SUMMARY
Improvements in functional endoscopic sinus surgery (FESS) and computed tomography (CT) have concurrently increased interest in the anatomy of the paranasal region. Common anatomical variations are not rare in patients with chronic paranasal sinusitis. The aim of this retrospective study was to analyze the incidence of anatomic variations of the lateral nasal wall in a series of 200 patients with persistent symptoms of rhinosinusitis, after failure of medical therapies, and their correlation with paranasal sinus disease. A detailed analysis of CT scans showed that 140 of 200 (70%) patients had anatomic variations. In particular, 122 patients (87%) were affected by common anatomic variations, and 18 patients (13%) with uncommon variations. There were 85 (60.7%) male and 55 (39.3%) females with ages ranging from 13 to 77 years (mean 45.5 years). The maxillary sinus was most commonly involved, followed by the anterior ethmoid, frontal sinus, posterior ethmoid and sphenoid sinus. Statistically significant association was found between the presence of common anatomic variations – septal deviation, bilateral concha bullosa, medial deviation of uncinate process, Haller cell, ethmoidal bulla hypertrophic, agger nasi cell – and the presence of sinus mucosal disease (p < 0.05). There was no significant correlation between other common and uncommon anatomic variations and mucosal pathologies. The associations were evaluated using the Fisher's exact test, and compared with those reported in the literature. Considering the results obtained, we believe that some anatomic variations may increase the risk of sinus mucosal disease. We therefore emphasize the importance of a careful evaluation of CT study in patients with persistent symptoms and recurrent chronic rhinosinusitis in order to identify those with anatomical variations that may have an increased risk of developing rhinosinusitis.
KEY WORDS: Anatomic variations, Chronic rhinosinusitis, Paranasal sinuses, Computed tomography, Endoscopic sinus surgery
RIASSUNTO
Il perfezionamento della chirurgia endoscopica funzionale dei seni paranasali (FESS) e della tomografia computerizzata (TC) hanno aumentato l'interesse per l'anatomia della regione dei seni paranasali. Varianti anatomiche comuni non sono rare nei pazienti affetti da rinosinusite cronica. Scopo di questo studio retrospettivo è stato quello di analizzare l'incidenza di varianti anatomiche della parete laterale del naso in una serie di 200 pazienti con sintomi persistenti di rinosinusite dopo il fallimento di terapie mediche e la loro correlazione con la patologia dei seni paranasali. Una dettagliata analisi della TC ha mostrato che 140/200 pazienti (70%) presentavano varianti anatomiche. In particolare, 122 pazienti (87%) erano affetti da varianti anatomiche comuni, mentre 18 pazienti (13%) da varianti anatomiche non comuni. Ottantacinque pazienti (60,7%) erano di sesso maschile mentre 55 (39,3%) di sesso femminile, di età compresa tra i 13 e i 77 anni (età media 45,5 anni). Il seno mascellare è stato quello più interessato, seguito dall'etmoide anteriore, dal seno frontale, dall'etmoide posteriore e dal seno sfenoidale. È stata riscontrata un'associazione statisticamente significativa fra la presenza delle seguenti varianti comuni quali deviazione del setto nasale, concha bullosa bilaterale, deviazione mediale del processo uncinato, cellula di Haller, bulla etmoidale ipertrofica, cellula dell'agger nasi e la presenza di patologia dei seni paranasali (p < 0,05). Non è stata trovata alcuna correlazione statisticamente significativa fra altre varianti anatomiche e la patologia dei seni paranasali. Le associazioni sono state valutate utilizzando il test esatto di Fisher, e confrontati con quelli riportati in letteratura. Considerando i risultati ottenuti, riteniamo che solo alcune varianti anatomiche possono aumentare il rischio di patologia sinusale. Abbiamo quindi enfatizzato l'importanza di un'attenta valutazione dello studio TC nei pazienti con sintomi persistenti e ricorrenti di rinosinusite cronica al fine di identificare i pazienti portatori di varianti anatomiche a maggior rischio per lo sviluppo di rinosinusite.
Introduction
Several authors have assessed the relationship between sinonasal anatomic variants and the incidence of rhinosinusitis 1-12. There is now worldwide interest among otolaryngologists in radiological definition of paranasal regional anatomy 6. Certain anatomic variations forming the lateral wall of the nose are very important because they can contribute to the blockage of the ostiomeatal units, drainage and ventilation, and can thereby increase the risk of sinus mucosal disease 13-15.
Moreover, anatomic variants with a potential impact on surgical safety occur frequently and need to be specifically sought as part of preoperative evaluation 8 16 17. Anatomic variations, such as deviation of the nasal septum, concha bullosa or paradoxical middle turbinate, ethmoidal bulla hypertrophic, agger nasi cell, lateral or medial bending of uncinate process (UP) and Haller cell are common and emphasized in routine evaluation of computed tomography (CT) images. Occasionally, some uncommon anatomic variations, in addition to those mentioned above, can increase the risk of surgical complications, especially when the operator lacks experience, and inadequate management of these anatomic conditions may be associated with residual disease or recurrence.
Appropriate radiologic imaging and accurate interpretation play an important role in the diagnosis and management of these conditions. CT plays a central role in the modern management of chronic rhinosinusitis due to its ability to delineate mucosal disease, to demonstrate a primary obstructive pathology and to image distal structures such as the posterior ethmoid sinus that cannot be viewed with direct endoscopy 18.
In this study, we reviewed the CT scans with at least one anatomical variation of 140 patients, of a total of 200 cases, suffering from chronic rhinosinusitis to investigate the common and uncommon anatomic variations and correlate them with the presence of radiologic evidence of sinus mucosal disease. Anatomic variants that have a potential impact on surgical safety were also assessed. Finally, we correlated the incidence of each anatomic variation with the presence of unilateral or bilateral sinusitis, and the results were compared with recent literature data.
