after 10 minutes, so flinching was divided into early phase (0-5 min) and late phase (10-60 min) (Fig. 1 and 2).
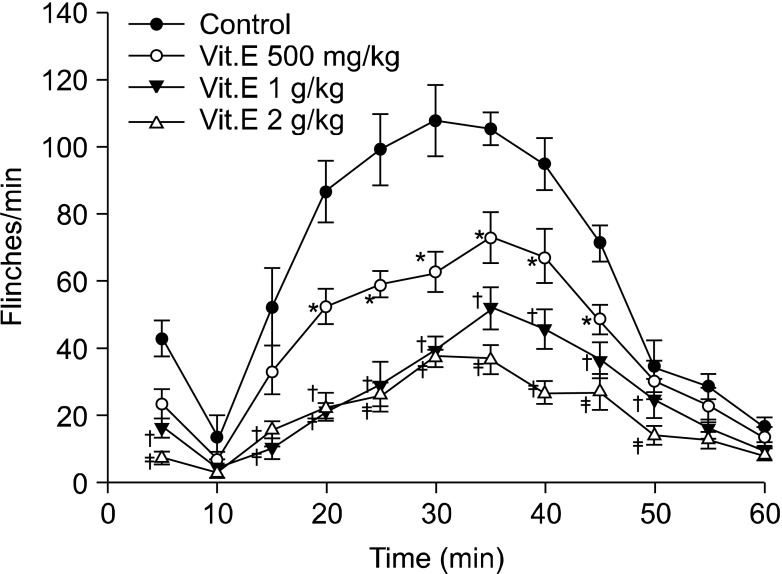
Fig. 1.
Time effect curve of intraperitoneal injection group. Vitamin E was administered 1 hour before formalin injection. Each point showed the mean ± SE and significant dose-dependent decreases in flinches in the early phase (0-5 min) and late phase (10-60 min). *P < 0.05, vitamin E 500 mg/kg compared with the control group. †P < 0.05, vitamin E 1 g/kg compared with the control group. ‡P < 0.05, vitamin E 2 g/kg compared with the control group.
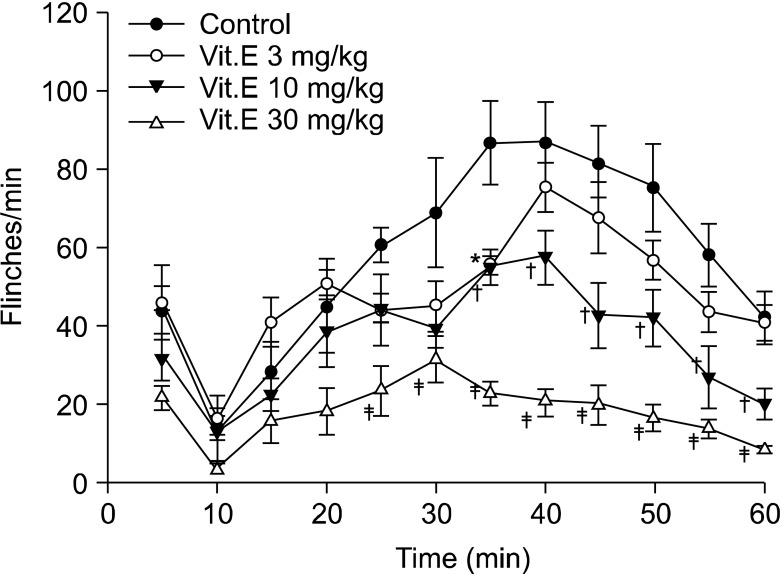
Fig. 2.
Time effect curve of intrathecal injection group. Vitamin E was administered 20 minutes before formalin injection. Each point showed the mean ± SE and significant dose-dependent decreases in flinches in the late phase (10-60 min). *P < 0.05, vitamin E 3 mg/kg compared with the control group. †P < 0.05, vitamin E 10 mg/kg compared with the control group. ‡P < 0.05, vitamin E 30 mg/kg compared with the control group.
