Abstract
Phosphorylation of epidermal growth factor receptor (EGFR) on tyrosine 845 by c-Src has been shown to be important for cell proliferation and migration in several model systems. This cross talk between EGFR and Src family kinases (SFKs) is one mechanism for resistance to EGFR inhibitors both in cell models and in the clinic. Here, we show that phosphorylation of tyrosine 845 on EGFR is required for proliferation and transformation using several cell models of breast cancer. Overexpression of EGFR-Y845F or treating cells with the SFK inhibitor dasatinib abrogated tyrosine 845 phosphorylation, yet had little to no effect on other EGFR phosphorylation sites or EGFR kinase activity. Abrogation of Y845 phosphorylation inhibited cell proliferation and transformation, even though extracellular signal-regulated kinase (ERK) and Akt remained active under these conditions. Importantly, cotransfection of mitogen-activated protein kinase (MAPK) kinase 3 and p38 MAPK restored cell proliferation in the absence of EGFR tyrosine 845 phosphorylation. Taken together, these data demonstrate a novel role for p38 MAPK signaling downstream of EGFR tyrosine 845 phosphorylation in the regulation of breast cancer cell proliferation and transformation and implicate SFK inhibitors as a potential therapeutic mechanism for overcoming EGFR tyrosine kinase inhibitor resistance in breast cancer.
Introduction
The epidermal growth factor receptor (EGFR) is a tyrosine kinase receptor identified as an oncogene in many types of solid tumors. Small-molecule inhibitors as well as monoclonal inhibitory antibodies have demonstrated clinical efficacy in a number of tumor types, including lung, colon, and pancreatic cancers (reviewed in [1]). Thirty percent of breast cancers overexpress EGFR and this overexpression correlates with poor prognosis [2–4]. However, studies have yet to show an efficacy for EGFR inhibition in breast cancer [5,6]. Mechanisms of resistance to EGFR inhibitors, either intrinsic or acquired, have been characterized in lung, colon, brain, and pancreatic tumors to involve the activation of other tyrosine kinases including Met and c-Src (reviewed in [1,7,8]). In breast cancer models, we have found that both Met and c-Src are mediators of EGFR inhibitor resistance [9,10].
EGFR and c-Src have been shown to functionally interact in several model systems including human mammary epithelial cells [11,12]. Specifically, ectopic expression of both EGFR and c-Src resulted in synergistic increases in proliferation, transformation, and tumorigenesis. The mechanism for this synergy is currently unknown; however, it was demonstrated that under the synergistic conditions two tyrosines in the intracellular domain of the EGFR were phosphorylated, namely, tyrosines 845 and 1101 [13]. EGFR tyrosine 845 is particularly interesting because of its location within the activation loop of the kinase domain of the EGFR. This tyrosine is highly homologous to autophosphorylated tyrosines found in the kinase domains of other tyrosine kinases [14], which have been shown to be critical to their activation. However, unlike the tyrosines in these other kinases, EGFR tyrosine 845 is not an autophosphorylation site and does not need to be tyrosine phosphorylated for the kinase to be active [13]. Instead, phosphorylation of EGFR tyrosine 845 is a direct substrate of Src [13]. Mutating this tyrosine to a nonphosphorylatable phenylalanine (Y845F) and subsequent expression in fibroblasts lead to an abrogation of EGF-induced DNA synthesis, even though receptor autophosphorylation, phosphorylation of EGFR substrates such as Shc, and downstream ERK activation are unaffected [13]. Taken together, these data suggest that tyrosine 845 phosphorylation is critical to the EGFR/c-Src biologic synergy and cross talk. Therefore, the identification of Y845-dependent signaling pathways is not only important to understand EGFR signaling but also important to the development of therapeutic interventions that disrupt the synergistic affects of EGFR and Src.
Here, we demonstrate that phosphorylation of Y845 on EGFR is required for cell growth and transformation in breast cancer cell lines. In addition, we show that EGFR remains tyrosine phosphorylated on all five autophosphorylation sites and is kinase active when tyrosine 845 phosphorylation is inhibited by either mutation to phenylalanine or treatment of cells with the Src family kinase (SFK) inhibitor dasatinib. Under either condition, EGFR was autophosphorylated and stimulated signaling through the Ras/MAPK and phosphomositide 3-kinase (PI3 kinase)/Akt pathways. Interestingly, p38 MAPK activity was inhibited upon loss of EGFR tyrosine 845 phosphorylation. Upstream of p38 MAPK phosphorylation, Gab1 phosphorylation was reduced, whereas Shc remained phosphorylated. Significantly, the decrease in cell proliferation because of the loss of EGFR tyrosine 845 phosphorylation could be overcome by ectopic coexpression of p38 MAPK and a constitutively active MAP kinase kinase 3 (MKK3), its direct activator. Taken together, these data suggest that Src synergism with EGFR occurs through Gab1 and p38 MAPK signaling pathway to enhance cell proliferation and transformation in breast cancer cells.
