Abstract
AIM: To evaluate the effect of double-balloon enteroscopy (DBE) on pancreas histology and levels of pancreatic enzymes.
METHODS: Conventional upper gastrointestinal endoscopy was performed on five control pigs. Oral DBE was performed with an EN-450T5 enteroscope on 20 pigs. Two experimental groups (10 pigs each) were defined according to DBE duration: 90 min for Group 1 and 140 min for Group 2. During oral insertion, the balloons were not inflated in the descending part of the duodenum to avoid the minor duodenal papilla. Serum amylase, lipase and C-reactive protein (CRP) levels were monitored before the procedure and repeated every 30 min until the exploration was finished, as well as 24 h and 7 d after. After the procedure and for a total of 7 d, the pigs were observed twice a day for signs of decreased activity, irritability, vomiting or anorexia. Gross and microscopic examination of the pancreas was performed on day 7.
RESULTS: All animals tolerated DBE without clinical manifestations of acute pancreatitis. Experimental groups had higher levels of enzymes than the control group at 24 h. Throughout the exploration, the amylase levels increased significantly above the baseline 24 h after DBE, although the increase was not statistically significant and did not reach 20% of the baseline. An increase in lipase and CRP was observed at 24 h after the procedure, although by day 7, all enzymatic levels had returned to baseline. No differences between Groups 1 and 2 were found for any enzyme and sampling site during and after the procedure. Similarly, no correlation between insertion depth and enzyme levels was observed. Direct in situ and post-removal inspection of the pancreas did not show any evidence of fluid collection, abscesses or hemorrhage. Histological examination of the pancreas from Groups 1 and 2 revealed the existence of focal areas (0.14-0.26 mm2) of ischemic necrosis in 47.4% of the animals. In the pigs with damaged pancreas, the left lobe (tail) was always affected. However, this only happened in 83.3% of the samples from the right lobe (head) and in 33.3% of the samples from the body of the pancreas. Significant differences were found between the left lobe (tail) and the body for the percentage of affected pancreas. Both the size of the lesions and the percentage of affected pancreas were higher in the left pancreatic lobe (tail). The presence of the lesions was not related to the exploration length.
CONCLUSION: The increase in pancreatic enzymes after DBE could be related to focal points of pancreatic ischemic necrosis due to mechanical stress.
Keywords: Endoscopy, Pancreatitis, Double-balloon enteroscopy, Experimental study, Pig model
INTRODUCTION
Double-balloon enteroscopy (DBE) has enabled endoscopic diagnosis and treatment in the small intestine, which had been very difficult for many years[1]. Yamamoto et al[2] introduced DBE in 2001 and its usefulness is already recognized in many countries. DBE is considered a well-tolerated and safe endoscopic technique[3-8], but an increase in pancreatic enzymes and potential pancreatitis are recognized as complications directly attributed to the procedure[1,7,9-12].
The mechanism for post-DBE pancreatitis remains unclear. Potential explanations might include: (1) pancreatic duct obstruction by direct oppression of the papilla with the inflated balloon[7,9]; (2) reflux of intestinal fluid into the pancreatic duct owing to an increase in intraduodenal pressure because of mechanical strain[12,13]; or (3) prolonged mechanical injury or ischemia on the pancreas as a result of repeated stretching and shortening of the endoscope and overtube[7,14-16].
Unfortunately, levels of pancreatic enzymes several days after the procedure and evaluation of potential lesions in the pancreas under normal clinical conditions are unknown in humans. Also, to the best of our knowledge, there have been no studies in animal models to clarify the etiology of pancreatic hyperamylasemia and pancreatitis post-DBE. This study was aimed at determining the effects of the DBE technique on the pancreatic enzymes and histology under nonpathological conditions. To assess if the timing of DBE influences the pancreatic enzyme markers, two experimental groups with different DBE duration (90 or 140 min) were established.
MATERIALS AND METHODS
Animals and protocols
Twenty-five Large White pigs (35-40 kg) were used. The day before DBE, animals were fasted, with no liquid restrictions, and given a laxative preparation. Animals were prepared and anesthetized for the endoscopic procedures. Intravenous saline solution was administrated to secure basic hydration during the DBE procedure. After 24 h fasting, each pig was intramuscularly premedicated with diazepam 0.1 mg/kg, ketamine 10 mg/kg and atropine 0.01 mg/kg. General anesthesia was induced with propofol 2 mg/kg intravenously and maintained with sevofluorane 1.8%-2% delivered via an endotracheal tube. Animals from the control group (n = 5) underwent conventional upper gastrointestinal (GI) endoscopy. In the remaining 20 pigs DBE was performed with an EN-450T5 enteroscope (Fujinon, Japan) by experienced endoscopists. The exploration depth was estimated according to the methodology established by May et al[17].