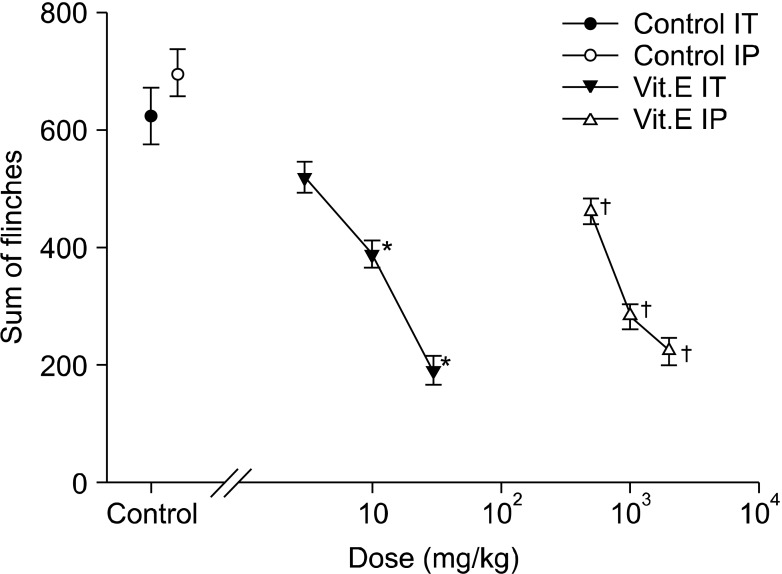
In the early phase of the intraperitoneal group, the intraperitoneal administration of vitamin E led to dose-reliant reduction of flinching, and compared with the intraperitoneal olive oil group, there was a statistically significant difference from the 1 g/kg group (Fig. 1 and 3). In the late phase, there was also a dose-reliant reduction of flinching. When the total sum of flinching during the late phase was used to compare the control group with the vitamin E groups of various dosages, there was a statistically significant difference from the 500 g/kg group (Fig. 1 and 4).
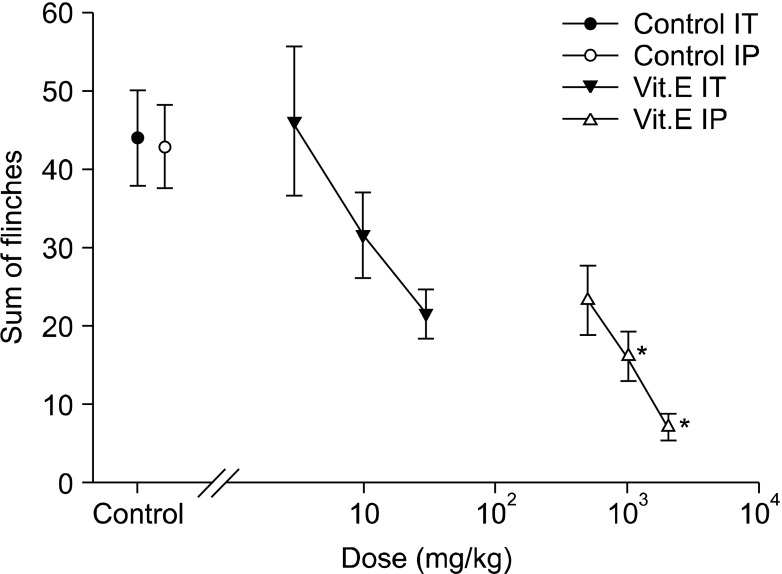
Fig. 3.
Dose-response curve of the early phase in intrathecal and intraperitoneal injection group respective. Each point showed the mean ± SE and non-significant difference between control and intrathecal injection group but significant dose-dependent decreases of flinches in intraperitoneal injection group. IT: intrathecal injection, IP: intraperitoneal injection. *P < 0.05, intraperitoneal injection compared with the control group.
Fig. 4.
Dose-response curve of the late phase in intrathecal and intraperitoneal injection group respective. Each point showed the mean ± SE and significant dose-dependent decreases of flinches in both injection group. IT: intrathecal injection, IP: intraperitoneal injection. *P < 0.05, intrathecal injection compared with the control group. †P < 0.05, intraperitoneal injection compared with the control group.
On the other hand, flinching in the early phase of the intrathecal group showed a tendency to reduce depending on the dosage of intrathecal administration, but there were no statistically significant differences compared with the intrathecal olive oil group (Fig. 2 and 3). In the late phase, flinching was reduced according to dose. When the total sum of flinching during the late phase was used to compare the control group with the vitamin E groups of various dosages, there was a statistically significant difference from the 10 mg/kg group (Fig. 2 and 4).
DISCUSSION
Rosland et al. [12] reported that when injecting formalin subcutaneously with a concentration of 1% or higher, there was a biphasic aspect in which the pain response was divided into early and late phases. The early phase reflects an acute pain response that is maintained for 5 minutes directly following injection. This response is due to the direct vitalization of primary afferent fibers (Aδ and C fiber), which convey signals to the superficial laminae of the spinal cord as a result of peripheral noxious stimulation from the formalin injection. The late phase is a state in which continuous severe pain occurs due to the inflammation of the peripheral tissue and central sensitization coming from repeated stimulation of C fibers, despite the low vitalization of primary afferent fibers. It is maintained from 10 to 60 minutes, and reflects the activation of wide dynamic range neurons of the dorsal horn; in other words, the hyperalgesia is caused by a continuous stimulus [13,14].