Material and methods
At the ENT Department in San Luigi Gonzaga Hospital of Orbassano, Turin, Italy, we reviewed 200 coronal and axial high-resolution CT scans, from patients with persistent symptoms of rhinosinusitis who had failed medical therapies. The study excluded patients with odontogenic sinusitis, mycetoma, massive or recurrent polyposis, cystic fibrosis and patients who had undergone endoscopic sinus surgery.
Each CT was reviewed by the authors, investigating the presence of anatomic variations and sites of involved sinuses based on findings of opacification of CT scans. A detailed analysis of CT scans showed 140 of 200 (70%) patients had common or uncommon anatomic variations. 122 patients (87%) were affected by common anatomic variations, and 18 patients (13%) with uncommon variations. Anatomic variations were graded according to the classification proposed by Sarna et al. 19.
A clinical diagnosis of chronic rhinosinusitis was made on physical examination and clinical criteria reported by the Task Force on Rhinosinusitis 20; all patients underwent examination by diagnostic nasal endoscopy using a nasal telescope. Clinical findings included posterior nasal drip, headache, facial pain over the area of the affected sinus, nasal obstruction, purulent rhinorrhoea and hyposmia.
The statistical analysis was useful to assess the relationship between common and uncommon anatomic variations and the presence of unilateral or bilateral sinusitis. Associations were evaluated using the Fisher's exact test. A chi-square was not appropriate for this data because the expected frequencies were less than 5 for more of 25% of the cells of the contingency tables (data not shown). Analyses were conducted using SAS System 9.2. Statistical significance was set at p < 0.05.
Eighty-five patients (85/140) affected by chronic sinusitis and anatomic variations underwent functional endoscopic sinus surgery, and the procedures were tailored appropriately if there was a common or uncommon anatomic variation.
Results
A total of 140 of 200 patients showing symptoms of chronic rhinosinusitis who had at least one sign on CT anatomic variation were included in this study. 85 (60.7%) patients were male and 55 (39.3%) were female with ages ranging from 13 to 77 years (mean 45.5 years).
The analysis of CT scans showed that the affected side was right in 40 (28.6%) patients, left in 84 (60%) patients and bilateral in 105 patients (75%). Nineteen (13.6%) patients had no signs of disease on CT. Regarding the CT prevalence of sinusal opacities in the group of 106 patients with sinusitis, 94 (67.1%) had maxillary sinusitis, 76 (54.3%) anterior ethmoid sinusitis, 31 (22.1%) frontal sinusitis, 14 (10%) posterior ethmoid sinusitis and 14 (10%) had sphenoid sinusitis. Pathology at osteomeatal complex (OMC) on CT scan was observed in 106 (75.7%) patients in this series (Table I).
Table I.
CT scans prevalence of sinusal opacities in the group of 140 patients with sinusitis (*OMC: osteomeatal complex).
| Sinusitis | Unilateral, N (%) | Bilateral, N (%) | Total, N (%) | ||
|---|---|---|---|---|---|
| Right, N (%) | Left, N (%) | Total, N (%) | |||
| Maxillary | 18 (12.8) | 40 (28.6) | 58 (41.4) | 36 (25.7) | 94 (67.1) |
| Anterior ethmoid | 12 (8.6) | 24 (17.1) | 36 (25.7) | 40 (28.6) | 76 (54.3) |
| Frontal | 6 (4.3) | 13 (9.3) | 19 (13.6) | 12 (8.5) | 31 (22.1) |
| Posterior ethmoid | 2 (1.4) | 3 (2.1) | 5 (3.5) | 9 (6.5) | 14 (10) |
| Sphenoid | 2 (1.4) | 4 (2.9) | 6 (4.3) | 8 (5.7) | 14 (10) |
| Closed OMC* | 18 (12.9) | 17 (12.1) | 35 (25) | 71 (50.7) | 106 (75.7) |
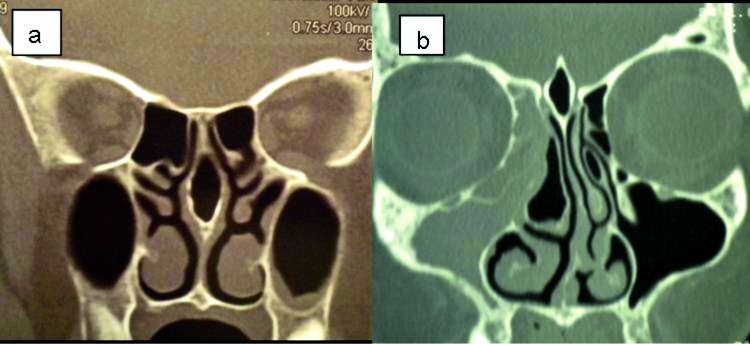
The most common anatomic variation (Table II) observed on CT scans was nasal septal deviation, which was presented by 82 patients (58.5%). Concha bullosa of the middle turbinate was the second most common variant, observed in 69 patients (49.3%), and was mostly seen on one side of the nasal wall (30.7%). A total of 46 patients (32.8%) had hypertrophic ethmoid bulla, whereas agger nasi cell was observed in 34 patients (24.3%). Considering the UP, its lateral deviation was found in 30 patients (21.4%), whereas its medial deviation was presented by 32 patients (22.8%); a hypertrophic UP was observed in 14 patients (10%) and atelectatic in 9 patients (6.3%). Haller cell was observed in 32 patients (22.8%); frontal cells were seen in 17 patients (12.1%), and Onodi cell in 12 patients (8.5%); paradoxical middle turbinate was observed in only 9 patients (6.4%); hypoplastic maxillary sinus was present in 8 patients (5.7%) and septated maxillary sinus in 7 patients (5%). With respect to the level difference between the ethmoid and cribriform plate, Keros type I was the most common and seen in 22 patients (15.7%), followed by type II in 11 (7.8%) and type III in 2 patients (1.4%). Pneumatization of crista galli was observed in 19 patients (13.6%) and pneumatization of the nasal septum in 13 patients (9.3%) (Fig. 1a, b).