Two experimental groups (10 pigs each) were defined according to DBE duration: 90 min for Group 1 and 140 min for Group 2. During the oral insertion of the scope and overtube, the balloons were not inflated in the descending part of the duodenum to avoid the major and minor duodenal papilla. Blood samples were taken before the procedure and during the exploration at 20 (control group only) 30, 60, 90 and 140 (Group 2 only) min, and also at 24 h and 7 d after DBE (Groups 1 and 2) and 24 h and 7 d after GI endoscopy (control group) to evaluate the serum concentrations of amylase, lipase and C-reactive protein (CRP). Animals were allowed to feed 24 h after DBE. After the procedure and for a total of 7 d, the pigs were observed twice a day for signs of decreased activity, irritability, vomiting or anorexia. On day 7, all the animals were euthanized with a pentobarbital overdose and the pancreases were removed. Each pancreas was examined in situ, palpated, removed from the cadaver and then sectioned to identify gross alterations. The right lobe (head) and left lobe (tail), as well as the body of the pancreas were preserved in 10% buffered formalin, trimmed into 1 cm × 1 cm × 1 cm tissue blocks (18-22 blocks per pancreas) and processed for histopathology after hematoxylin and eosin staining. Histology sections were studied under light microscopy (three fields per block of tissue) and when lesions were observed, the cross-sectional areas were measured with the SigmaScan Pro 5.0 program (Systat Software Inc., San Jose, CA, United States).
Ethical approval
All animals received humane care in compliance with the European Communities Council Directive (86/609/EEC). Protocols were approved by the local government Ethics Committee for Animal Research. The endoscopic equipment used was for research with animals only.
Statistical analysis
Data of enzyme levels were included in a spreadsheet and analyzed with SPSS 17.0 (SPSS, Chicago, IL, United States). Descriptive statistics were obtained and all the variables tested for normality (Kolmogorov-Smirnov test) before being subjected to analysis of variance (ANOVA) (linear model with repeated measures). Within-subject factors were the different timing of blood sampling and the inter-subject factor was the duration of the exploration (90 or 140 min). Tukey and Bonferroni tests were used to ascertain post hoc differences. The possible association between the experimental groups and the presence of lesions in the pancreas was checked with the χ2 test. In addition, the nonparametric Mann-Whitney test was used to ascertain any dependence between the size of the lesions and the portion of the pancreas.
RESULTS
Procedure evaluation
All the animals tolerated the procedure without any clinical manifestations of pancreatitis or distress. During the endoscopic exploration, passing the endoscope and the overtube into the duodenum was not difficult (< 3 min). Remnants of food in stomach did not make DBE more difficult. During the 7 d observation period after the procedure, the activity and dietary intake were normal in all the animals.
Estimations were calculated using the depth of insertion technique described by May et al[5]. The average insertion depth in Group 1 was lower than in Group 2: 268 cm (range: 209-336 cm) and 333 cm (range: 230-488 cm), respectively. Nevertheless, due to high data variability, the ANOVA for the insertion depth between the two groups was not significant (P = 0.181).
Biochemical evaluation
No statistical differences between sampling states in the control group were found. Experimental Group 1 had higher levels of enzymes than the control group at 24 h (Table 1). These differences were also present between the control group and Group 2.
Table 1.
Comparison of serum enzyme levels between double-balloon enteroscopy (Group 1) and control group at different sampling intervals
| Amylase (U/L) | Lipase (U/L) | CRP (U/L) | |
| t0 | |||
| Control | 1295.74 ± 120.55 | 14.66 ± 2.16 | 11.02 ± 3.92 |
| DBE | 2074.42 ± 296.82 | 17.74 ± 2.44 | 39.14 ± 13.02 |
| tend | |||
| Control | 1290.62 ± 158.58 | 9.38 ± 1.02 | 11.86 ± 3.8 |
| DBE | 2070.99 ± 281.31 | 8.84 ± 1.15 | 37.16 ± 12.1 |
| t24 h | |||
| Control | 1311.02 ± 88.56 | 19.48 ± 3.6 | 51.46 ± 26.02 |
| DBE | 2487.18 ± 364.46b | 26.73 ± 6.63 | 114.81 ± 31.84 |
| t168 h | |||
| Control | 1474.42 ± 143.16 | 8.86 ± 2.21 | 20.36 ± 10.52 |
| DBE | 2337.9 ± 300.72 | 5.71 ± 0.51 | 78.07 ± 31.6 |
t0: Samples before the procedure; tend: Samples at the end of the procedure [gastrointestinal endocopy (GI) in control group and double-balloon enteroscopy (DBE) in experimental group]; t24 h: Samples 24 h after the procedure (GI in control group and DBE in experimental group); t168h: Samples 7 d after the procedure (GI in control group and DBE in experimental group).