Materials and Methods
Reagents
Dasatinib was purchased from LC Laboratories (Woburn, MA). Doxycycline and EGF, as well as tissue culture supplements, were purchased from Sigma (St Louis, MO). Other reagents were purchased from Thermo Fisher (Pittsburgh, PA).
Cell Models and Transfections
MCF7 cells inducibly expressing wild-type (wt)-EGFR or the EGFR tyrosine 845 phenylalanine mutant were generated previously [15]. SUM149 cells are a triple-negative breast cancer cell line from a patient with inflammatory breast cancer and are a gift from Dr Stephen Ethier (Medical University of South Carolina, Charleston, SC).
Constitutively active MKK3 (MKK3EE) and p38 MAPK in pcDNA were provided by Roger Davis (University of Massachusetts) [16]. The p38 MAPK construct was FLAG-tagged as described previously [17].
Transfections into SUM149 cells were performed using FuGENE 6 according to the manufacturer's recommendations (Roche Applied Sciences, Indianapolis, IN).
Cell Proliferation Assays
Cells were plated at 35,000 cells/well in a six-well dish on day 0. On day 1, cells were either induced to express the EGFR constructs with 1 mg/ml doxycycline. The cells were treated every other day with 0, 0.1, 0.5, or 1 µM dasatinib for 8 days. For the transfection experiments, cells were transfected with wt-EGFR or Y845F-EGFR on day 1 and again on day 4. The number of cells present at day 4 and day 8 or 9 was measured using a Coulter counter. Each experiment was performed in triplicate at least three times. Statistics were performed using Student's t test.
Soft Agar Colony Formation Assays
Soft agar plates were prepared with a 0.5% agar bottom layer, a 0.45% agar cell layer, and a 0.35% top layer. One day after seeding, cells were treated with 1 mg/ml doxycycline or 0.5 µM dasatinib every other day with the addition of fresh media for 3 weeks. Colonies greater than 50 cells in size were counted on a microscope and normalized to the DMSO-treated control, which was set at 100% colony formation. Experiments were performed in triplicate at least three times. Student's t test was used to determine statistical significance.
Immunoprecipitation and Immunoblot analysis
Cells were lysed in CHAPS lysis buffer (10 mM CHAPS, 50 mM Tris (pH 8.0), 150 mM NaCl, and 2 mM EDTA with 10 µM NaOVa and 1x protease inhibitor cocktail (EMD Biosciences, Rockland, MA). For the immunoprecipitation assays, 0.5 mg of protein was used for immunoprecipitation using anti-EGFR antibodies (mab108, M. Weber, University of Virginia, Charlottesville, VA). Antibody-bound proteins were collected using 40 µl of protein A beads (Millipore, Billerica, MA) and washed three times with phosphate-buffered saline (PBS). Immunoblot analysis was performed after sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transfer to polyvinylidene fluoride (PVDF) membranes. Membranes were blocked in either 5% nonfat dry milk or BSA depending on the recommendations of the antibody manufacturer. Primary antibodies used in this study include EGFR, pY845 EGFR, pY992 EGFR, pY1068 EGFR, pY1086 EGFR, pY1148 EGFR, pY1173 EGFR, GRB2-associated binding protein 1 (Gab1), phospho-Gab1 (pGab1), Akt, phospho-Akt (pAkt), ERK, p38 MAPK, and phospho-p38 (pp38) MAPK from Cell Signaling (Danvers, MA); actin and FLAG from Sigma; phospho-ERK (pERK) and phospho-Src (pSrc) from Invitrogen (Carlsbad, CA); and Src (2–17; Dr Sally Parsons, University of Virginia). Anti-mouse and anti-rabbit IgG-HRP were used from Cell Signaling, and enhanced chemiluminescence reagents were from GE Healthcare Life Sciences (Piscataway, NJ).
