Abstract
Penicillium marneffei (P. marneffei) infection usually occurs with skin, bone marrow, lung or hepatic involvement. However, no cases of P. marneffei infection with chylous ascites have been reported thus far. In this report, we describe the first case of acquired immune deficiency syndrome (AIDS) which has been complicated by a P. marneffei infection causing chylous ascites. We describe the details of the case, with an emphasis on treatment regimen. This patient was treated with amphotericin B for 3 mo, while receiving concomitant therapy with an efavirenz-containing antiretroviral regimen, but cultures in ascitic fluid were persistently positive for P. marneffei. The infection resolved after treatment with high-dose voriconazole (400 mg every 12 h) for 3 mo. P. marneffei should be considered in the differential diagnosis of chylous ascites in human immunodeficiency virus patients. High-dose voriconazole is an effective, well-tolerated and convenient option for the treatment of systemic infections with P. marneffei in AIDS patients on an efavirenz-containing antiretroviral regimen.
Keywords: Chylous ascites, Penicillium marneffei, Acquired immune deficiency syndrome, Voriconazole, Efavirenz, Fungal sepsis
INTRODUCTION
Penicillium marneffei (P. marneffei) infection now represents one of the most common acquired immune deficiency syndrome (AIDS)-defining opportunistic infections in endemic areas of Southeast Asia[1-3]. The infection is associated with a high mortality rate if timely treatment with appropriate antifungal drugs is not administered[2,4]. P. marneffei usually occurs with skin, bone marrow, lung or hepatic involvement; however, no cases of P. marneffei infection with chylous ascites have yet been reported. In this report, we describe the first case of AIDS which has been complicated by a P. marneffei infection causing chylous ascites. We describe the details of the case with emphasis on treatment regimen.
CASE REPORT
A 47-year-old man, a native of Yunnan province, southwest China, infected with human immunodeficiency virus (HIV) who had a CD4 cell count of 66 cells/μL had a 1-year history of intermittent fever and a 6-mo history of abdominal distension. Culture of blood and ascitic fluid revealed P. marneffei. He was diagnosed with AIDS and P. marneffei infection. He had received antiretroviral therapy (ART) with stavudine (30 mg twice daily), lamivudine (300 mg once daily) and efavirenz (600 mg daily before bedtime). He was treated with amphotericin B 25 mg/d which was continued for 3 mo, but his condition did not improve. Persistent abdominal distension remained, so paracentesis was performed periodically to relieve the symptoms. Cultures in ascitic fluid were persistently positive for P. marneffei. He came to our hospital for further management. Examination revealed the presence of shifting dullness and paracentesis confirmed diagnosis of chylous ascites. Total serum protein, albumin, total cholesterol and triacylglycerol were 65.80 g/L, 37.70 g/L, 3.92 mmol/L and 0.95 mmol/L, respectively. The ascitic fluid had a milky appearance(Figure 1A) and showed 270 white blood cells/μL with 60% multinucleated cells and 40% lymphocytes, triglyceride 13.59 mmol/L (normal range ﹤1.70 mmol/L), and total protein 31.5 g/L. No malignant cells were found on pathology. Chest computer tomography scan showed no abnormalities while abdominal magnetic resonance imaging showed hepatosplenomegaly with ascites (Figure 1B). Treatment with oral voriconazole (400 mg every 12 h) was started and was continued for 3 mo. He continued on ART (stavudine, lamivudine and efavirenz), but he was recommended to take a lower dose of efavirenz (300 mg once daily before bedtime). In order to monitor efficacy and side effects, we performed therapeutic drug concentration monitoring (TDM) of both voriconazole and efavirenz. The results showed that the peak and trough concentrations of voriconazole were 2.31 mg/L and 1.42 mg/L, respectively and the peak and trough concentrations of efavirenz were 3720 ng/mL and 2680 ng/mL, respectively. While on voriconazole, he improved. All disease-related clinical symptoms and signs gradually disappeared. Fungal cultures of chylous ascites became negative after receiving 2 wk of voriconazole treatment. The values of laboratory tests including electrolytes, renal function and transaminases were within normal ranges during treatment. The infection resolved after treatment with voriconazole for 3 mo and was discontinued when recovery was thought to be achieved. He was recommended to take a normal dose of efavirenz (600 mg once daily) after discontinuation of voriconazole. Six months after initiation of ART, his CD4 cell count rose to 110 cells/μL. He refused to receive secondary prophylaxis with antifungal drugs. No relapse was found in an 8-mo follow-up.
Figure 1.