P < 0.01 vs control group.
Enzyme serum levels at the different sampling stages are displayed in Figure 1. To simplify notation, values at 30 and 60 min of the procedure are omitted because they were always similar to time 0.
Figure 1.
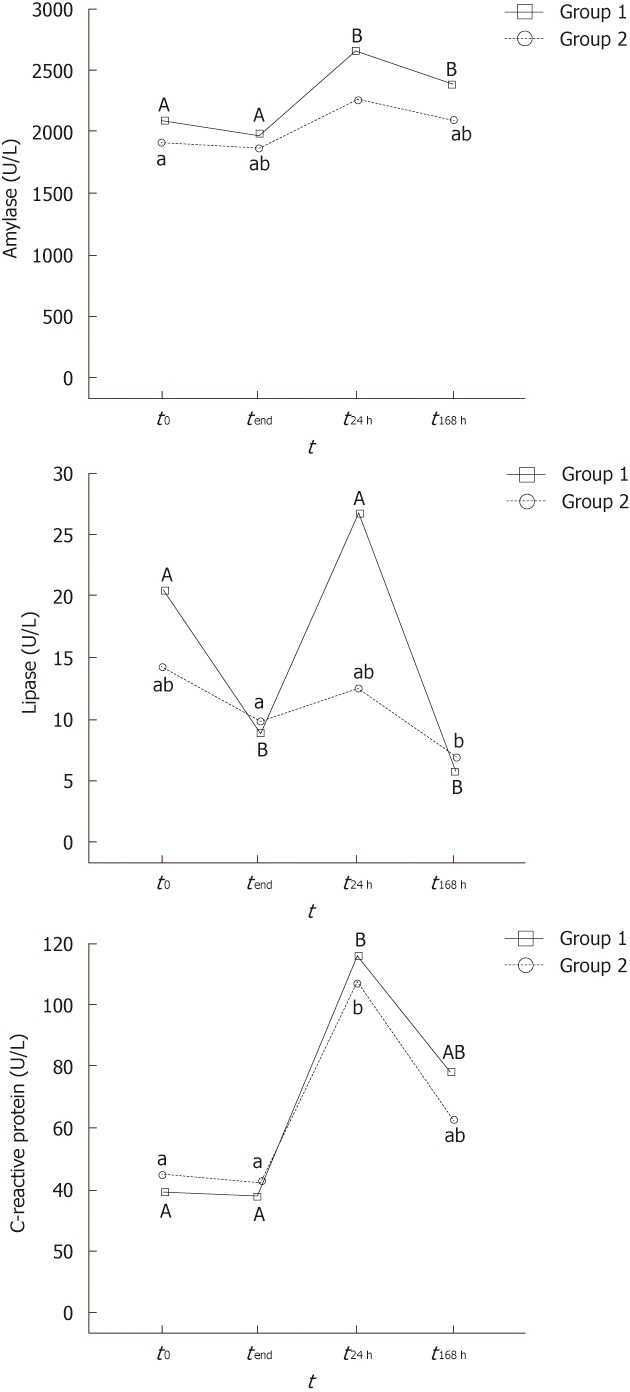
Plots representing the serum levels of amylase (A), lipase (B) and C-reactive protein (C) for Groups 1 and 2 at different sampling intervals. t0: Samples before the procedure; tend: Samples at the end of the procedure [gastrointestinal endocopy (GI) in control group and double-balloon enteroscopy (DBE) in experimental group]; t24 h: Samples 24 h after the procedure (GI in control group and DBE in experimental group); t168 h: Samples 7 d after the procedure (GI in control group and DBE in experimental group). Within-group differences: sampling stages with no coincident capital (Group 1) or normal case (Group 2) letters were significantly different (P < 0.05). No significantly different results between Groups 1 and 2 were found at any sampling stage.
All the animals had similar basal amylase levels before the procedure (approximately 2000 IU). Throughout the exploration, no significant changes in the amylase levels were noted (Figure 1A). However, the amylase levels increased significantly above the baseline 24 h after DBE, although the increase did not reach 20% of the baseline level. On day 7 after the procedure, the amylase level decreased progressively to the baseline, but it was still significantly higher in Group 1.
Lipase levels showed a variable trend during and after the exploration (Figure 1B). This is well illustrated in Group 1, where there was a significant decrease during the exploration, peak levels at 24 h after the procedure, and the lowest levels 7 d later.
CRP levels were significantly higher 24 h after DBE (more than twice the initial levels) (Figure 1C). However, CRP concentrations then decreased progressively towards the baseline, so no significant differences were found between the initial levels and 7 d after DBE.