In this study, intraperitoneal administration of vitamin E had a statistically significant effect on hyperalgesia as well as acute pain caused by formalin, while intrathecal administration had a significant effect on hyperalgesia caused by formalin but did not reduce acute pain. Similarly, Hacimuftuoglu et al. [11] reported that intraperitoneal administration of another ROS scavenger, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL), was effective for both acute pain and hyperalgesia caused by formalin, whereas intrathecal administration only had a significant effect on hyperalgesia and not on acute pain. On the other hand, intrathecal administration of an NMDA receptor antagonist, such as MK801 or (±)-2-amino-5-phosphonopentanoic acid (AP-5), and an AMPA receptor antagonist, such as NBQX or ACEA 2752, showed a statistically significant effect in both acute pain and hyperalgesia caused by formalin injection in the hind paw of mice [2,15]. Through these results, it can be determined that NMDA receptors at the spinal cord level are related to the acute pain response that appears in formalin tests, and this hypothesis is supported by reports in which NMDA receptor antagonists suppressed the secretion of substance-P [16]. In the research of Lee et al. [17], central sensitization caused at the spinal dorsal horn level by the ROS donor tert-butyl hydroperoxide (t-BOOH) was suppressed by PBN but not by D-AP5, a NMDA receptor antagonist. Therefore, it is evident that ROS scavengers, including vitamin E, do not operate as NMDA receptor antagonists.
In conclusion, the suppression of the acute pain response in the formalin test due to vitamin E is considered to indicate an effect on an area other than the central nervous system, including the spinal cord. Wang et al. [1] showed that an immediate pain response is caused when superoxide is directly injected in the hind paw of mice, showing that ROS apply to peripheral injury sites. In the research of Kim et al. [3], the ectopic discharge rate due to spinal nerve ligation measured in the dorsal root ganglion was not affected by the intraperitoneal injection of PBN, and thus it was reported that PBN does not have an effect on the ligation site or dorsal root ganglion. According to these results, the suppression of acute pain in formalin tests due to PBN is speculated to result from the effect on the peripheral injury site rather than the peripheral afferent nerve. It is also speculated that, similar to PBN, the peripheral injury site is the main area for the vitamin E effect of suppressing acute pain. To prove this point, there is a need for more research on the effects of vitamin E on the peripheral nerve as well as on the peripheral injury site.
In the late phase, due to hyperalgesia in the formalin test, the major cause is peripheral sensitization and central sensitization. The inflammation response in the peripheral injury site is considered to be the important mechanism for peripheral sensitization. Wang et al. [1] induced edema and hyperalgesia in the hind paw of mice by injecting carrageenan and increased inflammatory cytokines such as TNF-α and IL-1β in the exudation in the edema. Then they inverted this situation by injecting superoxide dismutase mimetic. Tiwari et al. [8] found that, in an alcoholic neuropathy model using mice, increased inflammatory cytokines in the serum and sciatic nerve were suppressed by vitamin E. Hence, they reported that oxidative stress caused by peripheral injury induces the production of inflammatory cytokines, and ROS scavengers such as vitamin E are related to their suppression.
On the other hand, ROS that cause central sensitization in the dorsal horn of the spinal cord are usually superoxide generated from mitochondrial phosphorylation and cyclooxygenase reaction; hydrogen peroxide produced from chemical dismutation; and nitric oxide (NO) produced from L-arginine due to NO synthase [17]. Through research results that show Ca2+-dependent mitochondrial superoxide production caused by NMDA receptor activation [18], it can be speculated that, from these three types, mitochondria is an important source of ROS that is involved in the occurrence of central sensitization of the spinal cord.
ROS are considered to play an important role in the manifestation of central sensitization. In pain models, including the neuropathic pain model, there is known to be an increase in NMDA receptors, especially the phosphorylated NMDA receptor 1 (pNR1) in the dorsal horn [19,20]. There are reports that administration of PBN caused a decline in pain response and the manifestation of pNR1 in the dorsal horn [21]. Also, in the research of Kim et al. [10], the administration of vitamin E resulted in suppression of pNR1 manifestation. In the research of Lee et al. [17], the inducement and maintenance of long-term prolongation, which is considered the electrophysiological basis for central sensitization induced at the spinal dorsal horn level, were suppressed after exposure to ROS scavengers such as PBN or TEMPOL. Through these results, it was reported that ROS play an important role in the inducement and maintenance of central sensitization in the spinal cord and also play the role of intracellular signaling molecules that vitalize various intracellular signaling cascades that appear after activation of NMDA receptors.
