Abstract
Background
Chewing of regurgitated food elicits in baboons life-long gastro-esophageal reflux (GER). The acid reflux transforms the multilayered squamous cell epithelium of the esophagus into columnar-lined mucosa with mucus-producing accessory glands. The function of this mucous gland metaplasia (MGM), which mimics Barrett’s mucosa with MGM in humans, is to buffer the gastric acid entering the esophagus during regurgitation. In a previous study of entire esophagi, the majority of baboons showed MGM. The gastric mucosa was not investigated.
Materials and Methods
Hematoxylin-eosin-stained sections from the esophagus, from the lesser gastric curvature and from the greater gastric curvature were collected separately from 50 adult baboons. The presence of MGM was assessed in each one of these locations.
Results
MGM was demonstrated in 92% (46/50) of blocks from the esophagus, in 98% (49/50) of blocks from the lesser curvature and in 90% (45/50) of those of the greater curvature (fundus).
Conclusion
The majority of the animals had MGM, not only in the esophagus but also in the proximal gastric mucosa. Rationally, MGM in baboons starts in the distal esophagus and proceeds downwards, towards the proximal stomach. The histogenesis of the MGM in Barrett’s mucosa in humans (that is Barrett’s mucosa type 2) remains elusive. Therefore the baboon might be an important animal model for studying the histogenesis of Barrett’s mucosa with MGM in humans, a recognized pre-cancerous lesion.
Keywords: Esophagus, baboon, gastric reflux, mucus metaplasia
At birth, the esophagus in baboons is covered with stratified squamous cell epithelium having discrete papillae and occasional accessory glands (1). After birth, the chewing of regurgitated food, that is rumination, elicits daily, life-long gastro-esophageal reflux (GER) (2, 3). This protracted reflux transforms the multilayered squamous cell-lined epithelium into columnar-lined mucosa with mucus-producing accessory glands (4). The function of this mucous gland metaplasia (MGM), which mimics Barrett’s mucosa with metaplastic mucus glands in humans (5), is to buffer the gastric acid entering the esophagus during regurgitation (2, 3). Regurgitation and rumination also takes place in other nonhuman primates, such as chimpanzees (6, 7) and gorillas (8, 9).
In a previous study in adult baboons, the majority of the esophagi examined had MGM (mean length 10.5 mm, range 1.0–45.0 mm (10). In that work, the entire esophagus, from the base of the tongue to the angle of His, was analyzed. One relevant question, however, remained unanswered: Is the mucosa of the proximal stomach in adult baboons normal or metaplastic?
To investigate this, both the esophagus and the proximal stomach were collected separately in a cohort of adult baboons.
Materials and Methods
Fifty adult female baboons (Papio spp), members of a colony at the Southwest National Primate Research Center, Southwest Foundation for Biomedical Research were investigated. The conditions of animal housing were reported elsewhere (3). Briefly, the baboons were housed in metal and concrete indoor-outdoor cages and fed with commercial monkey diets, occasionally supplemented with a variety of fruit and vegetables. Water was available ad libitum. The animal management was carried out in accordance with the Institutional Animal Care and Use Committee guidelines.
Four cm-long blocks each, from the esophagus, the lesser gastric curvature and the greater gastric curvature were individually removed adhered to cardboard and fixed in individual jars containing 4% neutral formalin. Sections were stained with hematoxylin and eosin (H&E).
Definitions MGM
Metaplastic mucosa: built with columnar epithelium on top and mucus-producing glands underneath. Fundic mucosa: Gastric mucosa showing pits lined with columnar epithelium occupying less than one quarter of the mucosal thickness. The glandular area showed mucin-producing neck cells, and two distinct transversal domains: a parietal cell domain and a chief cell domain (11).
Statistical analysis
The nonparametric Wilcoxon test was used for comparing difference in MGM frequency in the three localizations. Statistical significance was defined as p <0.05.