Table II.
Common anatomic variations in CT scans of 140 patients with chronic paranasal sinusitis.
| Common anatomic variations | Unilateral, N (%) | Bilateral, N (%) | Total, N (%) | ||
|---|---|---|---|---|---|
| Right, N (%) | Left, N (%) | Total, N (%) | |||
| Septal deviation | 36 (25.7) | 46 (32.85) | - | - | 82 (58.5) |
| Hypertrophic ethmoidal bulla | 9 (6.4) | 19 (13.6) | 28 (20) | 18 (12.8) | 46 (32.8) |
| Large agger nasi cell | 5 (3.6) | 7 (5) | 12 (8.6) | 22 (15.7) | 34 (24.3) |
| Middle turbinate | |||||
| Concha bullosa | 20 (14.3) | 23 (16.4) | 43 (30.7) | 26 (18.6) | 69 (49.3) |
| Paradoxical | 4 (2.8) | 3 (2.1) | 7 (5) | 2 (1.4) | 9 (6.4) |
| Uncinate process | |||||
| Lateral deviation | 10 (7.1) | 6 (4.3) | 16 (11.4) | 14 (10) | 30 (21.4) |
| Medial deviation | 10 (7.1) | 4 (2.9) | 14 (10) | 18 (12.8) | 32 (22.8) |
| Hypertrophy | 2 (1.4) | 7 (5) | 9 (6.4) | 5 (3.5) | 14 (10) |
| Atelectatic | 1 (0.7) | 4 (2.8) | 5 (3.5) | 4 (2.8) | 9 (6.3) |
| Haller's cell | 8 (5.7) | 8 (5.7) | 16 (11.4) | 16 (11.4) | 32 (22.8) |
| Onodi cell | 4 (2.8) | 6 (4.3) | 10 (7.1) | 2 (1.4) | 12 (8.5) |
| Maxillary sinus | |||||
| Hypoplastic | 1 (0.7) | 5 (3.6) | 6 (4.3) | 2 (1.4) | 8 (5.7) |
| Septated | 2 (1.4) | 4 (2.9) | 6 (4.3) | 1 (0.7) | 7 (5) |
| Roof of the ethmoid # | Type I : 22 (15.7) | Types II : 11 (7.8) | Types III : 2 (1.4) | 35 (25) | |
| Frontal cells * | Type I : 11 (7.8) | Type II : 6 (4.3) | Type III : 1 (0.7) | 17 (12.1) | |
| Pneumatization of crista galli | 19 (13.6) | ||||
| Pneumatization of nasal septum | 13 (9.3) | ||||
Roof of the ethmoid: Keros type I, olfactory fossa is 1-3 mm deep; Keros type II, olfactory fossa is 4-7 mm deep; Keros type III, olfactory fossa is 8-16 mm deep (41).
Frontal cell types: type I, single frontal recess above agger nasi cell; type II, tier of cells in frontal recess above agger nasi cell; type III, single massive cell pneumatizing cephalad into frontal sinus; type IV, single isolated cell within the frontal sinus (42).
Fig. 1.
Coronal CT scans. a) Nasal septal pneumatization. b) Extensive pneumatization of the crista galli or bulla galli. Right concha bullosa; left pneumatization of the uncinate process; deviation of the nasal septum convexity to the left can be seen; small bilateral Haller cells are also present.
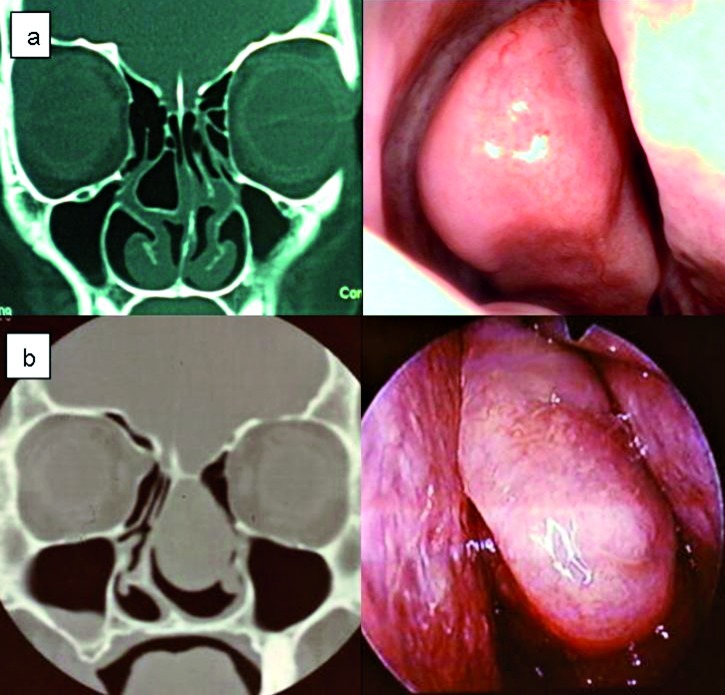
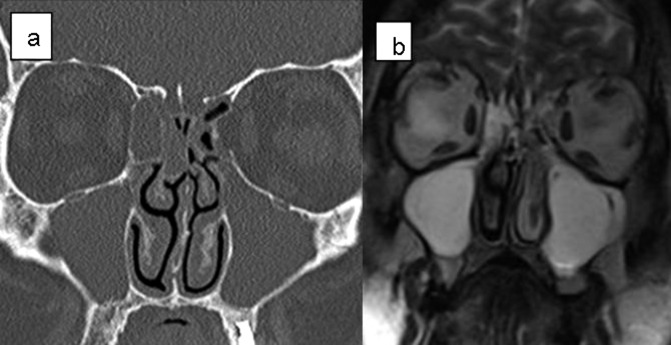
A total of 18 uncommon anatomic variations were seen (12.8%) in the 140 patients. These included pneumatization of UP in 4 cases (2.8%); massive concha bullosa of middle turbinate in 4 cases (2.8%) (Fig. 2a, b), massive pneumatization of frontal sinus in 3 cases (2.1%); lateralized maxillary sinus in 2 cases (1.4%); single sphenoid sinus in 2 cases (1.4%); separated uncinate process in 2 cases (1.4%) and nontraumatic protrusion of orbital contents through dehiscence of lamina papyracea in 1 case (0.7%) (Fig. 3a, b).