In the research of Kim et al. [10], 50 µl of Sudan Black B dye dissolved in olive oil was injected at the L5 vertebral level, and it expanded mostly from L1 to T7. Hence, the 50 µl of vitamin E dissolved in olive oil and administered can be considered to have mainly affected the spinal cord area. However, it is difficult to confirm that an intrathecal injection of vitamin E only affects the spinal cord through just the colored area, so the effect on the supraspinal level from diffusion of the medication cannot be discounted. For example, intracerebroventricular administration of PBN reversed the reduction of mechanical threshold caused by spinal nerve ligation and reduced hyperalgesia from capsaicin injected in the hind paw, and thus the effect of ROS scavengers on the supraspinal level was observed [3,22]. However Lee et al. [22] reported that in the hyperalgesia model induced by capsaicin, intrathecal injection of PBN resulted in a larger reduction of hyperalgesia than intraperitoneal or intracerebroventricular injection, and thus the spinal cord area is an important area in the manifestation of central sensitization caused by ROS. Therefore, in reducing hyperalgesia caused by formalin injection, ROS scavengers such as vitamin E usually are related to the suppression of central sensitization in the spinal cord, and this circumstance is thought to result from the suppression of oxidative stress caused by the increase of ROS related to activation of NMDA receptors within the cells.
In conclusion, the intraperitoneal and intrathecal administration of vitamin E suppresses hyperalgesia induced by formalin, and it is considered that intraperitoneal administration of vitamin E has a systemic effect to suppress acute pain and hyperalgesia.
References
- 1.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, et al. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 2.Yoon MH, Bae HB, Choi JI. Antinociceptive interactions between intrathecal gabapentin and MK801 or NBQX in rat formalin test. J Korean Med Sci. 2005;20:307–312. doi: 10.3346/jkms.2005.20.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, et al. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Balazs L, Leon M. Evidence of an oxidative challenge in the Alzheimer's brain. Neurochem Res. 1994;19:1131–1137. doi: 10.1007/BF00965146. [DOI] [PubMed] [Google Scholar]
- 5.Olanow CW. An introduction to the free radical hypothesis in Parkinson's disease. Ann Neurol. 1992;32 Suppl:S2–S9. doi: 10.1002/ana.410320703. [DOI] [PubMed] [Google Scholar]
- 6.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 7.Hong BH, Ko YK, Lee YJ, Han K, Kim Y, Lee W. Antinociceptive effects of vitamin E in formalin-induced nociceptive response in rats. Anesth Pain Med. 2011;6:59–62. [Google Scholar]
- 8.Tiwari V, Kuhad A, Chopra K. Tocotrienol ameliorates behavioral and biochemical alterations in the rat model of alcoholic neuropathy. Pain. 2009;145:129–135. doi: 10.1016/j.pain.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Kuhad A, Chopra K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology. 2009;57:456–462. doi: 10.1016/j.neuropharm.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, et al. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Hacimuftuoglu A, Handy CR, Goettl VM, Lin CG, Dane S, Stephens RL., Jr Antioxidants attenuate multiple phases of formalin-induced nociceptive response in mice. Behav Brain Res. 2006;173:211–216. doi: 10.1016/j.bbr.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Rosland JH, Tjølsen A, Maehle B, Hole K. The formalin test in mice: effect of formalin concentration. Pain. 1990;42:235–242. doi: 10.1016/0304-3959(90)91167-H. [DOI] [PubMed] [Google Scholar]
- 13.Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990;535:155–158. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- 14.Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T. Interaction between intrathecal morphine and glutamate receptor antagonists in formalin test. Eur J Pharmacol. 2000;395:203–210. doi: 10.1016/s0014-2999(00)00268-5. [DOI] [PubMed] [Google Scholar]
- 16.Cuesta MC, Arcaya JL, Cano G, Sanchez L, Maixner W, Suarez-Roca H. Opposite modulation of capsaicin-evoked substance P release by glutamate receptors. Neurochem Int. 1999;35:471–478. doi: 10.1016/s0197-0186(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 17.Lee KY, Chung K, Chung JM. Involvement of reactive oxygen species in long-term potentiation in the spinal cord dorsal horn. J Neurophysiol. 2010;103:382–391. doi: 10.1152/jn.90906.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 20.Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]