In Vitro Kinase Assays
Stable MCF7 cells were induced to express wt-EGFR or Y845F-EGFR with doxycycline for 48 hours. Cells were then serum starved for 24 hours and stimulated with 10 ng/ml EGF for 15 minutes. Cells were washed twice in PBS and lysed in solubilization buffer [50 mM HEPES (pH 7.5), 10% glycerol, 0.5% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 1 mM polymethylsufonyl fluoride, 50 µg/ml aprotinin, and 400 nM sodium orthovanadate]. Lysates were cleared by centrifugation, protein concentration was determined by the Bio-Rad Bradford Assay (Hercules, CA), and 0.5 mg of protein was used for an immunoprecipitation using anti-EGFR antibody mab108. Antibody-bound proteins were collected using 40 µl of protein A beads (Millipore) and washed three times in HTG buffer (20 mM HEPES (pH 7.5), 0.1% Triton X-100, and 10%glycerol). For the kinase assay, 40 µl of HTG buffer, 4 µl of MnCl2 (of 100 mM stock), and 10 µCi 32P-γATP were incubated with the immunoprecipitates for 10 minutes at 30°C. The beads were pelleted, and the supernatant was removed and discarded. The beads were washed twice with solubilization buffer and once with PBS. Forty microliters of sample buffer was added to the pellets, the samples were boiled, and proteins were separated using 7.5% SDS-PAGE. The gels were dried and exposed to film. Each experiment was repeated at least three times.
Rac Activity Assays
MCF7 or SUM149 cells were treated as indicated and lysed according to the manufacturer's recommendations (Millipore). Briefly, 10 µl of PAK-1 p21 binding domain (PBD) agarose was added to 500 µg of protein lysate for 1 hour at 4°C. Precipitates were washed three times with lysis buffer, and the pellet was resuspended in 2x sample buffer. Proteins were separated by SDS-PAGE and transferred to membranes. Immunoblots were performed using an anti-Rac-1 antibody. Positive and negative controls provided by the manufacturer were used (GTPγS or guanosine diphosphate [GDP]). Each experiment was repeated at least three times.
BrdU Incorporation Assays
MCF7 cells inducibly expressing wt-EGFR or Y845F-EGFR and SUM149 cells were transfected with constitutively active MKK3 and p38MAPK in a ratio of 1:5. For the MCF7 models, cells were induced to express wt-EGFR or Y845F-EGFR at the time of plating on coverslips and transfected 24 hours later. SUM149 cells were plated and, 24 hours later, treated with 0.5 µM dasatinib and transfected. After transfection for 24 hours, bromodeoxyuridine (BrdU; Invitrogen) was added to the cells for 4 hours. Cells were then fixed and stained for BrdU incorporation as well as for the FLAG tag on the transfected vectors using fluorescent-tagged antibodies (Invitrogen). Transfected cells were counted to determine the percent BrdU positive. Each experiment was repeated at least three times in duplicate. Student's t test was used to determine statistical significance.
Results
EGFR Tyrosine 845 Phosphorylation Is Required for Transformation of Breast Cancer Cells
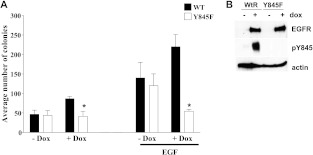
We have previously shown that inhibiting phosphorylation of tyrosine 845 on EGFR decreases DNA synthesis [11,12,15]. Using an MCF7 cell model engineered to inducibly express either wt-EGFR or a tyrosine to phenylalanine mutant at tyrosine 845 (Y845F-EGFR; Figure 1B), we sought to determine if EGFR tyrosine 845 phosphorylation regulated the ability of cells to grow in soft agar. As MCF7 cells express less than 10,000 EGFR molecules per cell, using these models will allow us to test the role of tyrosine 845 phosphorylation with little contribution of endogenous EGFR [18]. MCF7 cells induced to express wt-EGFR increased soft agar colony formation by 80% compared to the uninduced controls (Figure 1A, black bars + Dox), similar to the results obtained upon EGFR and c-Src co-overexpression in fibroblasts or mammary epithelial cells [11,12]. In contrast, expression of the Y845F-EGFR mutant abrogated this increase in soft agar colony formation, essentially inhibiting the biologic synergy that occurs with EGFR and c-Src co-overexpression (Figure 1A, white bars + Dox). Without inducing expression of wt-EGFR or Y845F-EGFR with doxycycline, both cell lines showed a similar increase in colony formation with EGF stimulation (Figure 1A). These results demonstrate for the first time that the phosphorylation of a single site on EGFR may regulate cellular transformation.
Figure 1.
Mutation of tyrosine 845 to phenylalanine on EGFR inhibits cellular transformation. (A) MCF7 cells were induced to express wt-EGFR (WT) or Y845F-EGFR (Y845F) with 1 mg/ml doxycycline (Dox) in the presence or absence of EGF while plated in soft agar to measure transformation. Doxycycline was added every other day for 3 weeks of the transformation assay. (B) MCF7 cells were induced to express wt-EGFR or Y845F-EGFR with 1 mg/ml doxycycline for 48 hours and lysed. Lysates were immunoblotted using anti-EGFR and anti-β-actin antibodies. P values were calculated using Student's t test. EGF, *P = .0316, +P = .0064. Each experiment was performed in triplicate at least three times.
