Abstract
Consistent with a popular theory of associative learning, the Pearce-Hall (1980) model, the surprising omission of expected events enhances cue associability (the ease with which a cue may enter into new associations), across a wide variety of behavioral training procedures. Furthermore, previous experiments from this laboratory showed that these enhancements are absent in rats with impaired function of the amygdala central nucleus (CeA). A notable exception to these assertions is found in feature negative (FN) discrimination learning, in which a “target” stimulus is reinforced when it is presented alone but nonreinforced when it is presented in compound with another, “feature” stimulus. According to the Pearce-Hall model, reinforcer omission on compound trials should enhance the associability of the feature relative to control training conditions. However, prior experiments have shown no evidence that CeA lesions affect FN discrimination learning. Here we explored this apparent contradiction by evaluating the hypothesis that the surprising omission of an event confers enhanced associability on a cue only if that cue itself generates the disconfirmed prediction. Thus, in a FN discrimination, the surprising omission of the reinforcer on compound trials would enhance the associability of the target stimulus but not that of the feature. Our data confirmed this hypothesis, and showed this enhancement to depend on intact CeA function, as in other procedures. The results are consistent with modern reformulations of both cue and reward processing theories that assign roles for both individual and aggregate error terms in associative learning.
Keywords: attention, associability, associative learning, feature negative discrimination, amygdala
Considerable behavioral evidence indicates that the disconfirmation of learned expectancies (“surprise”) can alter processing of stimuli present near the time of that surprise. Pearce and Hall (1980) proposed a model that related the associability of conditioned stimuli (CSs), their ability to participate in the formation of associations, to surprise. Within that model, the associability of a CS is proportional to the absolute value of the difference between the expected and actual value of the reinforcer on recent episodes that included that CS. Evidence suggests that such enhancements in CS associability that occur when reinforcers are unexpectedly omitted depend on the integrity of a neural system that includes the amygdala central nucleus (CeA) and its interactions with dopaminergic neurons of the substantia nigra pars compacta (SNc), cholinergic neurons of the substantia innominata (SI), and those latter neurons’ projections to posterior parietal cortex (PPC). Several behavioral phenomena attributed to these enhancements, including performance in a serial prediction task, unblocking with reinforcer downshifts, enhanced overshadowing after partial reinforcement, and augmented serial negative patterning learning, fail to occur in rats with disconnections among the elements of this system (see Holland & Maddux, 2010, for a review).
Much of the evidence for these assertions come from experiments that used a serial prediction task first used by Wilson, Boumphrey, and Pearce (1992). In that task, rats received Pavlovian conditioning training in which the predictive validity of a visual CS was manipulated by altering its relation with an auditory CS. Rats in a “consistent” condition received training in which a light consistently predicted a tone throughout training, whereas rats in a “surprise” condition received training in which the validity of the light in predicting the tone was suddenly reduced from a consistent to a 50% relationship by omitting the tone on half of the trials. According to the Pearce-Hall (1980) model, this violation should cause an increase in the associability of the light. Indeed, subsequent light-food learning was more rapid in rats in the surprise condition (Wilson et al, 1992). However, rats with disruptions in function of CeA or SI (Chiba et al., 1995; Holland & Gallagher, 1993a, 2006), or functional disconnections between these 2 regions (Han et al., 1999), between CeA and SNc (Lee et al., 2006, 2008), or between SI and PPC (Bucci et al., 1998) all fail to display this enhanced associability after surprise.
Similarly, rats with lesions of CeA (Holland & Gallagher, 1993b) or lesions that eliminate SI’s cholinergic innervation of PPC (Bucci et al., 1998) fail to show unblocking with reinforcer downshifts. Normally, prior training of a light-food relation “blocks” subsequent tone-food learning when a light + tone compound is paired with the same food reinforcer. However, under some circumstances, if the value of the reinforcer is reduced when the tone is added, tone-food learning is not blocked. This “unblocking” is especially likely when reinforcer value is lowered by omitting one of a series of reinforcer events (e.g., the second of two shocks or the second of two food deliveries; Dickinson, Hall, & Mackintosh, 1976; Holland, 1984, 1988). Within the Pearce-Hall model, this omission enhances the associability of the available cues, encouraging the association of the added tone with the remaining reinforcer. Because many learning theories (e.g., Rescorla & Wagner, 1972) predict that cues that accompany reductions in reinforcer value should acquire inhibitory learning, the observation of unblocking under these circumstances is an especially powerful confirmation of the importance of attentional processes specified by the Pearce-Hall (1980) model. Thus, observations that the same circuit-element disruptions that affect performance in the serial prediction task also affect unblocking when an expected reinforcer is omitted, support a role for this circuit in the enhancement of cue associability after the surprising omission of expected events.
An anomaly within this framework is the lack of effects of CeA lesions on performance in the feature negative (FN) or conditioned inhibition (Rescorla, 1969) procedure (Holland, Thornton, & Ciali, 2000; Holland, Chik, & Zhang, 2001). In this procedure, a CS is reinforced when it is presented alone, but nonreinforced when it is accompanied by another, “feature” or “conditioned inhibitor” cue. As in the serial prediction task, this procedure involves partial reinforcement of a CS, and as in unblocking, it involves a reduction in the value of the reinforcer between presentations of a CS alone and those of a compound of that CS and another cue. Indeed, reduction of the value of the reinforcer to zero on compound trials in the FN procedure might be construed as the ultimate reduction in reinforcer value, and hence might also be expected to enhance or maintain the associability of both cues. In that case, rats with CeA lesions might be expected to exhibit slower learning than intact rats in this task, because they would not benefit from enhancements in cue associability normally produced by the surprising omission of the expected reinforcer. Nevertheless, Holland et al. (2000) found no effect of CeA lesions on the acquisition of performance in a serial FN procedure, in which an auditory cue was reinforced with food when it was presented alone, but nonreinforced when it was preceded by a light feature stimulus, and Holland et al. (2001) found no effects of CeA lesions on the acquisition of a simultaneous FN discrimination, in which an auditory cue was reinforced with food when it was presented alone, but not when it was presented simultaneously with a light cue. This latter observation was especially noteworthy because other rats in the same study demonstrated CeA lesion deficits in learning after the surprise treatment procedure in the serial prediction task.
The present experiments were intended to further investigate the role of CeA in FN discriminations. In Experiments 1 and 2 we examined, in both sham- and CeA-lesioned rats, the acquisition of FN discriminations under training conditions that might be expected to influence the impact of surprise-induced associability enhancements. Regardless of those conditions, we found no effects of CeA lesions. In Experiment 3, we evaluated an alternative formulation of the Pearce-Hall (1980) model in which enhancement of the associability of the feature cue might not be anticipated in FN discrimination learning. Within that reformulation, the facilitatory effects of surprise on associability apply only to cues that generate the disconfirmed expectancy. This perspective corresponds to the casual view that attention is maintained to inconsistently treated cues (e.g., the target), but not to cues that consistently predict reinforcement or nonreinforcement (e.g., the feature). Accordingly, in Experiment 3 we directly compared the associability of feature and target cues after FN discrimination learning in CeA- and sham-lesioned rats.