Fig. 2.
Coronal CT scan and corresponding endoscopic image of concha bullosa. a) Large right concha bullosa with moderate deviation of the nasal septum convexity to the left. There is also small Haller cell on the right side, and Keros grade II. b) Masssive left mucopyocele of the concha bullosa of the middle turbinate presenting as a large nasal mass.
Fig. 3.
Coronal CT scans (a) and magnetic resonance imaging (b) reveal protrusion of the orbital contents through the dehiscence of lamina papyracea on the left side. The medial rectus muscle (arrow) could be a marker in differentiation of the ethmoiditis and orbital contents.
Multiparametric statistical analysis correlations are represented in Table IV. With regards to septal deviation, there was a statistically significant significance between left septal deviation and left maxillary sinusitis (p < 0.01). We also found a correlation between bilateral concha bullosa and bilateral maxillary sinusitis (p < 0.01). Considering the uncinate process, we investigated the correlation between hypertrophic UP, its lateral or medial deviation and the presence of anterior ethmoid sinusitis. It was seen that right and bilateral medial UP deviation was associated with right and bilateral anterior ethmoid sinusitis (p < 0.01 and p < 0.05, respectively). There was a statistically significance between right Haller cell and ipsilateral maxillary sinusitis, left Haller cell and left maxillary sinusitis and bilateral Haller cell and bilateral maxillary sinusitis (all p < 0.01).
Table IV.
Statistically significant correlation between anatomical variations and disease extension of sinusitis (Fisher's exact test).
| Anatomic variations | Sinusitis | p |
|---|---|---|
| Left septal deviation | Left maxillary | < 0.01 |
| Bilateral concha bullosa | Bilateral maxillary | < 0.01 |
| Medial deviation of uncinate process | Anterior ethmoid | |
| Right | Right | < 0.01 |
| Bilateral | Bilateral | < 0.05 |
| Haller's cell | Maxillary | |
| Right | Right | < 0.01 |
| Left | Left | |
| Bilateral | Bilateral | |
| Ethmoidal bulla hypertrophic | Anterior ethmoid | |
| Right | Right | < 0.01 |
| Bilateral | Bilateral | |
| Agger nasi cell | Frontal | |
| Right | Right | < 0.01 |
| Left | Left | < 0.05 |
A statistically significant correlation between right and bilateral hypertrophic ethmoid bulla and ipsilateral and bilateral anterior ethmoid sinusitis was also seen (p < 0.01). In addition, there was a statistically significant significance between right and left agger nasi cell and homolateral frontal sinusitis (p < 0.01 and p < 0.05).
No other statistically significant correlations were demonstrated between any other common and uncommon anatomic variations and ipsilateral, contralateral or bilateral sinusitis.
Discussion
Anatomic variations of paranasal sinus structures may predispose patients to recurrent sinusitis and, in selected cases, to headache 12. However, the relative importance of anatomic variations is still a matter of discussion and variable results have been reported 10.
Kim et al., Lerdlum et al., and Stallman et al. showed no specific association of anatomic variations in rhinosinusitis, and claimed that local, systemic, environmental factors or intrinsic mucosal disease were more significant in the pathogenesis of rhinosinusitis 3 21 22.
Nasal septal deviation is present in 20-31% of the general population, and severe deviation has been noted as a contributing factor for sinusitis 19 23. However, some studies have not demonstrated a causal relationship between nasal septal deviation and sinusitis 3 24. We found a statistically significant correlation between left septal deviation and left maxillary sinusitis (p < 0.01) (Table III).
Table III.
Uncommon anatomic variations in CT scans of 140 patients with chronic paranasal sinusitis.
| Uncommon anatomic variations | Right, N (%) | Left, N (%) | Bilateral, N (%) | Total, N (%) |
|---|---|---|---|---|
| Uncinate process | ||||
| Pneumatization | 1 (0.7) | 3 (2.1) | - | 4 (2.8) |
| Separated | 2 (1.4) | - | - | 2 (1.4) |
| Large concha bullosa of middle turbinate | 3 (2.1) | 1 (0.7) | 0 | 4 (2.8) |
| Massive pneumatization frontal sinus | - | 2 (1.4) | 1 (0.7) | 3 (2.1) |
| Lateralized maxillary sinus | - | - | 2 (1.4) | 2 (1.4) |
| Single sphenoid sinus | 2 (1.4) | |||
| Nontraumatic protusion of orbital contents through dehiscence of lamina papyracea | - | 1 (0.7) | - | 1 (0.7) |
Normal ethmoid bulla was detected in 94 of 140 patients (67.2%), compared to 17-89% of cases in previous reports 11 18. Ethmoid bulla hypertrophic – prominent – was present in 32.8% of patients in our study (Fig. 4). Krzeski et al. reported a frequency of 26.75% 11, while Scribano estimated that it is only 3.5% 25. In our study, there was significant correlation between hypertrophic ethmoidal bulla and sinusitis of anterior ethmoid (p < 0.01), which has not been reported in the literature (Table IV).