No differences between Groups 1 and 2 were found for any enzyme and sampling site during and after the procedure. Similarly, no correlation between insertion depth and enzyme levels was observed (P for Pearson coefficient was always > 0.3).
Histology evaluation
Direct in situ and post-removal inspection of the pancreas did not show any evidence of fluid collection, abscesses or hemorrhage (Figure 2).
Figure 2.
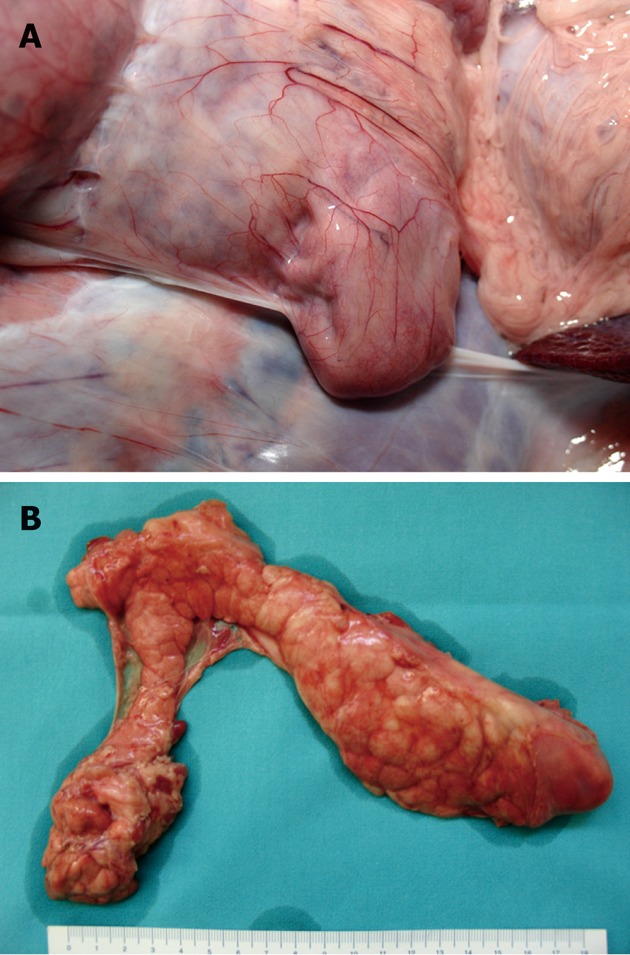
Gross anatomy of the porcine pancreas after double-balloon enteroscopy. A: In situ image of the left lobe (tail); B: Aspect of the whole pancreas immediately after removal from cadaver.
Light microscopy examination of tissue samples from the control group showed occasional small areas with infiltration and edema. However, the tissue samples from Groups 1 and 2 revealed the existence of limited areas of ischemic necrosis scattered throughout the parenchyma (Figure 3). This was observed in nine of the 19 pigs (47.4%). It should be noted that the histology samples from one pig were not included in the analysis due to bad processing. Some of the necrotic areas showed slight inflammatory cell infiltration around the sites of necrosis, but alterations to the pancreatic duct system were rare. In the nine pigs with damaged pancreas, the left lobe (tail) was always affected. However, this only happened in 83.3% of the samples from the right lobe (head) and in 33.3% of the samples from the body of the pancreas. The average area of lesions (μm2) related to each portion was: right lobe (head): 198274 ± 23952, body: 136782 ± 24163 and left lobe (tail): 260516 ± 32819. The percentage (%) of affected pancreas in each portion was: right lobe (head): 1.83, body: 0.60 and left lobe (tail): 3.11. Although the overall differences were not statistically significant, the average values for both parameters were higher in the left pancreatic lobe (tail). Significant differences were found between the left lobe and the body for the percentage of affected pancreas. Interestingly, the presence of the lesions was not related to the exploration length (90 or 140 min; Pearson χ2 and Fisher’s exact tests > 0.3).
Figure 3.