Experiment 1
Holland et al.’s (2001) observation of no effect of CeA lesions on simultaneous FN discrimination learning involved a feature cue that had previously been trained as the first element in the predictive validity task described in the Introduction. It is possible that this prior treatment of the feature contributed to their failure to observe a lesion effect. Experiment 1 was designed to examine the effects of CeA lesions on simultaneous FN discrimination learning in the absence of such confounds. After initial pairings of a noise target CS with a sucrose reinforcer, rats with neurotoxic or sham lesions of CeA received presentations of a nonreinforced simultaneous compound of the noise and a light stimulus, intermixed with reinforced presentations of the noise alone. If surprise-induced associability increments occur to the cues present at the time of surprise, then the associability of the light feature would be enhanced in sham-lesioned rats. If such an enhancement normally contributes to the acquisition of inhibition to that feature, then FN discrimination learning would be impaired by the CeA lesion.
Methods
Subjects
Thirty-one male, experimentally naive CD-strain rats (Charles River Laboratories, Raleigh, NC), approximately 350 g at the beginning of the experiment, served as subjects. The rats were individually housed in stainless steel cages in a vivarium that was maintained at 23° C, with the lights on from 6:00 a.m. to 8:00 p.m. daily. All experimental sessions were carried out during the light portion of the cycle, between 8:00 a.m. and 2:00 p.m. The rats were maintained at 85% of their ad lib weights by limiting their access to food; water was always available.
Apparatus
The apparatus consisted of eight individual chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls, clear acrylic sides and top, and a grid floor (0.48-cm stainless steel rods spaced 1.9 cm apart). A dimly illuminated food cup was recessed 2 cm to the right of the center of one end wall, 2 cm above the floor. Access to the food cup was through a 5 × 5 cm opening in the end wall, equipped with infrared photocells (sampled by computer circuitry at approximately 1 kHz), mounted flush with the wall. A jeweled 6-W lamp (panel light) was located 5 cm above that opening. An infrared activity monitor (Coulbourn Instruments, Allentown, PA) was mounted on the top of each chamber. Each experimental chamber was enclosed in a sound-resistant shell with an acrylic window for viewing the rats. A speaker, used to present the auditory CSs, was mounted on the inside wall of the shell, 10 cm above and 10 cm to one side of the experimental chamber, even with the wall that contained the food cup. Ventilation fans provided masking noise (70 DB).
Surgical procedures
Under Nembutal (50 mg/kg) anesthesia, rats received bilateral ibotenic acid or sham lesions of CeA. Infusions of 0.2 μl of 10 μg/μl ibotenic acid (Regis Chemical, Morton Grove, IL), dissolved in 0.1M phosphate-buffered saline (PBS), or PBS alone were made at a single injection site in each hemisphere, using a pulled glass micropipette connected to a Hamilton 2-μl syringe. Stereotaxic coordinates, relative to Bregma, were anterior-posterior −2.3, medial-lateral ±4.2, and dorsal-ventral −7.7. All surgical and experimental procedures were approved by the Animal Care and Use Committee of Duke University, where this experiment was conducted.
Behavioral Procedures
Initially, all rats were trained to eat from the recessed food cup. In a single 64-min session, there were 16 presentations of the reinforcer used throughout this experiment, the delivery of 0.3 ml of 0.2-M sucrose solution, on a variable-time 4-min schedule. As in most studies of conditioned inhibition, we first established conditioning to the stimulus to be used as the target cue in the FN discrimination, an 80-db white noise. There were five daily 64-min sessions in this phase, each including eight pairings of the noise and sucrose. Next, the rats were trained on a FN discrimination over the course of 32 daily sessions. In each of these 64-min sessions, rats received two 5-s noise-sucrose pairings and 6 nonreinforced simultaneous 5-s noise + light compound presentations, randomly intermixed, with intertrial intervals (ITIs) that averaged 8 min (range, 4–12 min).
Data analysis
The primary measure of conditioning was the percentage of time during the CS that the photocell in the food cup was activated. We reported the percentages of time spent in the food cup during the 5-s interval before CS presentations, during the 5-s reinforced cue(s), and during the 5-s nonreinforced light + noise compound. We also reported activity counts/min generated by the activity monitor during the same periods, for noise and light + noise compound trials. This measure provided a crude index of startle responding that occurs to the onset of novel auditory cues, and which in intact rats is maintained when those cues are paired with reinforcement (Holland, 1977; El-Amamy & Holland, 2007). Each of these measures was subjected to standard analyses of variance (ANOVAs), with lesion (CeA vs. sham) as a between-subject variable, and sessions or blocks of sessions, and (if appropriate) stimulus (compound vs target-alone) as repeated measures. The Greenhouse-Giesser correction was used for analysis of repeated measures to correct for any violations of sphericity.
Histological procedures
After completion of behavioral testing, the rats were deeply anaesthetized with sodium pentobarbital, and perfused transcardially with 0.9% 0.1-M PBS followed by 10% formalin. Brains were removed and stored in 0.1-M PBS containing 20% sucrose and 1% DMSO at 4 C. Using a sliding microtome, 40-μm sections were taken from each brain, mounted on slides and Nissl-stained to verify the CeA lesions.
Results
Histological results
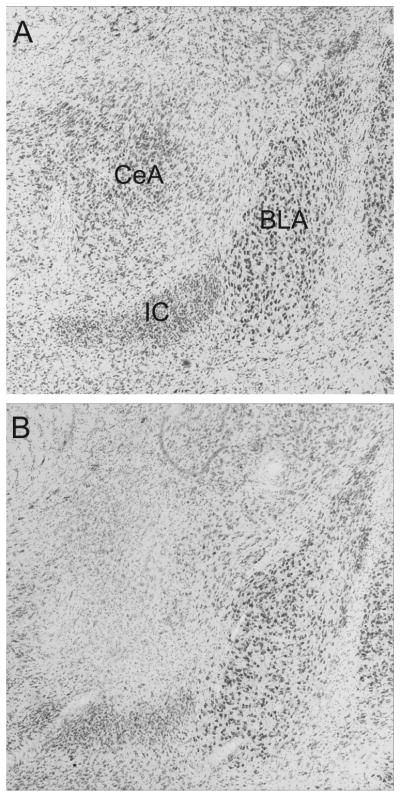
Lesion judgments were made blind to the behavioral data and treatment conditions. Rats with intended CeA lesions were excluded if there was less than about 40% destruction of CeA in either hemisphere, or if there was more than minimal damage to surrounding tissue, especially basolateral amygdala. Nine of 16 lesioned rats met this criterion, averaging 60.6 ± 5.0% damage. Sham-lesioned rats showed no evidence for CeA damage except for minimal damage around the injector track; all 15 sham-lesioned rats were accepted. Figure 1 shows sample sham and neurotoxic lesions.