Results
The results are presented in Table I.
Table I.
The mucosa phenotype (squamous cell, mucous gland metaplastic (MGM), or fundic) in the esophagus, the lesser curvature and the greater curvature of the stomach in 50 adult baboons.
| Mucosa phenotype |
Esophagus | Lesser curvature |
Greater curvature (fundus) |
|---|---|---|---|
| Squamous cell | 4 (8%) | -- | -- |
| Squamous cell/MGM | 46 (92%) | -- | -- |
| MGM | -- | 49 (98%) | 12 (24%) |
| MGM/fundic | -- | 1 (2%) | 33 (66%) |
| Fundic | -- | -- | 5 (10%) |
| Total | 50 | 50 | 50 |
Esophagus
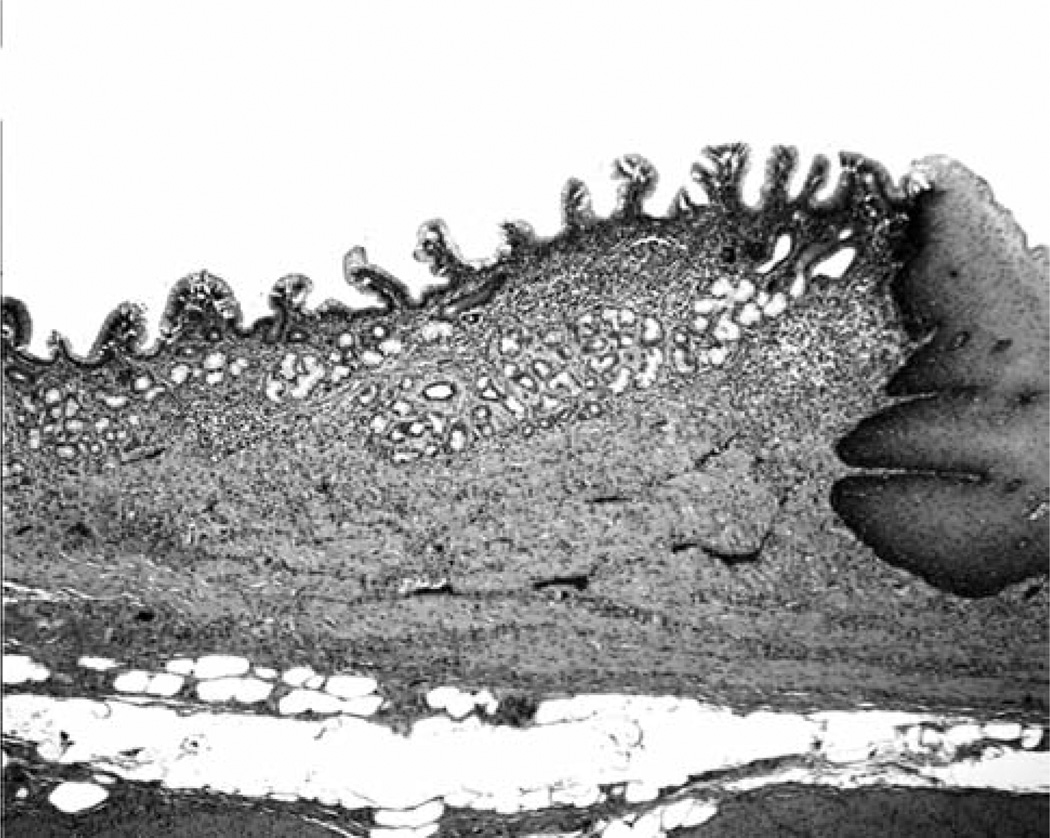
Four of the 50 oesophagi (8%) were lined with squamous-cell mucosa exclusively and the remaining 46 (92%) both by squamous-cell mucosa (proximal) and MGM (distal) (Figure 1).
Figure 1.