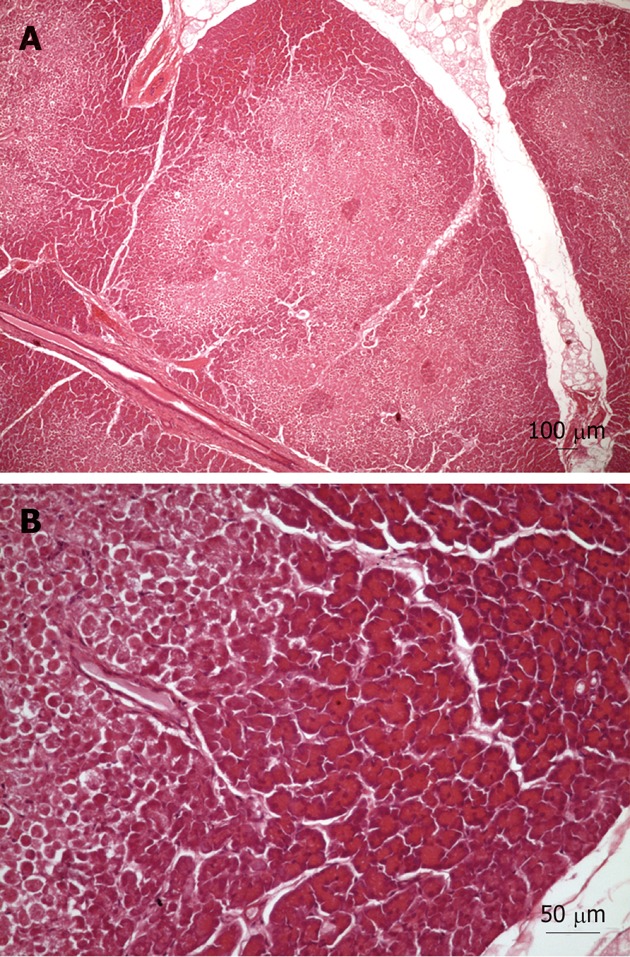
Light microscopy pictures of the porcine pancreas after double-balloon enteroscopy. A: Light microscopy of porcine pancreas after double-balloon enteroscopy showing located ischemic necrosis in pancreatic interlobular tissue; B: Magnification of previous image, view of margin between necrosis and viable tissue.
DISCUSSION
A low incidence (0.3%) of acute pancreatitis after diagnostic DBE has been reported in retrospective studies in Europe and Asia[18], and also in the United States[19,20]. However, latent hyperamylasemia without the development of pancreatitis occurs after peroral DBE more frequently than was previously thought[9,10,21].
The physiology and anatomy of the porcine pancreas is similar to that in humans, that is, it is a partially retroperitoneal organ and the pancreatic body wraps the portal vein. The firmness of the pancreatic parenchyma in swine is also similar to the gland in humans[22]. Thus, the porcine model has been used in many types of studies related to the pancreas such as endoscopic approaches to the pancreas[23-25], or experimental obstructive pancreatitis[26]. An ex vivo model for training has also been developed on porcine intestine[5,27]. Recently, the swine model has been validated for both DBE training and research, altogether improving the safety conditions of DBE in humans[28].
The characteristics, in terms of duration and insertion depth, of this study have been designed for comparison with DBE in humans. The DBE time length in Group 1 (90 min) and Group 2 (140 min) was selected in accordance with published average values from prospective studies in humans. Thus, Mehdizadeh et al[29] referred to a duration of 109.1 ± 44.6 min for the first 10 cases and 92.4 ± 37.6 min for subsequent cases. Similar times were referred to by other authors, namely, 75 min[14], 95 ± 42 min,[7] 115 ± 9 min[21], and 148 min[10,16]. Our results for the average insertion depth in both groups of animals were within the same range as those reported in previous works: 240 ± 100 cm[5], 250 ± 170 cm[30], 220 ± 90 cm[6], 270 ± 100 cm[7], and 351 ± 108 cm[21].
According to the literature, three authors have specifically measured amylase levels in patients before and after oral DBE[9,10,21]. Honda et al[9] found that 46% of patients undergoing DBE developed hyperamylasemia. Kopácová et al[10] investigated the levels of serum amylase, lipase and CRP both before and after DBE (4 h and 24 h). They found increased levels of amylase and lipase in 51.4% of the patients 24 h after the procedure. However, only 2.8% of them suffered acute pancreatitis. Pata et al[21] also checked levels of serum amylase and lipase both before and 4 h and 12 h after DBE. Just 4 h after the procedure, they found 25% of the patients had hyperamylasemia and hyperlipasemia, and 12.5% of the patients had pancreatitis. It is important to pay attention to the fact that the increases in the serum pancreatic amylase described in those three previous studies were twice or even three times higher than normal levels. In contrast, serum levels of amylase and lipase in the present work never reached twice the baseline level, and this could be related to the fact that the balloons were always inflated after the site of the pancreatic duct opening in the duodenum. In support of this, Pata et al[21] have described that amylase levels after DBE are negatively correlated to the depth at which the balloons are first inflated. Interestingly, no changes in the levels of serum amylase and lipase have been reported in patients when the first inflation of the balloons was done after reaching the ligament of Treitz[14,31,32]. On the other hand, some authors have described that substantial hyperamylasemia tended to be associated with longer duration of DBE[10,15,21,32]. However, we did not find such a difference, and the enzyme levels of Groups 1 and 2 did not vary significantly at any stage. Similar results have been reported by others authors[9,16].
