Abstract
Herein, we present a case of pneumoaorta and aortoduodenal fistula (ADF) caused by an endoluminal aortic prosthesis infection. An 82-year-old man underwent endovascular aneurysm repair with a stent graft to exclude a 5.1-cm abdominal aortic aneurysm. Three months after the index procedure, the patient was taken to the emergency department at a medical university hospital. He presented with a 2-d history of bloody diarrhea. An endoluminal aortic stent graft infection was diagnosed, and an ADF was identified. The patient died of septic shock despite emergency surgery and intensive care. When encountered, stent graft infections require appropriate antibiotics and graft explantation. The diagnosis of an ADF is important, and surgery remains the most effective management if septic shock presents despite conservative treatment.
Keywords: Aortoduodenal fistula, Endovascular aneurysm repair, Infection, Stent graft, Shock
INTRODUCTION
The use of stent grafts to treat abdominal aortic aneurysms (AAAs) has recently become more widely used[1-3]. Stent graft infection rates associated with use of the endovascular technique appear to be lower than those for conventional open repair (range: 0.3%-0.4%)[1,2] as demonstrated by Ducasse et al[3], who reported a frequency of infection of 0.43%. However, the incidence of infection resulting from the implantation of an endoluminal aortic prosthesis has been reported to be as high as 6%[4,5]. Although prophylactic antibiotics are routinely prescribed prior to an operation, the incidence of infection and possible sequelae remain difficult to predict. Surgical intervention with complete stent graft removal may provide the best outcome for patients with an infection[3]. The overall treatment strategy can be optimized with the early detection of an endovascular stent graft infection. Herein, we present the case of an elderly male patient with an endovascular stent graft infection who ultimately died of septic shock despite intensive care.
CASE REPORT
An 82-year-old male patient with type 2 diabetes initially presented with a bulging mass in the abdomen and localized pain. The patient underwent endovascular aneurysm repair with a stent graft (Zenith; Cook, Bloomington, IN) to exclude a 5.1-cm AAA in October 2010 (Figure 1A). The patient recovered without incident post-surgery and was discharged. Although the post-surgical protocol calls for an abdominal computed tomography (CT) scan at one month, the patient did not return to the cardiothoracic surgery clinic following his discharge.
Figure 1.
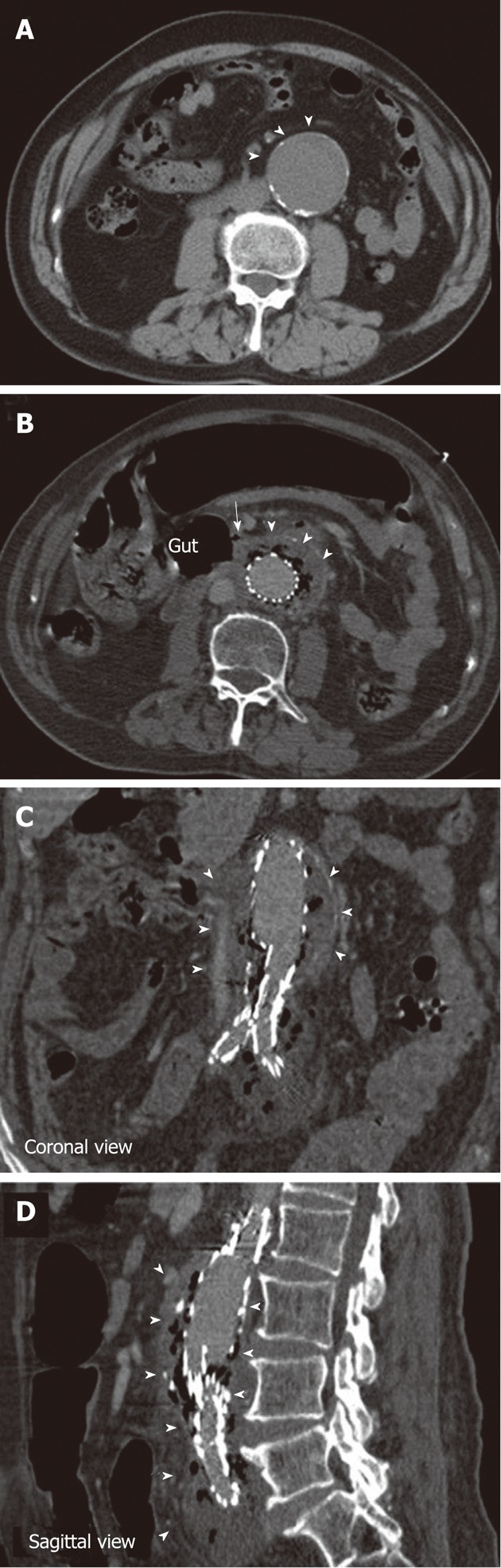
Abdominal computed tomography scans of the patient. A: The abdominal aortic aneurysm before stent-graft implantation (arrowheads); B: The aortoduodenal fistula (arrow); B-D: The necrotic tissue and associated gas formation (arrowheads).