Fig. 4.
Coronal CT scan and corresponding endoscopic image of left hypertrophic ethmoidal bulla. There is also right concha bullosa with lateral deviation of the uncinate process.
The term concha bullosa was coined by Zuckerlandl in 1862 to describe pneumatization of the middle turbinate, and its incidence was reported to range from 9% to 20% based on initial anatomical dissections. The significance of this most common anatomic variation of the middle turbinate lies in the potential secondary deformity of the turbinate, which increases the probability of obstruction of the middle meatus and lead to recurrent ethmoid sinusitis 1.
Bolger et al. reported three types of the middle turbinate pneumatization: the vertical lamella was pneumatized in 46.2% of cases ("lamellar cell") in the inferior bulbous portion in 31.2% of patients and in the entire middle turbinate in 15.7% of cases ("true" concha bullosa) 12.
Unilateral or bilateral concha bullosa was detected in 49.3% of patients in the present series. According to data from the literature, the incidence of positive CT findings for concha bullosa varies from 14% to 62% 1 3 4 11 12 16 18 25-27. In particular, incidences of 37.5%, 44% and 48.1%, respectively, were reported by Krzeski et al. 11, Stallman et al. 3 and Ozcan et al. 4.
There are different opinions in the literature concerning concomitance with mucosal pathologies. Herein, multivariate analysis showed that bilateral concha bullosa was associated with sinusitis bilateral maxillary (p < 0.05) (Table IV) in agreement with previous reports 4 28, while other studies found no direct relationship 3 8 29. Stallman JS et al. reported a significant relationship between the presence of concha bullosa and deviation of the nasal septal on the contralateral side (p < 0.0001) 3.
Paradoxical curvature of the middle turbinate is described as a convexity pointing toward the middle meatus, and is reported as a possible cause for closed OMC and mucosal pathologies 30. In our study, the incidence of middle paradoxical turbinate was 6.4%, and was not associated with mucosal pathologies. The rates of this variation in previous publications are highly variable, with incidences ranging from 3% to 40% 4 12 18 31. Nouraei et al., in a review of 278 CT scans, found only two (0.7%) cases with paradoxical curves 1.
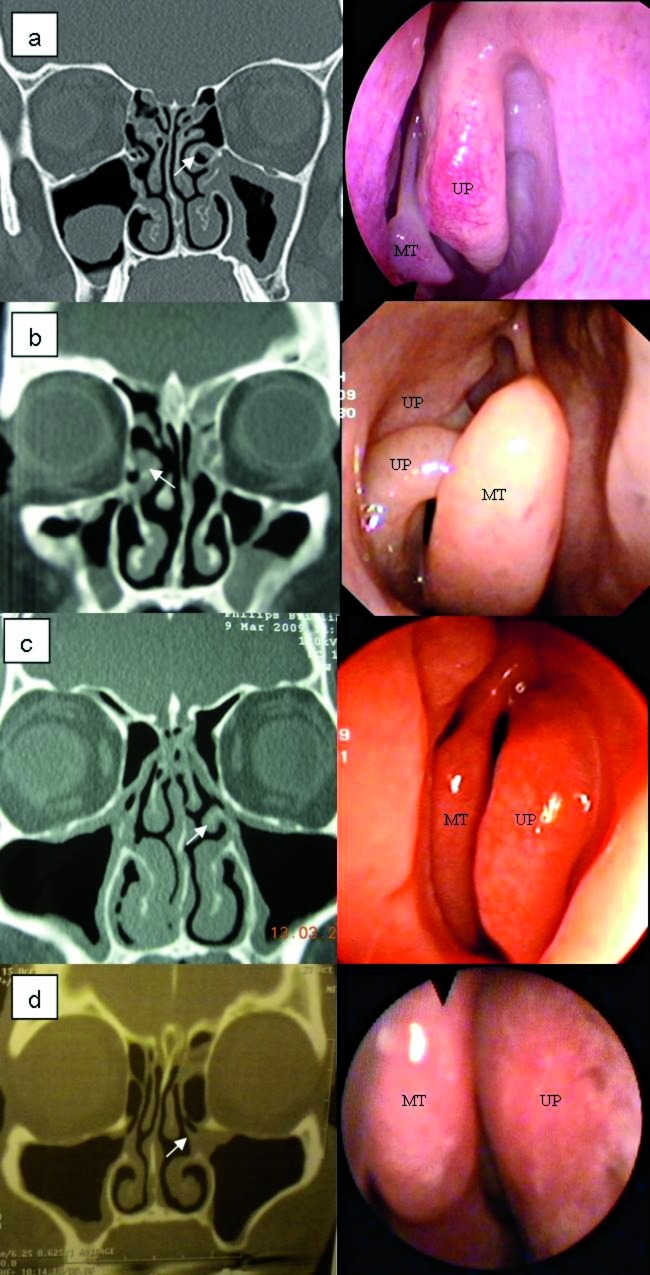
The UP is another important structure in relation to paranasal sinus drainage, and the incidence of variations in this structure is generally from 15.9% to 44.3% 11 13 32; in our study, it was 65% (Fig. 5a-d).