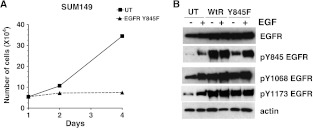
Previously, EGFR kinase activity has been shown to be required for proliferation in the SUM149 breast cancer cell line in which both EGFR and c-Src are overexpressed [9,19,20]. To support the data from the inducible MCF7 cell model, we transiently transfected Y845F-EGFR into SUM149 breast cancer cells and measured cell proliferation. Importantly, transient expression of Y845F-EGFR completely abrogated SUM149 cell proliferation (Figure 2A). This loss of cell proliferation is comparable to treatment of SUM149 cells with 1.0 µM gefitinib, a selective EGFR inhibitor [9]. At the time of plating for the proliferation assay, lysates were made from the remaining cells to verify EGFR overexpression and loss of EGFR tyrosine 845 phosphorylation (Figure 2B). As a control, transient overexpression of wt-EGFR resulted in constitutive phosphorylation of EGFR tyrosine 845 as well as EGFR tyrosine 1068 and tyrosine 1173, two EGFR autophosphorylation sites (Figure 2B). However, in Y845F-EGFR-expressing cells, EGF-induced EGFR tyrosine 845 phosphorylation was reduced compared to wt-EGFR, whereas EGFR tyrosine 1068 and tyrosine 1173 phosphorylation were unaffected (Figure 2B). The EGF-inducible EGFR tyrosine phosphorylation in the Y845F-EGFR-transfected cells represents the phosphorylation of endogenous EGFR. These data suggest that inhibition of EGFR tyrosine 845 phosphorylation by transfection of the Y845F-EGFR acts as a dominant negative with respect to proliferation.
Figure 2.
Transient expression of Y845F-EGFR decreases cell proliferation. (A) SUM149 cells were transiently transfected with Y845F-EGFR on day 0, and cells were counted on days 1, 2, and 4. (B) Cell lysates were prepared from companion plates transfected with the growth assay and cultured for 4 days of the growth assays. Equal protein was separated by SDS-PAGE and was immunoblotted using EGFR, pY845-EGFR, pY1068-EGFR, pY1173-EGFR, and actin antibodies. Cell proliferation assays were performed in triplicate at least three times, and immunoblot analysis was performed at least three times. UT = pcDNA3 transfected.
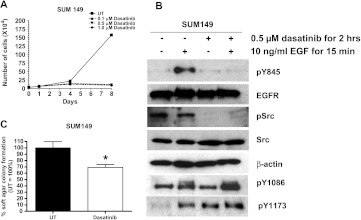
Tyrosine 845 on EGFR is not an autophosphorylation site and instead is phosphorylated by SFKs. We have previously shown that inhibiting SFK activity with the SFK inhibitor PP2 or a dominant negative c-Src in SUM149 cells abrogates cell proliferation and DNA synthesis [9]. Using a more clinically relevant SFK inhibitor, dasatinib, we wanted to determine the importance of SFK activity to the proliferation of SUM149 cells. By performing cell counts, 100 nM dasatinib was shown to be sufficient to block SUM149 cell proliferation (Figure 3A). These results demonstrate that inhibition of SFK activity in a breast cancer cell line with EGFR and c-Src co-overexpression is sufficient to abrogate cell proliferation.
Figure 3.
Inhibition of EGFR tyrosine 845 phosphorylation using dasatinib decreased cell growth and transformation. (A) SUM149 cells were plated on day 0, and dasatinib was added at 0.1, 0.5, or 1.0 µM on day 1 and every other day for 8 days. Cells were counted on days 1, 4, and 8. (B) SUM149 cells were treated with 0.5 µM dasatinib for 2 hours and 10 ng/ml EGF for 15 minutes. Cell lysates were prepared, separated by SDS-PAGE, and immunoblotted using pY845-EGFR, EGFR, pSrc, Src, actin, pY1173-EGFR, and pY1086-EGFR antibodies. (C) SUM149 cells were seeded in soft agar and treated with 0.5 µM dasatinib every other day for 3 weeks. UT = DMSO vehicle control. P value was calculated using Student's t test. *P = .0031. Growth and transformation assays were performed in triplicate at least three times. Immunoblot analysis was performed in at least three separate experiments.