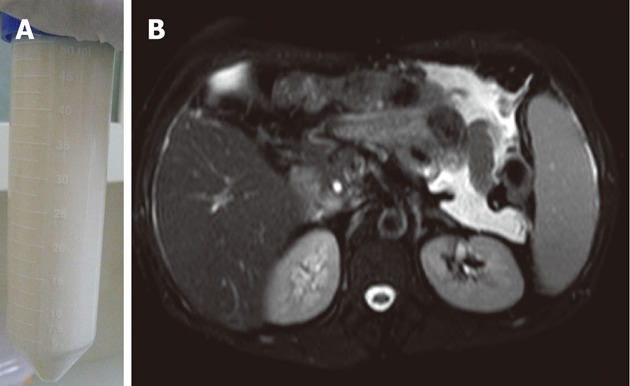
The auxiliary examination for the patient. A: The ascitic fluid had a milky appearance; B: Abdominal magnetic resonance imaging showed hepatosplenomegaly with ascites.
DISCUSSION
Prior to the epidemic of AIDS, penicilliosis was a rare event. The incidence of this fungal infection has increased markedly during the past few years, paralleling the incidence of HIV infection[1]. In China, P. marneffei infection is endemic in Guangdong, Guangxi, Yunnan, HongKong and Taiwan[5-7]. In recent years, with the increase of HIV infection, cases have been reported from non-endemic regions[3,8].
Chylous ascites is a rare complication of AIDS. It is caused by the leakage of chyle into the peritoneum due to rupture or obstruction of the thoracic duct. P. marneffei is a systemic fungal infection which usually occurs with skin, bone marrow, lung or hepatic involvement. This patient is the first case of AIDS which has been complicated by a P. marneffei infection causing chylous ascites. This case suggests that P. marneffei should be an important differential diagnosis in chylous ascites in HIV patients, especially in visitors to, or residents of, endemic areas for P. marneffei.
The recommended treatment for P. marneffei is amphotericin B for 2 wk, followed by oral itraconazole for a subsequent duration of 10 wk[9]. ART should be administered simultaneously to improve outcome[9], but avoiding adverse drug interactions between antifungals and antivirals is also important as these can complicate therapy. This patient had failed to respond to initial amphotericin B treatment. P. marneffei is highly susceptible to miconazole, itraconazole, ketoconazole, and 5-flucytosine. Amphotericin B has intermediate antifungal activity[9] which means some patients may not respond to treatment with it. Up to now, alternative treatment options for penicilliosis have not been established. A small case series[10] reported good outcomes with voriconazole in AIDS patients with systemic P. marneffei infections, but none of the patients in that study received ART simultaneously. Efavirenz is a mixed inducer and inhibitor of CYP3A4, 2C9 and 2C19. Concomitant use of itraconazole and efavirenz may result in subtherapeutic levels of itraconazole[9], so use of an alternate antifungal treatment may be necessary. Alternatively efavirenz can be replaced with a noninducing class of antiretrovirals. The limited number of antiviral drugs limits the choice of treatment of AIDS in China. We chose voriconazole as the alternative treatment option for penicilliosis. Voriconazole is metabolized primarily by CYP2C19, as well as CYP2C9 and CYP3A4[11]. Voriconazole is also known to inhibit these enzymes[11], and the manufacturer reports an extensive list of drugs that interact with voriconazole. Efavirenz reduces the plasma concentration of voriconazole which increases the plasma concentration of efavirenz, thus dose adjustments for voriconazole and efavirenz may be needed if concomitant use of these two agents is necessary[9]. It is recommended that clinicians increase voriconazole to 400 mg every 12 h and decrease efavirenz to 300 mg once daily before bedtime[9]. Therefore, we increased the voriconazole dose to 400 mg twice daily and decreased the efavirenz dose to 300 mg once daily before bedtime.
A relationship between progression of fungal infection and voriconazole concentrations was demonstrated in several studies[12-14]. They showed that monitoring voriconazole concentration and adjusting dosage to attain an appropriate plasma concentration is necessary to ensure antifungal effect and to avoid toxicity. TDM represents an important process to optimize the outcome of immunocompromised patients receiving triazoles[15]. The patient was treated with amphotericin B followed by 3 mo of high-dose voriconazole therapy, which resulted in a clinical cure. Treatment with high-dose voriconazole was well tolerated, with no discontinuations caused by drug-related adverse events. The patient also received ART containing low-dose efavirenz, without any relapse of P. marneffei infection. Treatment with low-dose efavirenz in this patient was also effective, with clinically significant increases in his CD4 counts. The results of this study suggest that high-dose voriconazole is an effective, well-tolerated, and convenient option for the treatment of systemic infections with P. marneffei in AIDS patients on efavirenz-containing ART. There may be a role for voriconazole and efavirenz serum concentration monitoring to ensure therapeutic efficacy when the drugs are used concomitantly.