Columnar-lined mucosa with mucous-gland metaplasia in a baboon oesophagus. Normal squamous-cell epithelium can be seen on the right (H&E, ×4).
Stomach
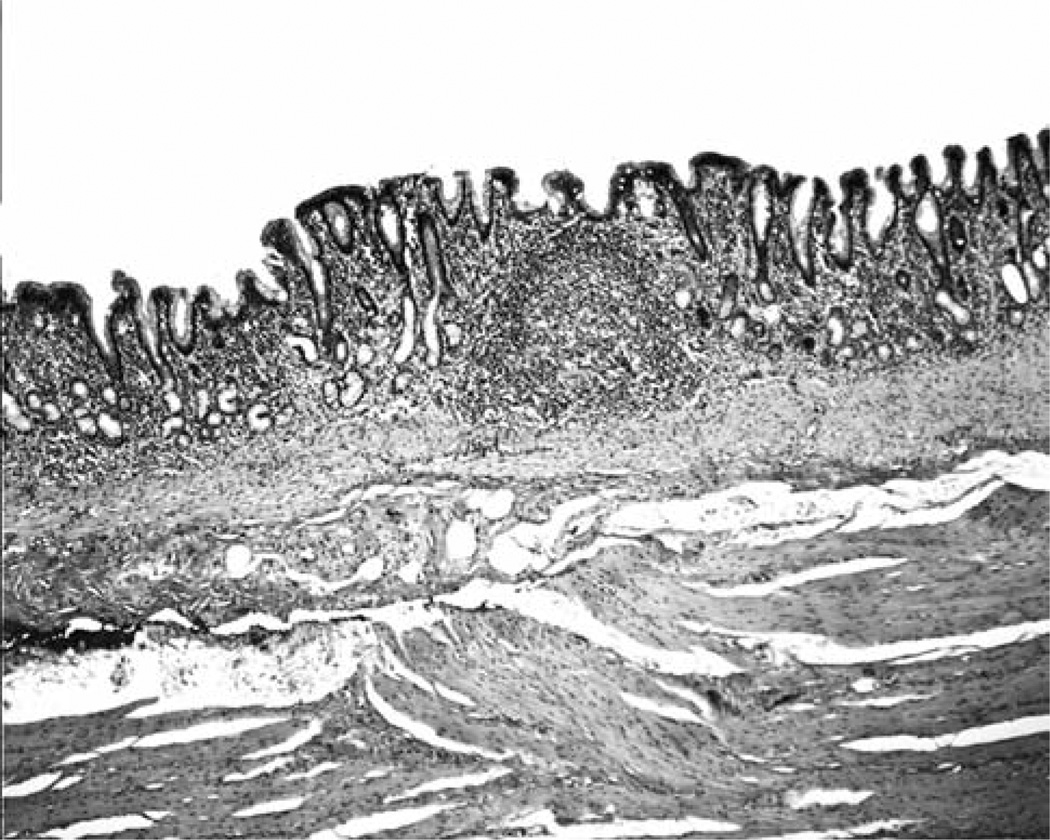
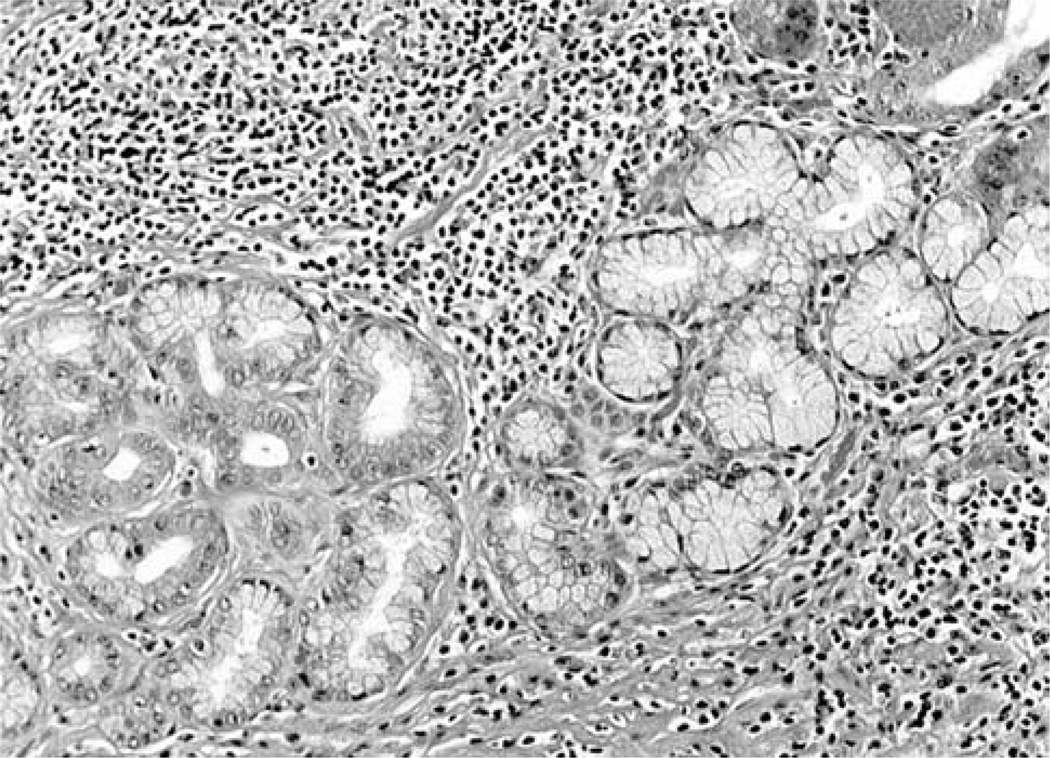
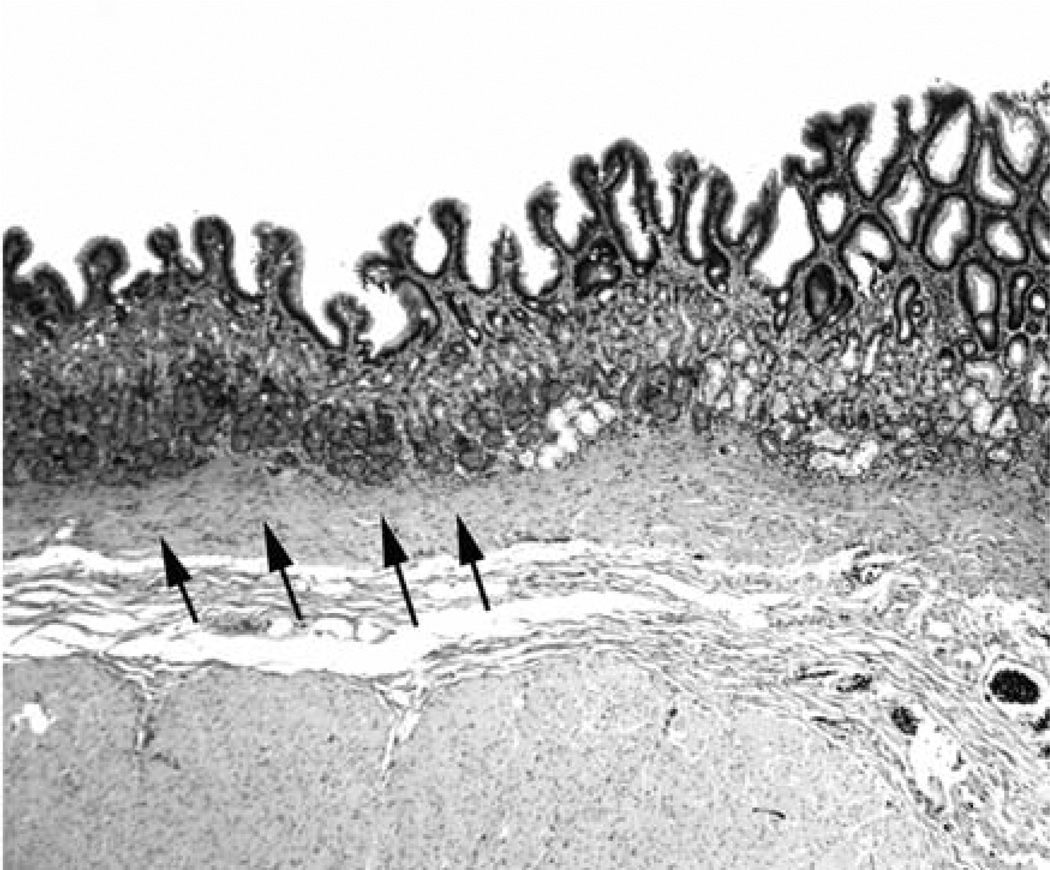
i) Lesser curvature: Forty-nine out of the 50 gastric blocks (98%) from the lesser curvature exhibited MGM exclusively (Figures 2 and 3) and the remaining one (2%) had both MGM (proximal) and fundic mucosa (distal). ii) Greater curvature (fundus): Twelve out of the 50 gastric blocks (24%) from the greater curvature exhibited MGM exclusively, 33 (66%) both MGM (proximal) and fundic mucosa (distal) (Figure 4), and the remaining 5 (10%) only fundic mucosa.
Figure 2.
Columnar-lined mucosa with mucous gland metaplasia along the lesser curvature of the stomach in a baboon (H&E, ×4).
Figure 3.
Higher power view of mucous gland metaplasia along the lesser curvature of the stomach in a baboon (H&E, ×10).
Figure 4.
Columnar-lined mucosa with mucous gland metaplasia and oxyntic mucosa (arrows) along the greater curvature of the stomach (fundus) in a baboon (H&E, ×4).