Figure 1.
Photomicrographs of typical sham (A) and neurotoxic (B) lesions of the amygdala central nucleus (CeA). BLA refers to the basolateral amygdala; IC to the intercalated nuclei.
Behavioral results
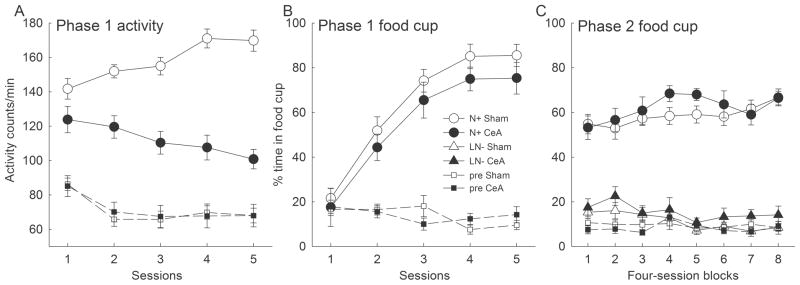
Figure 2 shows the acquisition of activity and food cup responses to the noise cue in initial training. Activity responding to the noise (Figure 2A) was maintained in intact rats but not CeA-lesioned rats. This pattern is consistent with previous findings that CeA lesions do not affect unconditioned startle responding to novel auditory cues, but impair the acquisition or maintenance of such orienting responses (ORs) to auditory cues paired with food (Gallagher et al., 1990; El-Amamy & Holland, 2007). ANOVA showed a significant main effect of lesion (F1,22 = 47.96, p < .001) and a lesion x session interaction (F4,88 = 11.05, p < .001), but no effect of session F < 1. ANOVA of pre-CS activity showed only a main effect of sessions (F4,88 = 11.48, p < .001; remaining ps > .88). By contrast, acquisition of food cup responding to the noise (Figure 2B) was unaffected by the CeA lesions. ANOVA showed only a main effect of sessions (F4,88 = 65.34, p < .001; other ps > .22). ANOVA of pre-CS food cup responding showed only a marginally significant effect of sessions (F4,88 = 2.33, p < .10; other ps > .12.) One sham-lesioned rat failed to acquire conditioned food cup responding in this phase and was dropped from the experiment.
Figure 2.
Results of Experiment 1. As in previous studies, rats with CeA lesions showed deficits in the acquisition of conditioned orienting responses (activity; Panel A) but not conditioned food-related responses (food cup; Panel B) to the reinforced noise (N+) conditioned stimulus (CS) during the initial acquisition phase of Experiment 1. Most important, discrimination of food cup responding between reinforced noise (N+) and nonreinforced light + noise (LN−) compound trials in feature negative discrimination training was unaffected by the lesions (Panel C). The abscissa points labeled “pre” refer to responding in the periods immediately prior to CS presentations. The designations CeA and sham refer to rats with neurotoxic or sham lesions of the amygdala central nucleus (CeA), respectively.
Figure 2C shows the acquisition of the FN discrimination, as reflected by food cup responding. The CeA lesions had no effect on discrimination learning, despite evidence from the previous phase (ORs) that those lesions were behaviorally effective. Discrimination performance was evaluated with a group x lesion x cue x session block ANOVA. Only the effect of cue (F1,22 = 349.48, p < .001) and the cue x blocks interaction (F7,154 = 9.47, p < .001) were significant; all other ps > .20. A group x lesion x block ANOVA of pre-CS responding showed no significant effects, Fs < 1, ps > .53.
Activity responding (not shown) to the noise alone gradually declined over the course of discrimination training, but the lesion difference found in acquisition was maintained. Activity responding to the light + noise compound was suppressed relative to the noise alone throughout training.
The lack of an effect of CeA lesions on the acquisition of the FN discrimination suggests that CS associability enhancements did not contribute to normal FN discrimination learning. However, this absence of associability enhancement may simply reflect the lack of surprise on nonreinforced compound trials. Responding to the compound was suppressed immediately on its first introduction. The rats may have attended exclusively to the added noise or spontaneously configured that compound as distinct from either of its elements. Thus, there would have been little if any “surprise” from omission of the reinforcer on compound trials. We examined this possibility in Experiment 2 by switching the roles of the light and noise cues, which altered the rate of learning about the target and compound cues.
Experiment 2
Experiment 2 was identical to Experiment 1, except that the roles of the light and noise were interchanged. That is, the noise was the feature and the light was the target element in the FN procedure. If the immediate suppression of responding to the compound in Experiment 1 was the result of much greater salience of the noise than of the light, then prior excitatory training of the more salient element would insure that initial presentations of the compound would engage strong expectation of food delivery, and hence substantial surprise when the food was omitted.
Method
The apparatus, surgical and histological procedures, data analysis, characteristics of the 20 rat subjects, and the behavioral training procedures were identical to those of Experiment 1, except that the roles of the light and noise stimuli were reversed.
Results
Histological results
In Experiment 2, 7 of 12 lesioned rats in Group FN met the criteria described in Experiment 1. All 8 sham-lesioned rats were accepted.
Behavioral results
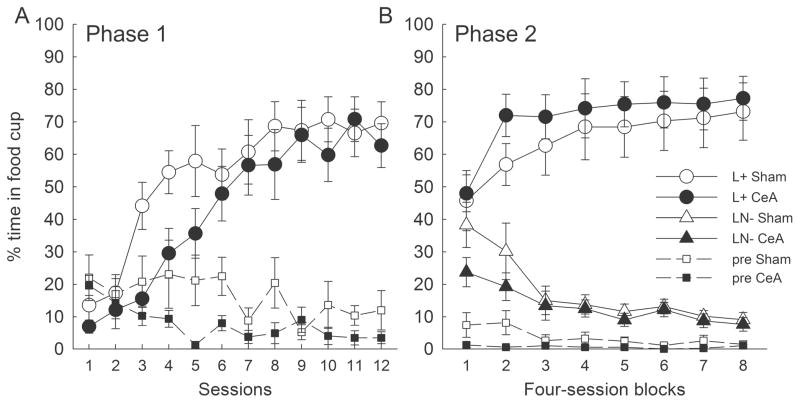
Figure 3A shows the acquisition of food cup behavior to the light CS in the initial training phase. As expected, acquisition of responding was considerably slower than acquisition of responding to the more salient noise in Experiment 1. ANOVA of food cup responding to the light showed only a main effect of session (F11,110 = 20.92, p < .001; other ps > .14.) The nonsignificantly-lower responding of lesioned rats likely reflected lower base rates of food cup behavior in those rats: ANOVA of pre-CS responding found a significant lesion effect (F1,10 = 8.27, p = .016) along with a marginally-significant main effect of session (F11,110 = 1.87, p = .051). Because ORs to visual cues (rearing) are not typically reflected in activity counts provided by our activity monitors, we did not report activity in this study.