Using lysates from SUM149 cells treated with dasatinib, we measured the ability of SFK inhibition to abrogate EGFR tyrosine 845 phosphorylation. Dasatinib treatment inhibited Src autophosphorylation on tyrosine 418 and EGFR tyrosine 845 phosphorylation (Figure 3B). However, similar to the results with transient transfection of Y845F-EGFR, phosphorylation of EGFR Y1086 and Y1173 was unaffected by dasatinib treatment. Therefore, inhibition of SFK activity blocked EGFR tyrosine 845 phosphorylation without altering autophosphorylation of EGFR. These results again support the hypothesis that inhibition of cell proliferation by loss of EGFR tyrosine 845 phosphorylation occurs independent of changes to EGFR autophosphorylation.
To determine if dasatinib inhibition of EGFR tyrosine 845 phosphorylation decreased cellular transformation, SUM149 cells were plated in soft agar and treated with 0.5 µM dasatinib every other day for 3 weeks. Dasatinib treatment decreased soft agar colony number by 40% compared to DMSO-treated controls (Figure 3C). Taken together, these results provide evidence for a role for EGFR and SFK cross talk in SUM149 cells to mediate proliferation and transformation through phosphorylation of EGFR tyrosine 845.
Loss of EGFR Tyrosine 845 Phosphorylation Does Not Alter EGFR Autophosphorylation or Kinase Activity
Tyrosine 845 on EGFR is a conserved tyrosine found in all tyrosine kinases located within the activation loop of the kinase domain [13]. In contrast to other tyrosine kinases, tyrosine 845 on EGFR is not required for its kinase activity [13]. Therefore, we would predict that inhibiting EGFR tyrosine 845 phosphorylation in the MCF7 cell models would not change autophosphorylation or kinase activity of EGFR. Indeed, all five EGFR autophosphorylation sites remained phosphorylated and EGFR kinase activity was maintained when Y845F-EGFR was expressed in MCF7 cells (see Figure W1). Similarly, transient expression of Y845F-EGFR in SUM149 cells did not abrogate phosphorylation of EGFR autophosphorylation site tyrosine 1068 (Figure 2B) and dasatinib did not inhibit phosphorylation of tyrosine 1086 (Figure 3C). Taken together, these data support that although phosphorylation of tyrosine 845 on EGFR is required for cell proliferation, DNA synthesis, and transformation in these breast cancer cell models, it is not required for autophosphorylation or kinase activity of EGFR. Therefore, inhibition of EGFR tyrosine 845 phosphorylation is sufficient to decrease cell proliferation, DNA synthesis, and transformation in the context of fully activated and phosphorylated EGFR.
Loss of EGFR Tyrosine 845 Phosphorylation Reduces Gab1 and p38 MAPK Phosphorylation
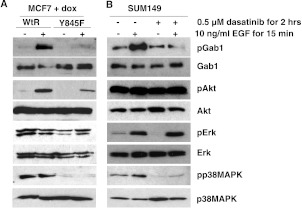
To understand what signaling pathways are being inhibited when EGFR tyrosine 845 phosphorylation is lost, we examined a panel of key EGFR signaling proteins, including Akt and the MAP kinases ERK and p38 MAPK. Interestingly, ERK and Akt remained phosphorylated when EGFR tyrosine 845 phosphorylation was abrogated by dasatinib treatment, whereas expression of Y845F-EGFR decreased Akt phosphorylation but not ERK phosphorylation (Figures 4, A and B, and W2). In contrast, the phosphorylation of the stress-associated MAPK, p38 MAPK, was lost under both basal and EGF-stimulated conditions (Figures 4, A and B, and W2). These data suggest that in the absence of endogenous EGFR in the MCF7 cells, signaling from Y845-EGFR phosphorylation is mediated downstream by both Akt and p38 MAPK phosphorylation. However, in the context of endogenous EGFR, Y845-EGFR phosphorylation is required for p38 MAPK activation.
Figure 4.
Signaling through Akt and MAPK but not p38 MAPK is maintained in the absence of EGFR tyrosine 845 phosphorylation. (A) MCF7 cells induced to express wt-EGFR or Y845F-EGFR or (B) SUM149 cells treated with dasatinib for 2 hours were treated with 10 ng/ml EGF for 15 minutes. Cell lysates were made, and subsequent separated protein was immunoblotted using pGab1-Tyr627, Gab1, pAkt-Ser473, Akt, pMAPK-Thr185/Tyr187, MAPK, pp38 MAPK-Thr180/Tyr182, and p38 MAPK antibodies. Each experiment was performed at least three independent times.