All patients who successfully complete treatment for penicilliosis should receive oral itraconazole as maintenance therapy to prevent relapse[9]. Discontinuation of secondary prophylaxis is recommended for AIDS patients who receive ART and have CD4 count > 100 cells/μL for > 6 mo [9]. This patient did not receive secondary prophylaxis with antifungal drugs. He had a CD4 count > 100 cells/μL 6 mo after initiation of ART. During the follow-up, no relapse of the fungal infection was observed.
In conclusion, this case report indicates that penicilliosis marneffei should be considered in the differential diagnosis of chylous ascites in HIV patients. High-dose voriconazole is an effective, well-tolerated, and convenient option for the treatment of penicilliosis marneffei in AIDS patients on efavirenz-containing ART. Further research into alternative treatment options for penicilliosis in AIDS patients is required.
Footnotes
Peer reviewers: Dr. Elena Vezali, Department of Hepatology, Hygeia Diagnostic and Therapeutic Center of Athens, Erythrou Staurou 4, Maroussi, 15123 Athens, Greece; Sri Prakash Misra, Professor, Department of Gastroenterology, Moti Lal Nehru Medical College, Allahabad 211001, India; Virendra Singh, MD, Professor, Department of Hepatology, Post Graduate Institute of Medical Education and Research, Chandigarh 160012, India; Diego Garcia-Compean, Professor, Faculty of Medicine, Autonomous University of Nuevo Leon, 64700 Monterrey, Mexico
S- Editor Gou SX L- Editor O’Neill M E- Editor Xiong L
References
- 1.Sirisanthana T, Supparatpinyo K. Epidemiology and management of penicilliosis in human immunodeficiency virus-infected patients. Int J Infect Dis. 1998;3:48–53. doi: 10.1016/s1201-9712(98)90095-9. [DOI] [PubMed] [Google Scholar]
- 2.Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19:95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ustianowski AP, Sieu TP, Day JN. Penicillium marneffei infection in HIV. Curr Opin Infect Dis. 2008;21:31–36. doi: 10.1097/QCO.0b013e3282f406ae. [DOI] [PubMed] [Google Scholar]
- 4.Supparatpinyo K, Nelson KE, Merz WG, Breslin BJ, Cooper CR, Kamwan C, Sirisanthana T. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated Penicillium marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob Agents Chemother. 1993;37:2407–2411. doi: 10.1128/aac.37.11.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liyan X, Changming L, Xianyi Z, Luxia W, Suisheng X. Fifteen cases of penicilliosis in Guangdong, China. Mycopathologia. 2004;158:151–155. doi: 10.1023/b:myco.0000041842.90633.86. [DOI] [PubMed] [Google Scholar]
- 6.Wong SS, Yuen KY. Penicilliosis in China. Mycopathologia. 2004;158:147–150. doi: 10.1023/b:myco.0000041898.18359.5a. [DOI] [PubMed] [Google Scholar]
- 7.Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of Penicillium marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008;14:103–109. [PubMed] [Google Scholar]
- 8.Antinori S, Gianelli E, Bonaccorso C, Ridolfo AL, Croce F, Sollima S, Parravicini C. Disseminated Penicillium marneffei infection in an HIV-positive Italian patient and a review of cases reported outside endemic regions. J Travel Med. 2006;13:181–188. doi: 10.1111/j.1708-8305.2006.00039.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207; quiz CE1-4. [PubMed] [Google Scholar]
- 10.Supparatpinyo K, Schlamm HT. Voriconazole as therapy for systemic Penicillium marneffei infections in AIDS patients. Am J Trop Med Hyg. 2007;77:350–353. [PubMed] [Google Scholar]
- 11.Gerzenshtein L, Patel SM, Scarsi KK, Postelnick MJ, Flaherty JP. Breakthrough Candida infections in patients receiving voriconazole. Ann Pharmacother. 2005;39:1342–1345. doi: 10.1345/aph.1E627. [DOI] [PubMed] [Google Scholar]
- 12.Smith J, Safdar N, Knasinski V, Simmons W, Bhavnani SM, Ambrose PG, Andes D. Voriconazole therapeutic drug monitoring. Antimicrob Agents Chemother. 2006;50:1570–1572. doi: 10.1128/AAC.50.4.1570-1572.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 14.Ueda K, Nannya Y, Kumano K, Hangaishi A, Takahashi T, Imai Y, Kurokawa M. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int J Hematol. 2009;89:592–599. doi: 10.1007/s12185-009-0296-3. [DOI] [PubMed] [Google Scholar]
- 15.Hope WW, Billaud EM, Lestner J, Denning DW. Therapeutic drug monitoring for triazoles. Curr Opin Infect Dis. 2008;21:580–586. doi: 10.1097/QCO.0b013e3283184611. [DOI] [PubMed] [Google Scholar]