Three months post-surgery, the patient was taken to an emergency department at a medical university hospital reporting a 2-d history of bloody diarrhea. Upon further examination, his blood pressure was measured at 121/55 mmHg, his body temperature was 37.8 °C, his pulse was 68/min, and his oxygen saturation was 88% as measured using a nasal cannula with 3 L/min of oxygen flow. A physical exam revealed pale conjunctiva and a distended abdomen. Furthermore, blood analysis revealed leukocytosis with a white blood cell count of 21 250 cells/mm3 and a hemoglobin level of 9.6 g/dL. Serum chemistry was unremarkable except for mildly elevated creatinine (1.3 mg/dL) with an estimated glomerular filtration rate of 56.45 mL/min. Empiric antibiotics were prescribed, including flomoxef and vancomycin. An abdominal CT scan disclosed a fistula between the aorta and the retroperitoneal duodenum, suggesting the formation of an aortoduodenal fistula (ADF) (Figure 1B). There was circumferential fluid collection with air surrounding the stent region, suggesting the presence of necrotic tissue and associated gas formation (Figure 1B-D). Due to the occurrence of refractory shock, an emergency operation was indicated. A bilateral axillary-femoral extra-anatomic bypass was performed with 8-mm polytetrafluoroethylene grafts. In addition, a retroperitoneal abscess and an abdominal aortic aneurysmal sac necrosis were debrided. The ADF was located at the bare-metal stent supra-renal fixation point. The stent graft was removed, and the aortic stump was closed just distal to the renal artery orifices. The tear at the third portion of the duodenum was repaired using LigaSure (Tyco-Healthcare, United States). A segmental resection of the duodenum and a side-to-end duodenojejunostomy were performed. The ADF was 1.5 cm × 1.3 cm in diameter.
Blood cultures revealed a mixed growth of Salmonella species, Bacteroides fragilis, Clostridium species and Gemella morbillorum. Bacterial culture of the necrotic tissue demonstrated a mixed Salmonella and Bacteroides infection. The patient died of septic shock two days after admission despite intensive care.
DISCUSSION
In the current case, a gas-forming bacterial infection resulted in the development of a pneumoaorta, which is uncommon. In one recent report[6], a 77-year-old man was diagnosed with a stent graft infection, and his CT scan demonstrated soft-tissue thickening and air present in the right anterolateral aspect of the aneurysm sac. Additionally, coagulase-negative staphylococci were identified in a blood culture. Another case report[7] described a stent graft infection due to Bacteroides fragilis. The patient’s condition was successfully managed with staged extra-anatomic revascularization followed by graft excision.
ADF is a well-recognized and dangerous condition[8-15]. According to a single-center review, five patients developed an ADF between 18 d and 1 year after successful endovascular aneurysm repair[12]. ADF has also been shown to occur as late as five years after endovascular aneurysm repair[13]. The patient in this study presented with ADF and bloody stool passage three months after the index procedure. ADFs and aortic aneurysms can be caused by biliary stent-induced retroperitoneal perforation[14]. However, the current patient did not report any prior gastrointestinal (GI) procedures, such as biliary stenting. The ADF etiology might have included infection and endoleak[12], but an endoleak was not observed on the CT scan. An ADF might further act as a connecting route between the GI tract and the aorta, causing bacterial propagation and infection-related deterioration. Therefore, the bloody stool passage was a possible sequela of the ADF formation[15].
In conclusion, prompt diagnosis and intervention are crucial for effectively treating a patient with an endovascular stent graft infection. A combination of the appropriate antibiotics and surgical repair is the best course for avoiding a fatal outcome. The most effective surgical intervention consists of a complete stent graft explantation followed by in situ reconstruction. Endovascular prosthesis implantation is a challenging technique for AAA, but the early recognition and detection of a possible stent infection may be more critical.