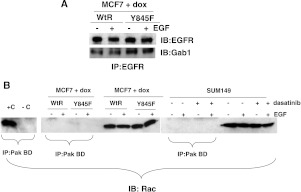
Phosphorylation of p38 MAPK has been shown to occur downstream of EGFR signaling through a Gab1/Rac-mediated pathway [21]. Therefore, we tested the phosphorylation status of Gab1 in the absence of EGFR tyrosine 845 phosphorylation. As predicted, Gab1 phosphorylation was reduced when EGFR tyrosine 845 phosphorylation was prevented (Figures 4, A and B, and W2). However, coimmunoprecipitation assays demonstrated that the association of Gab1 with EGFR remained unaltered (Figure 5A). These data are not surprising in that Gab1 has been shown to associate with EGFR through other phosphorylated tyrosines [22]. In addition, we tested the role of Rac1 activity in models of EGFR tyrosine 845 phosphorylation, as Rac1 has been shown to be a mediator between Gab1 and p38 MAPK signaling. However, Rac1 was not activated either basally or in the presence of EGF in either the MCF7 or the SUM149 cell model (Figure 5B), suggesting an alternative mediator for Gab1/p38 MAPK signaling.
Figure 5.
Gab1/Rac signaling is not mediating p38 MAPK activity. (A) MCF7 cells induced to express either wt-EGFR or Y845F-EGFR were treated with EGF for 15 minutes. Lysates were prepared and immunoprecipitated with anti-EGFR antibodies. Immunoprecipitates were washed and separated by SDS-PAGE. Immunoblot analysis using EGFR or Gab1 antibodies was performed. (B) MCF7 cells were induced to express either wt-EGFR or Y845F-EGFR, or SUM149 cells were treated with 0.5 µM dasatinib for 2 hours. Cells were stimulated with 10 ng/ml EGF for 15 minutes. Lysates were prepared using MLB lysis buffer provided by the manufacturer. Activated Rac was isolated from the lysates using the Rac-binding domain from PAK-1 (PAK BD). GTPγS (+C) or GDP (-C) was added to two of the SUM149 lysate reactions for positive and negative controls. In addition, whole-cell lysates were run next to the immunoprecipitations for expression controls. After gel electrophoresis, immunoblot analysis was performed using Rac antibodies. Each experiment was repeated at least three times.
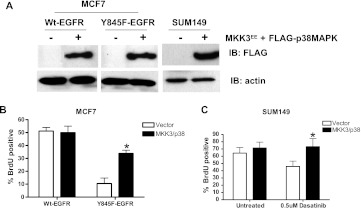
Constitutive Activation of p38 MAPK Restores Proliferation in the Absence of EGFR Tyrosine 845 Phosphorylation
To determine if p38 MAPK phosphorylation is a signaling link between EGFR tyrosine 845 phosphorylation and proliferation, we transiently cotransfected an activated form of MKK3, a direct activator of p38 MAPK, with a FLAG-tagged p38 MAPK into MCF7 and SUM149 cells (Figure 6A). We induced either wt-EGFR or Y845F-EGFR expression with doxycycline at the time of MKK3/p38 MAPK transfection, waited 48 hours, and measured BrdU incorporation in cells positive for FLAG-p38 MAPK (Figure 6B). Importantly, activation of p38 MAPK with the constitutively active MKK3 increased BrdU incorporation four-fold in MCF7 cells expressing Y845F-EGFR. Similarly, dasatinib-treated SUM149 cells showed a significant increased in DNA synthesis when p38 MAPK was activated (Figure 6C). Taken together, these data provide strong evidence for p38 MAPK activity as being a downstream mediator of EGFR tyrosine 845 phosphorylation required for proliferation and transformation.
Figure 6.
p38 MAPK activity compensates for loss of tyrosine 845 phosphorylation on EGFR. (A) MCF7 inducibly expressing wt-EGFR or Y845F-EGFR and SUM149 were transfected with MKK3EE and FLAG-tagged p38 MAPK at a 1:5 ratio. Lysates were collected, and immunoblot analysis was performed using anti-FLAG and anti-β-actin antibodies. (B) MCF7 cells induced to express wt-EGFR or Y845F-EGFR and (C) SUM149 cells were transfected with constitutively active MKK3EE and p38 MAPK on coverslips for 48 hours. A 4-hour BrdU pulse was followed by cell fixation and staining using anti-BrdU fluorescent antibodies and anti-FLAG followed by anti-mouse fluorescent antibodies. FLAG-positive cells were determined to be BrdU positive or negative. Untransfected wt-EGFR MCF7 cells (A) and untransfected, untreated SUM149 cells (B) were used as 100% BrdU-positive controls. Student's t test was used to identify statistical significance. Each experiment was repeated three times in duplicate. Student's t test was used to calculate P values. MCF7 cells, *P= .0005 and SUM149 cells, *P = .013.