The difference in the number of specimens with MGM between the esophagus and the stomach was non-significant. On the other hand, the difference in the number of specimens with MGM exclusively between lesser and greater curvatures was significant (p <0.05). The difference in the number of specimens with fundic mucosa between the greater and the lesser curvatures (p <0.05) was also significant.
Discussion
The present results demonstrated that the majority (92%) of the 50 baboon esophagi had MGM. This metaplastic transformation appears vital for buffering the gastric acid entering the distal esophagus in these ruminants (2, 3). Experimental studies in dogs indicate that it was possible for damage arising from GER to be repaired with columnar-lined epithelium creeping upwards from a pre-existing mucosa of the cardia (13). This possible mechanism seems not to apply to baboons, inasmuch as the cardia mucosa does not exist at birth in these animals (4). Some studies suggest that the metaplastic mucosa originates from normal esophageal glands (14). In baboons, there is no indication that MGM originated from the normal esophageal glands, as MGM lacks the serum-secreting cells normally present in the esophageal glands proper (10).
It is generally accepted that GER in baboons is generated by natural, daily regurgitation of gastric acid into the distal esophagus (2, 3). This mechanism leads to MGM in the distal esophagus in these animals (4, 12). Surprisingly, MGM was also found in the proximal gastric mucosa in the majority of the animals. Rationally, MGM in baboons starts in the distal esophagus and proceeds downwards, towards the proximal stomach. Nevertheless, further studies are necessary to monitor the initial location of MGM and its subsequent advancing vector along the mucosa of the upper digestive tract in these animals. Accordingly, esophageal and gastric specimens will be independently collected in new-born and young baboons at various ages for this purpose.
The histogenesis of MGM in Barrett’s mucosa in humans, namely Barrett’s mucosa type 2 (5), a lesion that mimics MGM in baboons (12), remains elusive. Therefore, the baboon might be an important animal model to study the histogenesis of Barrett’s mucosa with MGM in humans, a recognized pre-cancerous lesion (5).