Figure 3.
Results of Experiment 2. Neither food cup responding to the reinforced light conditioned stimulus (CS) during the initial acquisition phase (Panel A) nor feature negative discrimination learning (Panel B) was affected by CeA lesions. L+ refers to responding during the reinforced light and NL− refers to responding during the nonreinforced noise + light compound. The abscissa points labeled “pre” refer to responding in the periods immediately prior to CS presentations. The designations CeA and sham refer to rats with neurotoxic or sham lesions of the amygdala central nucleus (CeA), respectively.
Figure 3B shows the acquisition of the FN discrimination. As intended, discrimination learning was slower than in Experiment 1, but again learning was not affected by the CeA lesion. Discrimination performance was evaluated with a lesion x cue x session block ANOVA that contrasted responding to the nonreinforced light + noise compound with responding to the light alone. As in Experiment 1, the effect of cue (F1, 10 = 138.24, p < .001) and the cue x block (F7,70 = 34.12, p < .001) interaction were significant. Importantly, there were no effects or interactions involving lesion (ps > .18). As in Phase 1, a lesion x block ANOVA of pre-CS responding showed a significant main effect of lesion (F1, 10 = 5.93, p = .035), reflecting slightly lower pre-CS behavior in lesioned rats.
Unlike in Experiment 1, substantial responding occurred on compound trials early in FN discrimination training. Thus, there was ample opportunity for activation and disconfirmation of an expectancy of sucrose in Experiment 2. Nevertheless, CeA lesions had no effects, again suggesting that surprise-induced associability enhancements played little role in FN discrimination learning of sham-lesioned rats.
Experiment 3
Within the Pearce-Hall (1980) model, surprising omission of the reinforcer on compound trials in the FN procedure should enhance or maintain the associability of all cues present on that trial, including both feature and target. However, within an alternate formulation of that model, the facilitatory effects of surprise on associability might only apply to cues that generate the disconfirmed expectancy. Within this view, because the feature cues in Experiments 1 and 2 were never paired with food, they did not themselves generate an expectancy of food on nonreinforced feature + target trials, and hence their associabilities were not enhanced when food was omitted. The lack of an effect of CeA lesions on the rate of learning about the feature in the FN task is consistent with this view.
By contrast, according to this same logic, the associability of the target cue should be maintained because that cue is treated ambiguously and is the source of the disconfirmed food expectancy. However, the associability of the target cue should not profit from such mechanisms in rats with CeA lesions. Unfortunately, our FN procedures did not permit an easy comparison of target cue associability between lesioned and control rats. Not only was the FN discrimination procedure introduced after conditioned responding had already been acquired to the target, but any differences in the target’s associability in FN training would likely be expressed on both reinforced and nonreinforced trials, and hence would not be likely to be revealed in performance to the target.
In Experiment 3 (see Table 1 for an outline of its procedures), we examined the effects of CeA lesions on the associability of both the feature and target elements after FN discrimination learning, using a procedure employed previously to assess cue associability (e.g., Maddux et al., 2007; Maddux & Holland, 2011). After the completion of either FN or control discrimination training, in which the target was always reinforced and a control stimulus was presented alone on nonreinforced trials, we examined the ability of the feature, target, or control stimulus to both acquire new learning and to interfere with new learning about an additional cue. In separate groups of rats, the feature, target, or control stimulus was presented in compound with a novel stimulus, and reinforced with a new, larger and more palatable reinforcer. The higher the associability of the previously-trained cue, the more rapidly it should acquire associations with the new reinforcer, and the more it should interfere with the acquisition of associations between the novel stimulus and that reinforcer. From the alternative Pearce-Hall perspective just described, we would expect that in sham-lesioned rats, the associability of the target cue would be maintained at a higher level in FN discrimination training (in which it was both reinforced and nonreinforced on different trials) than in control discrimination training (in which it was consistently reinforced). Thus, in the final training phase, the FN target might acquire associations with the new reinforcer more rapidly and interfere more with acquisition of associations with the novel cue than the control target. Furthermore, this advantage of the FN target would not be present in CeA-lesioned rats. Finally, because the feature and control cues were treated unambiguously in all training conditions (always nonreinforced), their associabilities would be relatively low after either FN or control discrimination training, and hence would be unaffected by CeA lesions.
Table 1.
Outline of Procedures of Experiment 3
| Group | Discrimination training | Compound | Test |
|---|---|---|---|
| TA (ambiguous target) | T→sucrose, NT→nothing | LT→SUCROSE | L, T |
| F (feature) | T→sucrose, NT→nothing | LN→SUCROSE | L, N |
| C (control) | T→sucrose, N→nothing | LN→SUCROSE | L, N |
| TC (consistent target) | T→sucrose, N→nothing | LT→SUCROSE | L, T |
Note. T and N refer to two auditory stimuli, a 1,500-hz tone and a white noise; however, their roles were fully counterbalanced in each group (not shown above; see text). L refers to a visual stimulus; “sucrose” refers to delivery of 0.3 ml of 0.2 M sucrose solution; “SUCROSE” refers to delivery of 0.6 ml of 0.4 M sucrose solution.
As in previous experiments using this technique (Maddux et al., 2007; Maddux & Holland, 2011), the primary measure of the cues’ associabilities was their ability to interfere with learning about a novel cue. A major advantage of this indirect assessment is that the rate of learning about the novel cue could be assessed from identical starting points (zero) in all conditions. Given that the FN feature, the control stimulus, the partially-reinforced FN target and the consistently-reinforced control target all are likely to have different associative strengths at the end of discrimination training, it would be difficult to assess the amounts of new learning occurring to them during the final compound conditioning phase (see Rescorla, 2000, for detailed discussion of this issue).
Methods
The apparatus, data analysis, histological procedures, and subject characteristics were identical to those of Experiments 1 and 2.
Surgical procedures
The surgical procedures were similar to those of Experiments 1 and 2 except that they were conducted under isoflurane anesthesia, the ibotenic acid was obtained from Sigma-Aldrich, the infusions were made with a 27-gauge needle attached to the Hamilton syringe, and the dorsal-ventral coordinate used was −7.9. All experimental procedures were approved by the Animal Care and Use Committee of Johns Hopkins University, where this experiment was performed.