Footnotes
Peer reviewer: Dr. Guideng Li, Department of Biological Chemistry, University of California, 3101 Hewitt Hall, Irvine, CA 92697-4120, United States
S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Dattilo JB, Brewster DC, Fan CM, Geller SC, Cambria RP, Lamuraglia GM, Greenfield AJ, Lauterbach SR, Abbott WM. Clinical failures of endovascular abdominal aortic aneurysm repair: incidence, causes, and management. J Vasc Surg. 2002;35:1137–1144. doi: 10.1067/mva.2002.124627. [DOI] [PubMed] [Google Scholar]
- 2.Faries PL, Brener BJ, Connelly TL, Katzen BT, Briggs VL, Burks JA, Gravereaux EC, Carroccio A, Morrissey NJ, Teodorescu V, et al. A multicenter experience with the Talent endovascular graft for the treatment of abdominal aortic aneurysms. J Vasc Surg. 2002;35:1123–1128. doi: 10.1067/mva.2002.123324. [DOI] [PubMed] [Google Scholar]
- 3.Ducasse E, Calisti A, Speziale F, Rizzo L, Misuraca M, Fiorani P. Aortoiliac stent graft infection: current problems and management. Ann Vasc Surg. 2004;18:521–526. doi: 10.1007/s10016-004-0075-9. [DOI] [PubMed] [Google Scholar]
- 4.Jackson MR, Clagett GP. Aortic graft infection. In: Cronenwett JL, Rutherford RB, editors. Decision making in vascular surgery. Philadelphia, PA: WB Saunders; 2001. pp. 186–191. [Google Scholar]
- 5.Sharp WJ, Hoballah JJ, Mohan CR, Kresowik TF, Martinasevic M, Chalmers RT, Corson JD. The management of the infected aortic prosthesis: a current decade of experience. J Vasc Surg. 1994;19:844–850. doi: 10.1016/s0741-5214(94)70009-5. [DOI] [PubMed] [Google Scholar]
- 6.Sharif MA, Lee B, Lau LL, Ellis PK, Collins AJ, Blair PH, Soong CV. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;46:442–448. doi: 10.1016/j.jvs.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Baker M, Uflacker R, Robison JG. Stent graft infection after abdominal aortic aneurysm repair: a case report. J Vasc Surg. 2002;36:180–183. doi: 10.1067/mva.2002.123331. [DOI] [PubMed] [Google Scholar]
- 8.Suezawa T, Aoki A, Tago M, Iga N, Miyahara K, Wato M, Inaba T, Kawai K. Endovascular repair and pharmacotherapy of an inflammatory abdominal aortic aneurysm complicated by primary aortoduodenal fistula. Ann Vasc Surg. 2011;25:559.e7–559.11. doi: 10.1016/j.avsg.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Jayarajan S, Napolitano LM, Rectenwald JE, Upchurch GR. Primary aortoenteric fistula and endovascular repair. Vasc Endovascular Surg. 2009;43:592–596. doi: 10.1177/1538574409335275. [DOI] [PubMed] [Google Scholar]
- 10.Pulvirenti E, Toro A, Patanè D, Scolaro A, Di Carlo I. Surgery of the aortoduodenal fistula: two cases with survival. G Chir. 2009;30:157–159. [PubMed] [Google Scholar]
- 11.Papacharalambous G, Skourtis G, Saliveros A, Karagannidis D, Makris S, Panousis P, Ktenidis K. Endovascular treatment of a primary aortoduodenal fistula: 2-year follow-up of a case report. Vasc Endovascular Surg. 2007;41:265–270. doi: 10.1177/1538574407300919. [DOI] [PubMed] [Google Scholar]
- 12.Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther. 2008;15:441–448. doi: 10.1583/08-2377.1. [DOI] [PubMed] [Google Scholar]
- 13.Ruby BJ, Cogbill TH. Aortoduodenal fistula 5 years after endovascular abdominal aortic aneurysm repair with the Ancure stent graft. J Vasc Surg. 2007;45:834–836. doi: 10.1016/j.jvs.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 14.Lee TH, Park DH, Park JY, Lee SH, Chung IK, Kim HS, Park SH, Kim SJ. Aortoduodenal fistula and aortic aneurysm secondary to biliary stent-induced retroperitoneal perforation. World J Gastroenterol. 2008;14:3095–3097. doi: 10.3748/wjg.14.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sukawa Y, Goto A, Okuda H, Suzuki K, Hasegawa Y, Yonezawa K, Abe T, Shinomura Y. Unexplained melena associated with a history of endovascular stent grafting of abdominal aortic aneurysms: aortoduodenal fistula. Endoscopy. 2009;41 Suppl 2:E84. doi: 10.1055/s-0029-1214484. [DOI] [PubMed] [Google Scholar]