The mechanical stress to the small intestine, mesentery and pancreas has also been suspected[7,9] to cause increased levels of amylase, lipase and CRP. Thus, a plausible explanation for the increased levels of enzymes[9,12,13] is an effect of the increased intraluminal pressure on the pancreatic ducts allowing intestinal fluid to progress towards the pancreas, and as such, should be kept in mind. However, the histological injuries found in this study are more likely related to an ischemic process in the vascular supply to the pancreas. The continuous pressure of the small intestine and the mesentery during the push and pull maneuvers could compromise the vascular supply to the pancreas, resulting in an increase in the pancreatic enzymes and unspecific inflammatory factors such as CRP. Along these lines, several works have reported pancreatic vascular restriction as a potential mechanism for hyperamylasemia after oral DBE[11,16,33]. On the other hand, the larger and more frequent areas of ischemic necrosis in the left lobe (tail) of the pig pancreas seem to be related to the particular vasculature of this pancreatic portion. In pigs, the left lobe (tail) is supplied by a single artery, which is a branch of the splenic artery. A similar situation is found in humans where the main artery of the tail of the pancreas is the major pancreatic artery. Such anatomical particularity predisposes the left pancreatic lobe to suffer from hypoxia or even ischemia if there is any mechanical restriction to the blood supply through this artery. Although this explanation requires further specific research, it is interesting to highlight that computed tomography has revealed that human pancreatitis is predominantly located in the tail of pancreas[15,34]. Considering the variance of the enzyme levels and that this was a non-survival animal model study, the number of animals could be a limitation of this work.
In conclusion, the inflation of the balloons after the duodenal papilla diminished the iatrogenic effects on the pancreas. However, minor enzymatic alterations and focal lesions in the pancreas remained, which on the other hand, failed to cause any clinical signs of pancreatitis. A vascular component is probably involved in the etiology of DBE-related pancreatic alterations, but this topic needs further research aimed at evaluating the effects of DBE exploration maneuvers on the vascular supply to the pancreas.
COMMENTS
Background
Double-balloon enteroscopy (DBE) has enabled endoscopic diagnosis and treatment in the small intestine, but an increase in pancreatic enzymes and potential pancreatitis are recognized as complications directly attributed to the procedure.
Research frontiers
Unfortunately, levels of pancreatic enzymes several days after the DBE procedure and evaluation of potential lesions in the pancreas under normal clinical conditions are unknown in humans. In this study, the authors demonstrated that focal ischemic lesions in the pancreatic parenchyma, and minor enzymatic alterations were related with DBE procedure in a porcine model.
Innovations and breakthroughs
Previous reports have highlighted the importance of the amylase levels in patients before and after oral DBE. Increased levels of amylase and lipase in 51.4% of the patients 24 h after the procedure have been reported. However, only 2.8% of them suffered acute pancreatitis. This is believed to be the first study in an animal model aimed at clarifying the etiology of pancreatic hyperamylasemia and pancreatitis after DBE. Furthermore, the study suggested that a vascular component was probably involved in the etiology of pancreatic alterations after DBE.
Applications
By understanding the etiology of post-DBE pancreatic hyperamylasemia, this study demonstrates the need for further research aimed at evaluating the effects of DBE on the vascular supply to the pancreas.
Terminology
DBE is recognized as the gold standard method for total exploration of the small intestine. Based on the already existent push endoscopy, DBE is a form of deep endoscopy that not only allows the exploration but also treatment of the most common digestive disorders of the small intestine, such as obscure gastrointestinal bleeding, tumors, Crohn’s disease and polyps. The equipment consists of an endoscope and an overtube; both of them with a latex balloon attached to the tip. The two balloons are inflated and deflated in an alternating sequence so as to allow the endoscope to progress (pushing phase) or fold the explored intestine behind the balloons (pulling phase).
Peer review
This is an important issue because DBE might be associated with an increase of serum pancreatic enzymes or even complicated by acute pancreatitis. The mechanism of post-DBE pancreatitis has not been fully explained yet. That is why such an experimental study is important to understand possible pathogenetic mechanisms.