Discussion
From these studies, we have identified p38 MAPK activation as a key event in the synergistic signaling between EGFR tyrosine 845 phosphorylation and Src. Specifically, we demonstrated that loss of EGFR tyrosine 845 phosphorylation, either by mutation or kinase inhibition of SFKs, dramatically decreased cell growth and transformation. This loss of EGFR tyrosine 845 phosphorylation resulted in decreased p38 MAPK phosphorylation, with a decrease in Akt phosphorylation in the MCF7 cells expressing low levels of endogenous EGFR. Interestingly, constitutive activation of p38 MAPK in the presence of the EGFR tyrosine 845 to phenylalanine mutation or the SFK inhibitor dasatinib rescued proliferation. Taken together, these data provide strong evidence for the co-overexpression of EGFR and SFKs and the subsequent phosphorylation of EGFR tyrosine 845 in proliferation and transformation mediated by p38 MAPK activation.
The role of p38 MAPK in cancer has been thought to occur through negative regulation of the cell cycle and senescence, suggesting that p38 MAPK is a tumor suppressor gene [23]. In addition, p38 MAPK activation in response to chemotherapy has been thought to work as a mechanism for inducing apoptosis (reviewed in [24]). These data imply that loss and inactivation of p38 MAPK would mediate tumor growth and survival. However, our data demonstrate that in the context of EGFR and c-Src coexpression, p38 MAPK activation regulates cell proliferation and anchorage-independent growth. In support of our studies, activation of p38 MAPK has been shown to stimulate proliferation in response to specific stimuli including DNA damage, osmotic stress, and inflammation [25]. In addition, p38 MAPK activity was found to be upregulated in breast, head and neck, lymphoma, glioma, and squamous cell carcinomas (reviewed in [26]). Specifically, phosphorylation of p38 MAPK was found to be a negative prognostic indicator in human epidermal growth factor receptor 2 (HER2)-negative, lymph node-positive breast cancers [27]. A number of p38 MAPK inhibitors are in Phase I and II clinical trials (reviewed in [26]).
Although p38 MAPK is often considered as a stress-induced kinase, EGFR signaling has been shown to mediate p38 MAPK activation. One mechanism by which EGFR activates p38 MAPK signaling is through c-Src [28]. Src is directly activated by EGFR, phosphorylating c-Src on tyrosine 416/418. As discussed above, EGFR and c-Src when expressed at high levels stimulate the activation of synergistic signaling pathways [11,12]. Mikami et al. found that Src phosphorylates MKK3 and MKK6, the kinases that phosphorylate and activate p38 MAPK [28]. Our data demonstrate that when EGFR and c-Src are expressed and activated in our breast cancer models, p38 MAPK is activated. Yet, when EGFR and c-Src cross talk is eliminated by loss of EGFR tyrosine 845 phosphorylation, either by mutation or Src kinase inhibition, p38 MAPK phosphorylation is abrogated. This loss of p38MAPK phosphorylation correlates with a decrease in proliferation. Significantly, introducing a constitutively active MKK3 to induce p38MAPK phosphorylation was sufficient to reverse the reduction in phosphorylation when EGFR tyrosine 845 phosphorylation was inhibited. Therefore, we have established a novel link between EGFR and c-Src cross talk and proliferation through p38 MAPK phosphorylation.
Resistance to targeted therapies, such as EGFR inhibitors, in the clinic has become a significant challenge in the treatment of cancer. Identifying biomarkers and characterizing activated signaling pathways will improve the selection of the best targeted therapies for patients. Here, we have shown in breast cancer cells that phosphorylation of EGFR on tyrosine 845 by SFK is critical to the proliferation and transformation of these cells. In addition, activation of p38 MAPK overcame the need for EGFR tyrosine 845 phosphorylation. Therefore, EGFR tyrosine 845 phosphorylation may be a good biomarker for SFK inhibitor therapy.
Supplementary Material
Footnotes
This work was supported by Susan G. Komen for the Cure Career Catalyst award (KG081416; J.L.B.).
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.transonc.com.