Behavioral Procedures
Discrimination training
After food cup training identical to that of the previous experiments, all rats received 5 64-min sessions to pretrain one of two auditory cues as a signal for sucrose. Each session included 8 presentations of a 5-s 70-db white noise or a 72-db 1500 Hz tone paired with sucrose delivery. Next, all rats received 16 64-min discrimination training sessions, each of which included 2 reinforced presentations of the auditory cue trained in the previous phase, and 6 nonreinforced trials. In groups TC (target consistent) and C (control) the nonreinforced trials were 5-s presentations of the other auditory cue (tone or noise), and in groups TA (target ambiguous) and F (feature) they comprised a simultaneous compound of the two auditory stimuli. The amount of training was selected so that FN discrimination learning would be incomplete, with substantial prediction error still occurring on feature + target compound trials throughout the phase.
Associability assessment
All rats then received 4 64-min sessions of compound conditioning designed to evaluate the ability of the various auditory cues to overshadow conditioning of a novel visual cue, a 5-s intermittent (3 hz) presentation of the panel light used in Experiments 1 and 2. That stimulus was compounded with the cue trained as the feature in Group F, the cue trained as the target in groups TC and TA, and the control cue in group C. Each session included 8 compound presentations, each reinforced by the delivery of 0.4 ml of 0.4-M sucrose. The use of a larger amount of a more concentrated sucrose as the reinforcer was intended to encourage conditioning beyond the levels already attained in Phase 2 in all groups. Finally, all rats received a single 64-min test session, which included 4 nonreinforced presentations of the light alone, and then 4 nonreinforced presentations of the auditory cue member of the compound from the previous phase, presented alone.
Results
Histological results
In Experiment 3, 32 of 44 lesioned rats met the criteria described in Experiment 1. Data from all 28 sham-lesioned rats were accepted. The final numbers of rats in each group were: Group F, 6 sham, 8 CeA; group C, 6 sham, 7 CeA; group TA, 8 sham, 8 CeA; Group TC, 8 sham, 9 CeA.
Behavioral results
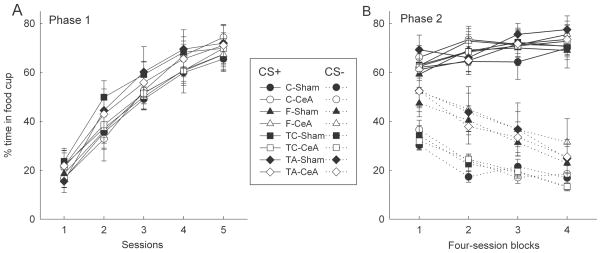
Figure 4A shows the results of the initial training phase. The rats acquired food cup behavior rapidly to the auditory cue. ANOVA with eventual training group, lesion, cue identity (tone or noise), and sessions as variables showed only a significant effect of sessions (F4,176 = 161.88, p < .001) and an interaction of group, cue, and sessions (F12,176 = 2.14, p = .017). Neither the effect of lesion nor any of its interactions was significant (ps > .435).
Figure 4.
CeA lesions had no effects on conditioned food cup responding in either the initial acquisition (Panel A) or discrimination training (Panel B) phases of Experiment 3. CS+ and CS− refer to reinforced and nonreinforced conditioned stimuli. Rats in Groups F and TA received feature negative discrimination training, whereas rats in Groups C and TC received simple discrimination training (see text and Table 1 for more details). The designations CeA and sham refer to rats with neurotoxic or sham lesions of the amygdala central nucleus (CeA), respectively.
Figure 4B shows the results of discrimination training. As in Experiments 1 and 2, CeA lesions did not affect the acquisition of the FN discrimination, thus extending the generality of those results to learning when the features and targets were of similar salience. Although there was significant generalization from the reinforced to the nonreinforced auditory stimuli, performance on the control (T+, C−) discrimination was superior to that on the FN (T+, FT−) discrimination. A discrimination (FN vs. control) x lesion x target cue identity x contingency (reinforced cue vs nonreinforced cue) x block ANOVA showed significant main effects of discrimination (F1,52 = 10.14, p = .003), discrimination contingency (F1,52 = 251.07, p < .001), and blocks (F3,156 = 9.97, p < .001). In addition, the discrimination x blocks (F1,52 = 9.90, p = .003), contingency x blocks (F3,156 = 100.61, p < .001) and discrimination x contingency x blocks (F3,156 = 4.67, p = .004) interactions were significant, consistent with the more rapid learning of the simple discrimination. Neither the effect of lesion nor any of its interactions was significant (ps > .192). ANOVA of pre-CS responding (not shown) revealed only a significant discrimination x blocks interaction (F3,156 = 4.60, p = .004; other ps > .229.)
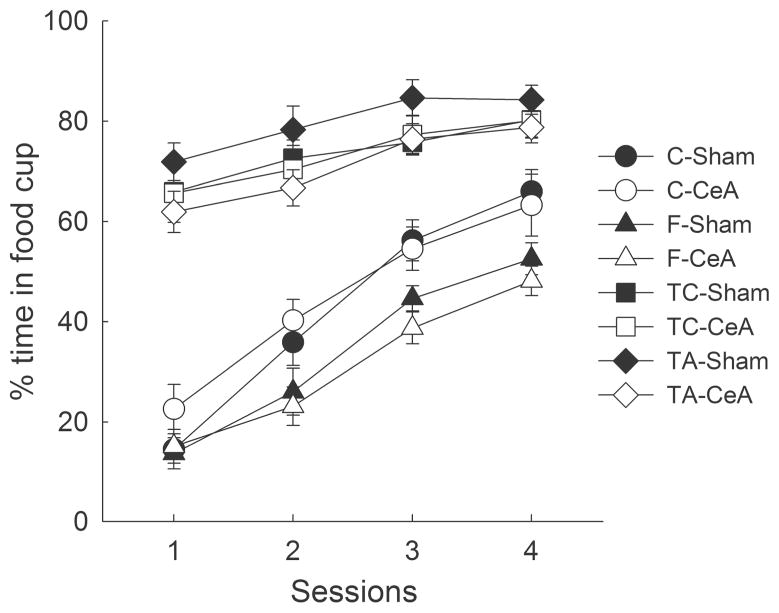
Figure 5 shows the acquisition of food cup behavior to the auditory + visual cue in the compound conditioning phase, designed to establish new learning to the visual and auditory cues. A group x lesion x target identity (tone or noise) x session ANOVA showed the effects of group (F3,44 = 81.26, p < .001), sessions (F3,132 = 151.49, p < .001) and the group x sessions interaction (F9,132 = 12.38, p < .001) to be significant. Neither the effect of lesion nor any of its interactions was significant (ps > .306). ANOVA of pre-CS behaviors showed no significant effects or interactions (ps > .130).
Figure 5.
CeA lesions had no effects on the acquisition of food cup responding during the compound conditioning (overshadowing) phase of Experiment 3. In separate groups of rats, a novel light was compounded with either the feature (F) or target (TA) stimulus from a feature negative discrimination, or a consistently reinforced (TC) or nonreinforced (C) stimulus from the control discrimination, and paired with the delivery of a large magnitude high concentration sucrose reinforcer (see text and Table 1 for more details.) The designations CeA and sham refer to rats with neurotoxic or sham lesions of the amygdala central nucleus (CeA), respectively.