Footnotes
Supported by PI070712 Project (Instituto de Salud Carlos III, Ministerio de Sandiad y Consumo, Spain); and BIO-MED 07/08-0019 Project (Consejería de Educación, Ciencia e Investigación de la Región de Murcia, Spain)
Peer reviewer: Jan Bureš, MD, PhD, Professor, Second Department of Medicine Gastroenterology, Charles University in Praha, Faculty of Medicine at Hradec Králové, University Teaching Hospital, Sokolská 581, 50005 Hradec Králové, Czech Republic
S- Editor Lv S L- Editor Kerr C E- Editor Xiong L
References
- 1.Yano T, Yamamoto H. Current state of double balloon endoscopy: the latest approach to small intestinal diseases. J Gastroenterol Hepatol. 2009;24:185–192. doi: 10.1111/j.1440-1746.2008.05773.x. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, Iino S, Ido K, Sugano K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216–220. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A, et al. Endoscopic diagnosis and treatment of small intestinal diseases using the double-balloon Endoscopy. Gastrointestinal Endoscopy. 2004;59:P100–P100. [Google Scholar]
- 4.Gerson LB. Double-balloon enteroscopy: the new gold standard for small-bowel imaging? Gastrointest Endosc. 2005;62:71–75. doi: 10.1016/s0016-5107(05)00507-9. [DOI] [PubMed] [Google Scholar]
- 5.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/s0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 6.Di Caro S, May A, Heine DG, Fini L, Landi B, Petruzziello L, Cellier C, Mulder CJ, Costamagna G, Ell C, et al. The European experience with double-balloon enteroscopy: indications, methodology, safety, and clinical impact. Gastrointest Endosc. 2005;62:545–550. doi: 10.1016/j.gie.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield, and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42–48. doi: 10.1055/s-2005-921188. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto H, Kita H. Enteroscopy. J Gastroenterol. 2005;40:555–562. doi: 10.1007/s00535-005-1645-5. [DOI] [PubMed] [Google Scholar]
- 9.Honda K, Itaba S, Mizutani T, Sumida Y, Kanayama K, Higuchi N, Yoshinaga S, Akiho H, Kawabe K, Arita Y, et al. An increase in the serum amylase level in patients after peroral double-balloon enteroscopy: an association with the development of pancreatitis. Endoscopy. 2006;38:1040–1043. doi: 10.1055/s-2006-944831. [DOI] [PubMed] [Google Scholar]
- 10.Kopácová M, Rejchrt S, Tachecí I, Bures J. Hyperamylasemia of uncertain significance associated with oral double-balloon enteroscopy. Gastrointest Endosc. 2007;66:1133–1138. doi: 10.1016/j.gie.2007.03.1085. [DOI] [PubMed] [Google Scholar]
- 11.Lo SK, Simpson PW. Pancreatitis associated with double-balloon enteroscopy: how common is it? Gastrointest Endosc. 2007;66:1139–1141. doi: 10.1016/j.gie.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Groenen MJ, Moreels TG, Orlent H, Haringsma J, Kuipers EJ. Acute pancreatitis after double-balloon enteroscopy: an old pathogenetic theory revisited as a result of using a new endoscopic tool. Endoscopy. 2006;38:82–85. doi: 10.1055/s-2005-921179. [DOI] [PubMed] [Google Scholar]
- 13.Aktas H, Mensink PB, Haringsma J, Kuipers EJ. Low incidence of hyperamylasemia after proximal double-balloon enteroscopy: has the insertion technique improved? Endoscopy. 2009;41:670–673. doi: 10.1055/s-0029-1214976. [DOI] [PubMed] [Google Scholar]
- 14.May A, Ell C. Push-and-pull enteroscopy using the double-balloon technique/double-balloon enteroscopy. Dig Liver Dis. 2006;38:932–938. doi: 10.1016/j.dld.2006.07.101. [DOI] [PubMed] [Google Scholar]
- 15.Matsushita M, Shimatani M, Uchida K, Okazaki K. Mechanism of acute pancreatitis after peroral double-balloon enteroscopy. Endoscopy. 2007;39:480; author reply 481. doi: 10.1055/s-2007-966258. [DOI] [PubMed] [Google Scholar]
- 16.Kopacova M, Tacheci I, Rejchrt S, Bartova J, Bures J. Double balloon enteroscopy and acute pancreatitis. World J Gastroenterol. 2010;16:2331–2340. doi: 10.3748/wjg.v16.i19.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May A, Nachbar L, Schneider M, Neumann M, Ell C. Push-and-pull enteroscopy using the double-balloon technique: method of assessing depth of insertion and training of the enteroscopy technique using the Erlangen Endo-Trainer. Endoscopy. 2005;37:66–70. doi: 10.1055/s-2004-826177. [DOI] [PubMed] [Google Scholar]