References
- 1.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors—impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrero JM, Ramaioli A, Largillier R, Formento JL, Francoual M, Ettore F, Namer M, Milano G. Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-up. Ann Oncol. 2001;12:841–846. doi: 10.1023/a:1011183421477. [DOI] [PubMed] [Google Scholar]
- 3.Klijn JG, Berns PM, Schmitz PI, Foekens JA. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev. 1992;13:3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71:67–75. doi: 10.1023/a:1013397232011. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Albanell J, Ruiz A, Lluch A, Gascon P, Guillem V, Gonzalez S, Sauleda S, Marimon I, Tabernero JM, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 6.Tan AR, Yang X, Hewitt SM, Berman A, Lepper ER, Sparreboom A, Parr AL, Figg WD, Chow C, Steinberg SM, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol. 2004;22:3080–3090. doi: 10.1200/JCO.2004.08.189. [DOI] [PubMed] [Google Scholar]
- 7.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 8.Kopetz S, Shah AN, Gallick GE. Src continues aging: current and future clinical directions. Clin Cancer Res. 2007;13:7232–7236. doi: 10.1158/1078-0432.CCR-07-1902. [DOI] [PubMed] [Google Scholar]
- 9.Mueller KL, Hunter LA, Ethier SP, Boerner JL. Met and c-Src cooperate to compensate for loss of epidermal growth factor receptor kinase activity in breast cancer cells. Cancer Res. 2008;68:3314–3322. doi: 10.1158/0008-5472.CAN-08-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller KL, Yang ZQ, Haddad R, Ethier SP, Boerner JL. EGFR/Met association regulates EGFR TKI resistance in breast cancer. J Mol Signal. 2010;5:8. doi: 10.1186/1750-2187-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimri M, Naramura M, Duan L, Chen J, Ortega-Cava C, Chen G, Goswami R, Fernandes N, Gao Q, Dimri GP, et al. Modeling breast cancer-associated c-Src and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer Res. 2007;67:4164–4172. doi: 10.1158/0008-5472.CAN-06-2580. [DOI] [PubMed] [Google Scholar]
- 12.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci USA. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinog. 2005;44:262–273. doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- 16.Whitmarsh AJ, Cavanagh J, Tournier C, Yasuda J, Davis RJ. A mammalian scaffold complex that selectively mediates MAP kinase activation. Science. 1998;281:1671–1674. doi: 10.1126/science.281.5383.1671. [DOI] [PubMed] [Google Scholar]
- 17.Vomastek T, Iwanicki MP, Burack WR, Tiwari D, Kumar D, Parsons JT, Weber MJ, Nandicoori VK. Extracellular signal-regulated kinase 2 (ERK2) phosphorylation sites and docking domain on the nuclear pore complex protein Tpr cooperatively regulate ERK2-Tpr interaction. Mol Cell Biol. 2008;28:6954–6966. doi: 10.1128/MCB.00925-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu YH, Richert N, Ito S, Merlino GT, Pastan I. Characterization of epidermal growth factor receptor gene expression in malignant and normal human cell lines. Proc Natl Acad Sci USA. 1984;81:7308–7312. doi: 10.1073/pnas.81.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willmarth NE, Ethier SP. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J Biol Chem. 2006;281:37728–37737. doi: 10.1074/jbc.M606532200. [DOI] [PubMed] [Google Scholar]
- 20.Irwin ME, Mueller KL, Bohin N, Ge Y, Boerner JL. Lipid raft localization of EGFR alters the response of cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol. 2010;226:2316–2328. doi: 10.1002/jcp.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallin A, Demoulin J-B, Nishida K, Hirano T, Rönnstrand L, Heldin C-H. Gab1 contributes to cytoskeletal reorganization and chemotaxis in response to platelet-derived growth factor. J Biol Chem. 2004;279:17897–17904. doi: 10.1074/jbc.M312996200. [DOI] [PubMed] [Google Scholar]
- 22.Mattoon D, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004;2:24. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle. 2006;5:824–828. doi: 10.4161/cc.5.8.2685. [DOI] [PubMed] [Google Scholar]
- 24.Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10:125–129. doi: 10.1016/j.molmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 27.Esteva FJ, Sahin AA, Smith TL, Yang Y, Pusztai L, Nahta R, Buchholz TA, Buzdar AU, Hortobagyi GN, Bacus SS. Prognostic significance of phosphorylated P38 mitogen-activated protein kinase and HER-2 expression in lymph node-positive breast carcinoma. Cancer. 2004;100:499–506. doi: 10.1002/cncr.11940. [DOI] [PubMed] [Google Scholar]
- 28.Mikami F, Gu H, Jono H, Andalibi A, Kai H, Li JD. Epidermal growth factor receptor acts as a negative regulator for bacterium nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via an Src-dependent p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2005;280:36185–36194. doi: 10.1074/jbc.M503941200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.