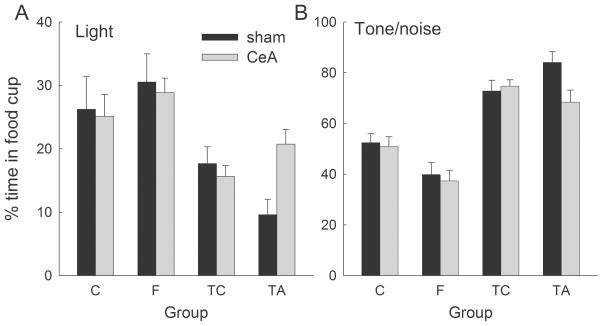
Figure 6A shows the first of the two primary results of Experiment 3, those from the test of responding to the visual cue alone. ANOVA showed a significant effect of group (F3,52) = 11.10, p < .001.) Consider first the performance of rats in groups TC and TA. These rats received training of the light in compound with either the target previously trained within a simple (control) discrimination (TC) or the target that was previously trained within a FN discrimination (TA). In both of these groups the target overshadowed (or blocked) conditioning to the light more than either the feature (group F) or control (group C) cue. Post-hoc contrasts supporting this description, which used the Tukey honestly significant difference (HSD) procedure for unequal ns (with Spjotvoll/Stoline correction), were significant (ps < .032). Furthermore, in sham-lesioned rats, the target trained within the FN discrimination (TA) overshadowed conditioning to the light more than the target that was consistently reinforced (TC; p = .051). This difference was not observed in CeA-lesioned rats in these groups, which displayed a nonsignificant difference in the opposite direction (p = .201). The effect of lesion was significant in group TA (p = .008) but not group TC (p = .610), and the effect of lesion was significantly larger in group TA than in group TC (p = .024).
Figure 6.
Results of the test session of Experiment 3. (Panel A) Responding to the light stimulus that was first introduced in the prior compound conditioning (overshadowing) phase. Lower levels of responding to the light indicate greater overshadowing by the cue that accompanied it in compound conditioning. CeA lesions interfered with the ability of the target of a feature-negative discrimination (Group TA) to overshadow conditioning to that light, but not with the ability of a consistently-reinforced stimulus to do so (Group TC). (Panel B) Responding to the auditory stimulus that accompanied the light stimulus during compound conditioning. In both panels, group designations indicate the nature of training to the auditory cue used in the overshadowing phase. CeA lesions interfered with the ability of a target of a feature-negative discrimination (Group TA) to acquire additional conditioned responding in that phase. TA and F refer to the target and feature from a feature negative discrimination, respectively, and TC and C refer to the consistently reinforced and nonreinforced stimuli (respectively) from a simple discrimination. The designations CeA and sham refer to rats with neurotoxic or sham lesions of the amygdala central nucleus (CeA), respectively.
This pattern of data is consistent with the possibility that the surprising omission of food on feature + target compound trials within a FN discrimination enhanced the associability of the target, increasing its ability to overshadow the light, and that this enhancement was absent in rats with CeA lesions. By contrast, performance of rats that received training of the light in compound with the feature from the previously trained FN discrimination was not affected by the CeA lesion (p = .702), consistent with the assertion that the surprising omission of reinforcement on feature + target compound trials within the FN discrimination did not enhance the associability of the feature. This last assertion is also consistent with our failure to observe effects of the lesion on acquisition of FN discriminations in all 3 of the present experiments.
Responding prior to the light test trials ranged from 1.3 ± 0.9% to 4.8 ± 1.8% in the 8 groups. A group x lesion ANOVA showed no significant main effects or interaction (ps > .183).
Figure 6B shows test responding to the auditory elements of the auditory + visual compound cue. ANOVA showed a significant effect of group (F3,52) = 38.22, p < .001.) Consider first the performance of rats in groups TC and TA. Not surprisingly, the previously-trained target cues in these two groups evoked more responding than the feature or control cues in Groups F and C (HSD ps < .001). More important, although these rats began compound training with comparable levels of responding (Figure 4B), among sham-lesioned rats the target cue evoked more responding in Group TA than in Group TC in test (p = .050), consistent with that cue’s greater overshadowing of conditioning to the light (Figure 6A). This difference was not observed in CeA-lesioned rats in these groups, which displayed a nonsignificant difference in the opposite direction (p = .251). The effect of lesion was significant in group TA (p = .007) but not in group TC (p = .724), and the effect of lesion was significantly larger in group TA than in group TC (p = .029). Finally, the auditory cue previously trained as a feature (group F) evoked less responding (p = .023) than the control cue (Group C), consistent with its having acquired conditioned inhibition in the discrimination training phase. The CeA lesion had no effect on performance of either of these groups, nor the difference between them (ps > .683).
A groups x lesion ANOVA of responding prior to the auditory test trials, ranging from 0.6 ± 0.4% to 3.9 ± 1.3% across the various conditions, showed no significant main effects or interaction (ps > .143).
Discussion
In three experiments, the acquisition of FN discriminations was not affected by CeA lesions. Regardless of the modalities or relative saliences of the feature and target cues, or the rate of FN discrimination learning, performance of CeA- and sham-lesioned rats was very similar. Nevertheless, impairments in the acquisition of conditioned ORs (Experiment 1) and in the acquisition of new learning in the compound training phase of Experiment 3 showed that the CeA lesions were behaviorally effective. Because in many other testing situations CeA lesions have been found to interfere with surprise-induced enhancements in cue associability, these results imply that such enhancements do not contribute substantially to the rate of FN discrimination learning.
Within the highly-successful Pearce-Hall (1980) model, the associability of a stimulus is maintained or enhanced if that stimulus is accompanied by surprise (prediction error), but reduced if expectations are confirmed. In the present experiments, as initial conditioning of a target cue proceeds, the reinforcer becomes better predicted and the associability of the target cue declines. Later, when that cue is combined with a novel feature cue and nonreinforced early in FN discrimination training, the surprising omission of the reinforcer should produce both a recovery of the target’s associability, and maintenance or enhancement of the associability of the novel feature. This augmented associability of the feature’s associability should contribute to the rate of inhibitory learning about the feature in intact rats, but not in CeA-lesioned rats, in contrast to our observations of no such effects of these lesions.
A simple resolution of this apparent contradiction relies on altering the Pearce-Hall (1980) model so that the effects of prediction error are not applied to all cues present, but are confined to the cue that generates the disconfirmed prediction. Thus, only stimuli that themselves have ambiguous consequences will show enhanced associability. In the case of FN discrimination learning, although early in training the feature accompanies prediction error on nonreinforced feature + target trials, it is in fact consistently nonreinforced, and hence should lose rather than gain associability in this framework. Given considerable evidence that lesions of CeA have no effects on such losses in associability (e.g., Holland & Gallagher, 1993a,b; Holland & Maddux, 2010), this modification of the Pearce-Hall (1980) model is consistent with our observations that CeA lesions do not affect the rate of acquisition of FN discriminations.