- 18.Mensink PB, Haringsma J, Kucharzik T, Cellier C, Pérez-Cuadrado E, Mönkemüller K, Gasbarrini A, Kaffes AJ, Nakamura K, Yen HH, et al. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613–615. doi: 10.1055/s-2007-966444. [DOI] [PubMed] [Google Scholar]
- 19.Gerson LB, Tokar J, Chiorean M, Lo S, Decker GA, Cave D, Bouhaidar D, Mishkin D, Dye C, Haluszka O, et al. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177–1182, 1177-1182. doi: 10.1016/j.cgh.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Sunada K, Yamamoto H. Double-balloon endoscopy: past, present, and future. J Gastroenterol. 2009;44:1–12. doi: 10.1007/s00535-008-2292-4. [DOI] [PubMed] [Google Scholar]
- 21.Pata C, Akyüz U, Erzin Y, Mutlu N, Mercan A, Dirican A. Post-procedure elevated amylase and lipase levels after double-balloon enteroscopy: relations with the double-balloon technique. Dig Dis Sci. 2010;55:1982–1988. doi: 10.1007/s10620-009-0956-4. [DOI] [PubMed] [Google Scholar]
- 22.Traverso LW, MacFarlane S. Pancreas autotransplantation--unsuitability of the swine as a model. Transplantation. 1987;44:450–451. doi: 10.1097/00007890-198709000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Aslanian H, Salem RR, Marginean C, Robert M, Lee JH, Topazian M. EUS-guided ethanol injection of normal porcine pancreas: a pilot study. Gastrointest Endosc. 2005;62:723–727. doi: 10.1016/j.gie.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Yamao K, Okubo K, Hara K, Sawaki A, Mizuno N, Tajika M, Kawai H, Ashida R. Endoscopic ultrasound-guided ethanol injection in the pancreas in a porcine model: a preliminary study. J Gastroenterol Hepatol. 2008;23:e1–e6. doi: 10.1111/j.1440-1746.2007.04918.x. [DOI] [PubMed] [Google Scholar]
- 25.Imazu H, Sumiyama K, Ikeda K, Uchiyama Y, Aihara H, Kakutani H, Kaise M, Ang TL, Omar S, Tajiri H. A pilot study of EUS-guided hot saline injection for induction of pancreatic tissue necrosis. Endoscopy. 2009;41:598–602. doi: 10.1055/s-0029-1214823. [DOI] [PubMed] [Google Scholar]
- 26.Lamme B, Boermeester MA, Straatsburg IH, van Buijtenen JM, Boerma D, Offerhaus GJ, Gouma DJ, van Gulik TM. Early versus late surgical drainage for obstructive pancreatitis in an experimental model. Br J Surg. 2007;94:849–854. doi: 10.1002/bjs.5722. [DOI] [PubMed] [Google Scholar]
- 27.May A. Performing Double-Balloon Enteroscopy: The Utility of the Erlangen EndoTrainer. Techniques in Gastrointestinal Endoscopy. 2008;10:54–58. [Google Scholar]
- 28.Latorre R, Soria F, Lopez Albors O, Sarria R, Ayala I, Delgado I, Perez-Cuadrado E. Anatomy study of the pig intestine aimed to define a swine model for double balloon enteroscopy. Surgical and Radiologic Anatomy. 2009;31:169–170. [Google Scholar]
- 29.Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, Schembre D, Chen G, Semrad C, Kamal A, Harrison EM, et al. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. 2006;64:740–750. doi: 10.1016/j.gie.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Ell C, May A, Nachbar L, Schneider M, Gasbarrini A, di Caro S, Cellier C, Landi B. Prospective european multicenter trial for evaluation of push-and-pull enteroscopy in patients with small bowel diseases. Gastrointestinal Endoscopy. 2005;61:AB105. doi: 10.1055/s-2005-870126. [DOI] [PubMed] [Google Scholar]
- 31.Mensink PB, Haringsma J, Kuipers EJ. Low incidence of Hyperamylasemia after proximal double balloon enteroscopy: Adjustment of insertion technique. Gastrointestinal Endoscopy. 2008;67:AB286. doi: 10.1055/s-0029-1214976. [DOI] [PubMed] [Google Scholar]
- 32.Aktas H, Mensink P, Haringsma J, Kuipers EJ. Single Balloon Enteroscopy: Low Incidence of Procedure Related Hyperamylasemia and Complications. Gastrointestinal Endoscopy. 2009;69:AB197–AB197. [Google Scholar]
- 33.Lo SK. Technical matters in double balloon enteroscopy. Gastrointest Endosc. 2007;66:S15–S18. doi: 10.1016/j.gie.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 34.Jarbandhan SV, van Weyenberg SJ, van der Veer WM, Heine DG, Mulder CJ, Jacobs MA. Double balloon endoscopy associated pancreatitis: a description of six cases. World J Gastroenterol. 2008;14:720–724. doi: 10.3748/wjg.14.720. [DOI] [PMC free article] [PubMed] [Google Scholar]