Fig. 5.
Coronal CT scan and corresponding endoscopic images of possible variations of uncinate process (UP, arrows). a) Left pneumatization UP; b) Right separated UP; c) Hypertrophy left UP; d) Medial deviation UP (UP = uncinate process; MT = middle turbinate).
Medial deflection of UP was previously described in 3-19% of cases 11 33. Herein, it was observed in 22.8% of patients, while lateral deflection of UP was observed in 21.4% of cases. We found that the UP was pneumatized in 2.8% of patients. The rate of UP pneumatization in previous studies has been reported to be from 1-9% 1 12 18 31. Our study showed that medial deviation of UP was statistically associated with anterior ethmoidal sinusitis (p < 0.05) (Table IV).
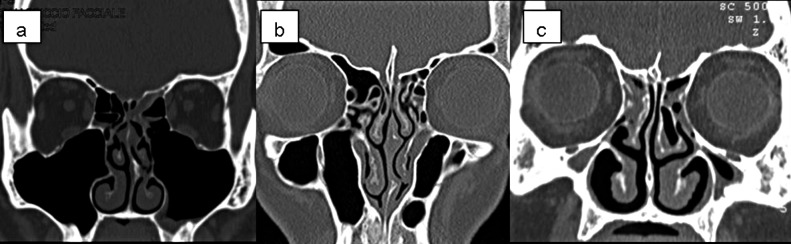
Maxillary sinus hypoplasia (MSH) is the most important anatomical variation among those involving the maxillary sinus (MS). MSH typing was done by Bolger et al. in 1990 34. Since MSH is often associated with orbital enlargement, thickening of the bony sinus wall, mucosal pathology, anterior ethmoidal cell variation or frontal sinus hypoplasia, it is important to identify these anatomical variations for proper surgical planning to prevent complications 2 34. The incidence of MS septae was found to vary from 20-31% in previous reports 2 35. In our study, the maxillary sinus was hypoplastic in 5.7% and septated in 5%, less than that previously reported, while over-pneumatization or lateralized variation was found in 1.4% of patients (Fig. 6a-c). There was no significant correlation between these anatomical variations and mucosal pathologies, in agreement with literature data 2.
Fig. 6.
Coronal CT scans of the maxillary sinuses. a) Bilateral lateralized; b) Bilateral septated; c) Bilateral hypoplasia of the maxillary sinuses.
The OMC is a functional entity of the anterior ethmoid complex that represents the final common pathway for drainage and ventilation of the frontal, maxillary and anterior ethmoid cells. Thus, anatomical variations that redirect nasal airflow or narrow the OMC have been implicated in the development of chronic rhinosinusitis 36. In this study, patients with pathologies at OMC (106/140) had involvement of multiple sinuses and were found to have increased symptom severity.
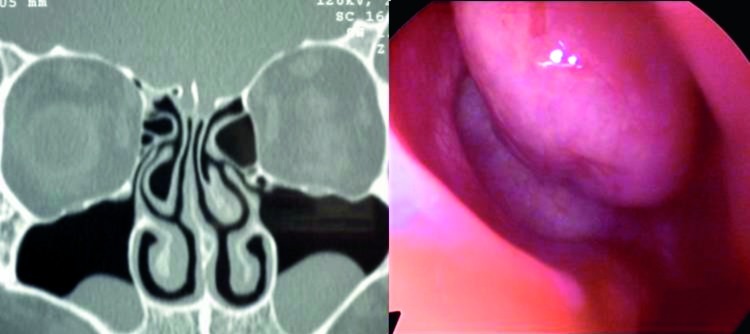
Another common anatomic variant was the presence of infraorbital ethmoid cells, also known as Haller cells. These are found between the maxillary sinus and the orbit and can increase the risk of orbital injury during ethmoidectomy 31. Haller cells are a clinically significant anatomic variation because they have been implicated as a possible aetiologic factor in recurrent maxillary sinusitis due blockage of the OMC (Fig. 7) 6. In previous studies, a variable incidence of Haller cells has been noted. In particular, Kennedy et al. 16 and Meloni et al. 36 both reported rates of 10%, while Arslan et al. 30 reported an incidence of 6% and Bolger et al. 12 an incidence of 45.1%. Possible reasons for this discrepancy include differences in interpretation of Haller cells, or in the technique of CT scanning.
Fig. 7.
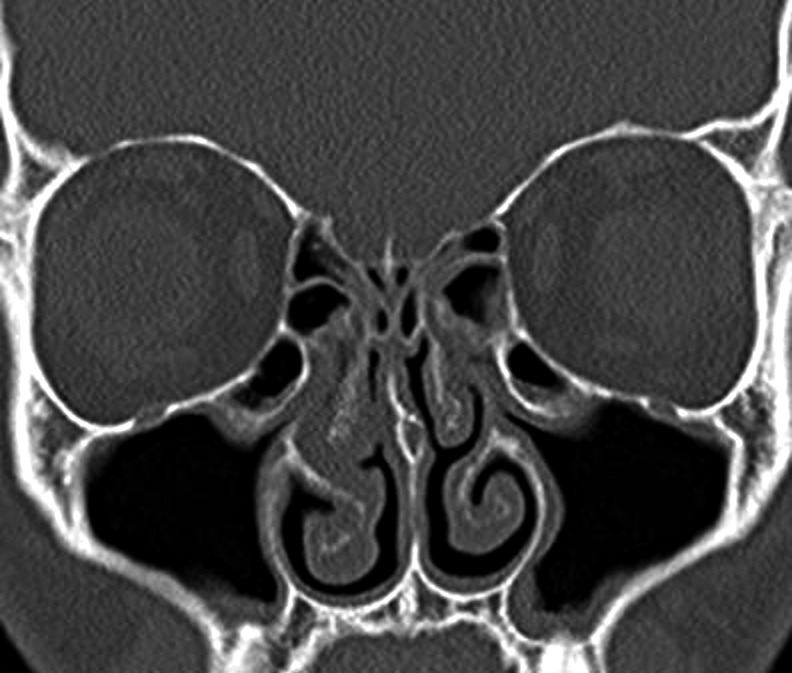
Coronal CT scan of bilateral large Haller's cell and closed osteomeatal complex.