By contrast, in FN discrimination procedures the target cue is itself both reinforced and nonreinforced, and thus should benefit from surprise-induced enhancements in associability. However, it is unlikely that more rapid learning about the target cue would contribute much to the rate of acquisition of FN discriminations, because enhanced extinction learning on nonreinforced tirals would be balanced by faster excitatory learning on reinforced trials. To evaluate the assertion that the associability of a FN target benefits from CeA-dependent enhancements, in Experiment 3 we examined the associability of the feature and target stimuli from a FN discrimination independent of the learning of that discrimination, by examining their ability to acquire new learning and to overshadow learning about a novel cue. Our observations that a FN target both acquired more learning and interfered more with learning about a novel cue than a FN feature, a consistently-reinforced target, or a control stimulus, and that those differences were CeA-dependent, support that assertion. Thus, the present data suggest a reformulation of the Pearce-Hall (1980) model, consistent with the casual claim that for purposes of new learning, animals come to attend less to consistent predictors of reinforcement but more to inconsistent predictors.
Although this revision of the Pearce-Hall model is consistent with our failures to find CeA lesion effects on FN discrimination learning as well as our observations of lesion effects on the serial prediction (Holland & Gallagher, 1993b, 2006) and serial negative patterning tasks (Holland et al., 2000), at first glance it seems to conflict with the observation of such effects in unblocking experiments (Holland & Gallagher, 1993a). By the same logic as described here for FN learning, in unblocking studies, alterations in associability would be limited to the originally trained (blocking) cue, and not the added cue. However, our revised Pearce-Hall (1980) model is consistent with an account of unblocking offered by Holland and Kenmuir (2005), who noted that all examples of successful unblocking with downshifts in reinforcer value (and many with upshifts) involve the serial delivery of reinforcer events, for example, shifting down from the delivery of a food pellet followed 5-s later by more pellets, to the delivery of a single pellet. Holland and Kenmuir (2005) asserted that the surprising omission or presentation of the second of two expected reinforcers in unblocking tasks may enhance processing of the first reinforcer rather than of the cues that preceded that reinforcer. Thus, Holland and Kenmuir (2005) argued that the unblocking task involves alterations in reward processing rather than of cue processing, as suggested by Pearce and Hall (1980). However, unlike reward-processing accounts such as the Rescorla-Wagner (1972) model, which assume that reward prediction error directly determines the magnitude and direction (excitation or inhibition) of change in associative strength, Holland and Kenmuir (2005) suggested that the effectiveness of the first reward was altered indirectly, as a consequence of the (unsigned) prediction error related to the second reward, in the same manner as such an unsigned prediction error signal is thought to alter cue associability in the Pearce-Hall (1980) model. Although Pearce and Hall (1980) emphasized prediction and prediction error information provided by the cues, the first reinforcer could provide that information equally well, and hence directly benefit from increased processing produced by the surprising presentation or omission of the second reinforcer. Notably, that first reinforcer itself provides the prediction that is later disconfirmed by omission (or presentation) of the second reinforcer, and thus would benefit from CeA-dependent associability enhancements in our revised formulation of the Pearce-Hall (1980) model.
Holland and Kenmuir (2005) presented two kinds of data in support of their claim. First, they observed asymmetries in the enhancements of learning observed in an unblocking experiment involving downshifts in reward from a serial (food1→food2) to a single (food1) reinforcer. Sham-lesioned rats that experienced such a downshift when a new stimulus (noise) was introduced acquired both excitatory associations between that cue and food1 and inhibitory associations between that cue and the (omitted) food2. By contrast, rats with CeA lesions showed normal inhibitory learning about the added cue, but no excitatory learning. If lesioned rats failed to acquire noise-food1 associations because the associability of the noise was not enhanced in those rats, then they should also have displayed slower inhibitory learning than sham-lesioned rats. By contrast, if the CeA lesions interfered with surprise-induced enhancements in the efficacy of food1, then only the excitatory learning would be impaired, as we observed. Second, Holland and Kenmuir (2005) found that in sham-lesioned rats, surprising omission of food2 made food1 more effective as a reinforcer for learning about another cue that had never before been presented. Rats first received either light→food1→food2 (downshift) or light→food1→nothing (control) training. Then, all rats received light→food1→nothing training in a second phase, followed by assessment of the rate of learning with noise→food1 pairings. Rats in the downshift condition acquired noise-food1 associations more rapidly, suggesting that the surprising omission of food2 in the second phase of training made food1 more effective as a reinforcer in the final test phase. Interestingly, that enhancement of food1’s reinforcing power was also found to be CeA-dependent. In this regard, recent electrophysiological data from an unblocking-like reinforcer shift study are notable. Calu et al. (2011) found that neurons in CeA responded to shifts from an 8 drop to a 1-drop reinforcer. However, those neurons fired in response to presentations of the 1-drop reinforcer, and not to presentations of the cue that signaled it.
Holland and Kenmuir’s (2005) results suggested that the surprising omission of the second of a two-reinforcer sequence enhanced processing of the first reinforcer, which was placed in an ambiguous relation with the second reinforcer, rather than of the added cue which was consistently associated with only the first reinforcer. This interpretation of the basis of unblocking effects is consistent with our revision of the Pearce-Hall (1980) model. Interestingly, by attributing excitatory learning in downshift unblocking experiments to enhanced processing of the remaining reinforcer, it also implies that such CeA-dependent excitatory learning would not occur in cases that did not permit disconfirmation of predictions provided by the lower-valued reinforcer itself. Indeed, when reinforcer value is varied by altering intensity (Wagner et al.,1980) or concentration (Cotton, Goodall, & Mackintosh, 1982) of a single reinforcing event, the typical outcome is inhibitory learning about the added cue, as predicted by the Rescorla-Wagner (1972) model, rather than excitatory learning, as predicted by Pearce and Hall (1980).