Acknowledgements
Thanks are due to Ms. Marie Silva and Ms. Michaelle Hohmann from the Histology Laboratory and to Mr. Jesse Martinez and Mr. Jacob Martinez from the Necropsy Unit, Southwest National Primate Research Center, San Antonio, TX, for invaluable help.
References
- 1.Rubio CA, Dick EJ, Hubbard GB. The columnar-lined mucosa in the distal esophagus. A preliminary study in baboons. In Vivo. 2009;23:273–275. [PMC free article] [PubMed] [Google Scholar]
- 2.Glover E, Leland M, Hubbard G. An association between gastric regurgitation and disease in nonhuman primates. Am J Primatol. 2005;66:174–179. [Google Scholar]
- 3.Glover E, Leland MM, Dick EJ, Hubbard G. Gastroesophageal reflux disease in baboons (Papio sp.): a new animal model. J Med Primatol. 2008;37:18–24. doi: 10.1111/j.1600-0684.2007.00217.x. [DOI] [PubMed] [Google Scholar]
- 4.Rubio CA, Dick EJ, Schlabritz-Loutsevitch NE, Hubbard G. The columnar-lined mucosa at the gastroesophageal junction in non-human primates. Int J Clin Exp Pathol. 2009;2:481–488. [PMC free article] [PubMed] [Google Scholar]
- 5.Playford RJ. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett's oesophagus. Gut. 2006;55:442–443. doi: 10.1136/gut.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker K, Easley S. An analysis of regurgitation and reingestion in captive chimpanzees. Appl Anim Behav Sci. 1996;49:403–415. [Google Scholar]
- 7.Morgan L, Howell S, Fritz J. Regurgitation and reingestion in a captive chimpanzee (Pan troglodytes) Lab Anim. 2003;22:42–45. [Google Scholar]
- 8.Gould E, Bres M. Regurgitation in gorillas possible model for human eating disorders (rumiation/bulimia) J Dev Behav Pediatr. 1986;7:314–319. doi: 10.1097/00004703-198610000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gould E, Bres M. Regurgitation and reingestion in captive gorillas. description and intervention. Zoo Biol. 1986;5:241–250. [Google Scholar]
- 10.Rubio CA, Orrego A, Dick EJ., Jr Further studies on the frequency and length of the glandulo-metaplastic esophageal mucosa in baboons. In Vivo. 2009;23:955–958. [PMC free article] [PubMed] [Google Scholar]
- 11.Rubio CA, Owston M, Orrego A, Dick EJ. A simple method to record parietal cells in the fundic mucosa in baboons. In Vivo. 2010;24:705–707. [PMC free article] [PubMed] [Google Scholar]
- 12.Rubio CA, Owston M, Orrego A, Dick EJ. Further studies on Barretts mucosa in baboons: metaplastic glandular cells produce sialomucin. Anticancer Res. 2010;30:4123–4226. [PMC free article] [PubMed] [Google Scholar]
- 13.Gillen P, Keeling P, Byrne PJ. Experimental columnar metaplasia in the canine oesophagus. Br J Surg. 1988;75:113–115. doi: 10.1002/bjs.1800750208. [DOI] [PubMed] [Google Scholar]
- 14.Bremner CG, Lynch VP, Ellis F., Jr Barrett’s esophagus: congenital or acquired? An experimental study of esophageal mucosal regeneration in the dog. Surgery. 1970;68:209–216. [PubMed] [Google Scholar]
- 15.Chandrasoma PT, Der R, Ma Y, Peters J, Demeester T. Histologic classification of patients based on mapping biopsies of the gastroesophageal junction. Am J Surg Pathol. 2003;27:929–936. doi: 10.1097/00000478-200307000-00008. [DOI] [PubMed] [Google Scholar]