In our study, the incidence of Haller cells was 22.8%, and we found a statistically significant relationship with maxillary sinusitis (p < 0.01), in agreement with what reported by Van Alyea 38.
The reported prevalence of the agger nasi cell varies widely among investigators. In anatomic dissection, Messerklinger 14 encountered the agger nasi cell in 10- 15% of specimens. Kantarci et al. 7, however, noted this cell in 47% of specimens, while Krzeski 11 reported its presence in 52.9% of cases and Van Alyea 38 in 89% of individuals. Kennedy and Zinreich noted the presence of the agger nasi cell in nearly all patients evaluated 16. Similarly, Bolger et al. reported that it was present in 98.5% of cases 12. In our study, agger nasi cells were detected in 24.3% of cases, and by multivariate analysis was associated with frontal sinusitis. The incidence rates reported in the literature, from 3% to 100% 11 12 16 18 40, may in part be related to the different method of analysis employed by Krzeski et al. 11.
Although the sphenoethmoid (Onodi) cell is an anatomic variant that is not associated with sinusitis, its presence poses an increased incidence of surgical complications for risk of injury to optic nerves or carotid arteries 1 30. In our work, these cells were present in 8.5% of patients. Nouraei et al. reported an incidence of 4.7% 1, while Stallman et al. reported an incidence of Onodi cells from 3.4-51% 3.
According to Keros' classification of the roof of the ethmoid, in our study there were 15.7% of patients in group I, 7.8% in group II and 1.4% in group III 41.
Conclusions
The results of this retrospective study highlight the statistically significant correlation between some anatomical variations of the lateral wall of the nose and pathologies of the paranasal sinus. This investigation also reinforces the fact that careful assessment and CT in patients with chronic rhinosinusitis is needed, especially in those undergoing endoscopic surgery, to identify the presence of anatomical variations in the paranasal sinus that may be correlated with rhinosinusitis. This also helps to identify and treat variations that may be associated with persistence or recurrence of disease. Finally, we believe that some anatomical variations of the paranasal sinus can play an important role in the pathogenesis of chronic rhinosinusitis, and thus may increase the risk of sinus mucosal disease.
References
- 1.Nouraei SAR, Elisay AR, DiMarco A. Variations in paranasal sinus anatomy: implications for the pathophysiology of chronic rhinosinusitis and safety of endoscopic sinus surgery. J Otolaryngology Head Neck Surg. 2009;38:32–37. [PubMed] [Google Scholar]
- 2.Selcuk A, Ozcan KM, Akdogan O, et al. Variations of maxillary sinus and accompanyng anatomical and pathological structures. J Craniofac Surg. 2008;19:159–164. doi: 10.1097/scs.0b013e3181577b01. [DOI] [PubMed] [Google Scholar]
- 3.Stallman JS, Lobo JN, Som PM. The incidence of concha bullosa and its relatioship to nasal septal deviations and paranasal sinus disease. Am J Neuroradiol. 2004;25:1613–1618. [PMC free article] [PubMed] [Google Scholar]
- 4.Ozcan KM, Selcuk A, Ozcan I, et al. Anatomical variations of nasal turbinates. J Craniofac Surg. 2008;19:1678–1682. doi: 10.1097/SCS.0b013e318188a29d. [DOI] [PubMed] [Google Scholar]
- 5.Ercan I, Cakir BO, Sayin I, et al. Relationship between the superior attachment type of uncinate process and presence of agger nasi cell: a computer-assisted anatomic study. Otolaryngol Head Neck Surg. 2006;134:1010–1014. doi: 10.1016/j.otohns.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 6.Zinreich SJ, Kennedy DW, Rosenbaum AE, et al. Paranasal sinuses: CT imaging requirements for endoscopic surgery. Radiology. 1987;163:769–775. doi: 10.1148/radiology.163.3.3575731. [DOI] [PubMed] [Google Scholar]
- 7.Kantarci M, Karasen RM, Alper F, et al. Remarkable anatomic variations in paranasal sinus region and their clinical importance. Eur J Radiol. 2004;50:296–302. doi: 10.1016/j.ejrad.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Danese M, Duvoisin B, Agrifoglio A, et al. Influence des variantes anatomiques nasosinusales sur les sinusites recidivantes, persistantes ou chroniques. J Radiol. 1997;78:651–657. [PubMed] [Google Scholar]
- 9.Chao TK. Uncommon anatomic variations in patients with chronic paranasal sinusitis. Otolaryngol Head Neck Surg. 2005;132:221–225. doi: 10.1016/j.otohns.2004.09.132. [DOI] [PubMed] [Google Scholar]
- 10.Nair S. Correlation between symptoms and radiological findings in patients of chronic rhinosinusitis: a modified radiological typing system. Rhinology. 2009;47:181–186. [PubMed] [Google Scholar]