It is important to recognize that our suggested revision of the Pearce-Hall (1980) model, which was prompted by apparent discrepancies in the effects of CeA lesions on FN discrimination learning, unblocking, and serial prediction tasks, is not unique. For example, Pearce and Mackintosh (2010) proposed a model of conditioning in which one component of a cue’s associability is determined in a Pearce-Hall (1980) fashion, except using a cue-specific prediction error, as we suggest here. If that component of Pearce and Mackintosh’s (2010) model (which also includes another associability component, which follows rules originally specified by Mackintosh (1975), and a Rescorla-Wagner (1972) mechanism, by which changes in a cue’s associative strength are determined by an aggregate prediction error) depends on CeA function, then the pattern of data reported here in Experiment 3 would be expected. Similarly, Esber and Haselgrove (2011) suggested that associability changes always involve a cue-specific error term, but proceed independently for each cue outcome. Thus, within that model, asymptotically a partially-reinforced cue will be more associable than a consistently reinforced or nonreinforced stimulus, because the partially-reinforced cue is paired with two separate outcomes, reinforcement and nonreinforcement, whereas the latter cues are each paired with only a single outcome. Thus, the partially-reinforced target in a FN discrimination should be more associable than either the consistently-nonreinforced feature cue or a consistently-reinforced control cue, as we observed in our sham-lesioned rats in Experiment 3. If CeA is involved in the use of cue-specific negative prediction errors for changing cue associability, then the lesion effects we observed in Experiment 3 would follow as well. It remains to be seen how well such new models can describe the wide range of associative learning phenomena attributed to attentional processes. However, it is notable that even outside the arena of so-called attentional effects, recent data indicate that both aggregate and cue-specific error terms are necessary to account for the occurrence of “surprise” and the allocation of its effects among various cues present at the time of surprise (e.g., Rescorla, 2000).
Finally, the question of CeA’s role in the use of cue-specific negative prediction errors for changing cue associability remains. Although considerable evidence shows that negative prediction error information is coded in CeA (e.g., Calu et al., 2010; Lee, Gallagher, & Holland, 2010) and that CeA function at the time of surprise is critical to enhanced cue associability (Holland & Gallagher, 2006), it is unlikely that cue associability information itself is represented in CeA. Using the serial prediction task described in the introduction of this article (Wilson et al., 1992), Holland and Gallagher (2006) found that transient inactivation of CeA function at the time of surprise prevented cue associability enhancements that were assessed in a subsequent learning test phase conducted while CeA function was intact. By contrast, CeA inactivation during that test phase alone had no effect on the expression of enhanced cue associability that was previously induced by surprise while CeA function was intact. Thus, CeA appears to alter cue processing elsewhere in the brain (e.g., SI, PPC, SNc, or other regions; Holland & Maddux, 2010) by providing a prediction error signal at the time of surprise. The present results suggest that this signal acts exclusively on processing of the cue that provided the prediction from which that error was derived. We can only speculate about the basis of that specificity. For example, CeA might propagate individual, cue-specific error signals to each of its sensory inputs, or broadcast the aggregate error signal indiscriminately to all sensory inputs, but only induce plasticity in pathways that are already active in generating predictions. Further research is needed to distinguish among these and other possibilities.
References
- Bucci DJ, Holland PC, Gallagher PC. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. Journal of Neuroscience. 1998;18:8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Haney RZ, Holland PC, Schoenbaum G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron. 2010;68:991–1001. doi: 10.1016/j.neuron.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. Journal of Neuroscience. 1995;15:7315–7322. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton MM, Goodall G, Mackintosh NJ. Inhibitory conditioning resulting from a reduction in the magnitude of reinforcement. Quarterly Journal of Experimental Psychology. 1982;34B:163–180. doi: 10.1080/14640748208400884. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Hall G, Mackintosh NJ. Surprise and the attenuation of blocking. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:313–322. [Google Scholar]
- El-Amamy H, Holland PC. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. European Journal of Neuroscience. 2007;25:1557–1567. doi: 10.1111/j.1460-9568.2007.05402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esber GR, Haselgrove M. Reconciling the influence of predictiveness and uncertainty on stimulus salience: a model of attention in associative learning. Proceedings of the Royal Society B: Biological Sciences. 2011;278:2553–2561. doi: 10.1098/rspb.2011.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Holland PC, Gallagher M. Disconnection of the amygdala central nucleus and substantia innominata/nucleus basalis disrupts increments in conditioned stimulus processing in rats. Behavioral Neuroscience. 1999;113:143–151. doi: 10.1037//0735-7044.113.1.143. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Unblocking in Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:476–497. [PubMed] [Google Scholar]
- Holland PC. Excitation and inhibition in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:261–279. [PubMed] [Google Scholar]
- Holland PC, Chik Y, Zhang Q. Inhibitory learning tests of conditioned stimulus associability in rats with lesions of the amygdala central nucleus. Behavioral Neuroscience. 2001;115:1154–1158. [PubMed] [Google Scholar]
- Holland PC, Thornton JA, Ciali L. The influence of associability changes in negative patterning and other discriminations. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:462–476. doi: 10.1037//0097-7403.26.4.462. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behavioral Neuroscience. 1993;107:246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Effects of amygdala central nucleus lesions on blocking and unblocking. Behavioral Neuroscience. 1993;107:235–245. doi: 10.1037//0735-7044.107.2.235. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. Journal of Neuroscience. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Kenmuir C. Variations in unconditioned stimulus processing in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:155–171. doi: 10.1037/0097-7403.31.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Maddux J-M. Brain systems of attention in associative learning. In: Mitchell CJ, LePelley ME, editors. Attention and learning. Oxford: Oxford University Press; 2010. pp. 305–349. [Google Scholar]
- Lee HJ, Gallagher M, Holland PC. The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learning and Memory. 2010;17:531–538. doi: 10.1101/lm.1889510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, OMJ, Gallagher M, Holland PC. Role of substantia nigra-amygdala connections in surprise-induced enhancement of attention. Journal of Neuroscience. 2006;26:6077–6081. doi: 10.1523/JNEUROSCI.1316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Youn JM, Gallagher M, Holland PC. Temporally-limited role of substantia nigra-central amygdala connections in surprise-induced enhancement of learning. European Journal of Neuroscience. 2008;27:3043–3049. doi: 10.1111/j.1460-9568.2008.06272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Maddux JM, Kerfoot EC, Chatterjee S, Holland PC. Dissociation of attention in learning and action: Effects of lesions of the amygdala central nucleus, medial prefrontal cortex, and posterior parietal cortex. Behavioral Neuroscience. 2007;121:63–79. doi: 10.1037/0735-7044.121.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JM, Holland PC. Dissociations between medial prefrontal cortical subregions in the modulation of learning and action. Behavioral Neuroscience. 2011;125:383–395. doi: 10.1037/a0023515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Pearce JM, Mackintosh NJ. Two theories of attention: a review and a possible integration. In: Mitchell CJ, LePelley ME, editors. Attention and learning. Oxford: Oxford University Press; 2010. pp. 11–39. [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Rescorla RA. Associative changes in excitors and inhibitors differ when they are conditioned in compound. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:428–438. doi: 10.1037//0097-7403.26.4.428. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Wagner AR, Mazur JE, Donegan NH, Pfautz PL. Evaluation of blocking and conditioned inhibition to a CS signaling a decrease in US intensity. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:376–385. [PubMed] [Google Scholar]
- Wilson PN, Boumphrey P, Pearce JM. Restoration of the orienting response to a light by a change in its predictive accuracy. Quarterly Journal of Experimental Psychology. 1992;44B:17–36. [Google Scholar]