- 11.Krzeski A, Tomaszewska E, Jakubczyk I, et al. Anatomic variations of the lateral nasal wall in the computed tomography scans of patients with chronic rhinosinusitis. Am J Rhinol. 2001;15:371–375. [PubMed] [Google Scholar]
- 12.Bolger WE, Butzin CA, Parsons DS. Paranasal sinus bony anatomic variations and mucosal abnormalities: CT analysis for endoscopic sinus surgery. Laryngoscope. 1991;101:56–64. doi: 10.1288/00005537-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Bayram M, Sirikci A, Bayazit YA. Important anatomic variations of the sinonasal anatomy in light of endoscopic surgery: a pictorial review. Eur Radiol. 2001;11:1991–1997. doi: 10.1007/s003300100858. [DOI] [PubMed] [Google Scholar]
- 14.Messerklinger W. On the drainage of the normal frontal sinus of man. Acta Otolaryngol (Stockh) 1967;63:176–181. doi: 10.3109/00016486709128748. [DOI] [PubMed] [Google Scholar]
- 15.Catalano GB, Conticello S, Galletti C, et al. Clinica Otorinolaringoiatrica. Bologna: Monduzzi Editore; 1993. [Google Scholar]
- 16.Kennedy DW, Zinreich SJ. The functional endoscopic approach to inflammatory sinus disease: current perpectives and technique modifications. Am J Rhinol. 1988;2:89–96. [Google Scholar]
- 17.Stammberger HR, Posawetz W. Functional endoscopic sinus surgery. Concept, indications and results of the Messerklinger technique. Arch Otorhinolaryngol. 1990;247:63–66. doi: 10.1007/BF00183169. [DOI] [PubMed] [Google Scholar]
- 18.Earwaker J. Anatomic variants in sinonasal CT. Radiographics. 1993;13:381–415. doi: 10.1148/radiographics.13.2.8460226. [DOI] [PubMed] [Google Scholar]
- 19.Sarna A, Hayman LA, Laina FJ, et al. Coronal imaging of the osteomeatal unit: anatomy of 24 variants. J Comput Assist Tomogr. 2002;26:153–157. doi: 10.1097/00004728-200201000-00027. [DOI] [PubMed] [Google Scholar]
- 20.und VJ, Kennedy DW, et al. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(part 2):S35–S40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Jung Cho M, Lee JW, et al. The relationship between anatomic variations of paranasal sinuses and chronic sinusitis in children. Acta Otolaryngol. 2006;126:1067–1072. doi: 10.1080/00016480600606681. [DOI] [PubMed] [Google Scholar]
- 22.Lerdlum S, Vachiranubhap B. Prevalence of anatomic variation demonstrated on screening sinus computed tomography and clinical correlation. J Med Assoc Thai. 2005;88(Suppl 4):110–115. [PubMed] [Google Scholar]
- 23.Hamdan AL, Bizri AR, Jaber M, et al. Nasoseptal variation in relation to sinusitis: a computerized tomographic evaluation. J Med Libian. 2001;49:2–5. [PubMed] [Google Scholar]
- 24.Scribano E, Ascenti G, Loria G, et al. The role of the ostiomeatal unit anatomic variations in inflamatory disease of the maxillary sinuses. Eur J Radiol. 1997;24:172–174. doi: 10.1016/s0720-048x(96)01073-x. [DOI] [PubMed] [Google Scholar]
- 25.Lam WW, Liang EY, Woo JK, et al. The etiological role of concha bullosa in chronica sinusitis. Eur Radiol. 1996;6:550–552. doi: 10.1007/BF00182491. [DOI] [PubMed] [Google Scholar]
- 26.Nadas S, Duvoisin B, Landry M, et al. Concha bullosa: frequency and appearance on CT and correlation with sinus disease in 308 patients with chronic sinusitis. Neuroradiology. 1995;37:234–237. doi: 10.1007/BF01578264. [DOI] [PubMed] [Google Scholar]
- 27.Calhoun KH, Waggenspack GA, Simpson CB, et al. CT evaluation of the paranasal sinuses in symptomatic and asymptomatic populations. Otolaryngol Head Neck Surg. 1991;104:480–483. doi: 10.1177/019459989110400409. [DOI] [PubMed] [Google Scholar]
- 28.Lam WW, Liang EY, Woo JK, et al. The etiological role of concha bullosa in chronic sinusitis. Eur Radiol. 1996;6:550–552. doi: 10.1007/BF00182491. [DOI] [PubMed] [Google Scholar]
- 29.Polavaram R, Devaiah AK, Sakai O, et al. Anatomic variants and pearls-funcional endoscopic sinus surgery. Otolaryngol Clin N Am. 2004;37:221–242. doi: 10.1016/S0030-6665(03)00161-0. [DOI] [PubMed] [Google Scholar]
- 30.Arslan H, Aydinlioglu A, Bozkurt M, et al. Anatomic variations of the paranasal sinuses: CT examination for endoscopic sinus surgery. Auris Nasus Larynx. 1999;26:39–48. doi: 10.1016/s0385-8146(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 31.Basak S, Karaman CZ, Akdilli A, et al. Evaluation of some important anatomical variations and dangerous areas of the paranasal sinuses by CT for safer endonasal surgery. Rhinology. 1998;36:162–167. [PubMed] [Google Scholar]
- 32.Zinreich SJ. Imaging of inflammatory sinus disease. Otolaryngol Clin North Am. 1993;26:535–547. [PubMed] [Google Scholar]
- 33.Bolger WE, Woodruff WW, Jr, Morehead J, et al. Maxillary sinus hypoplasia: classification and description of associated uncinate process hypoplasia. Otolaryngol Head Neck Surg. 1990;103:759–765. doi: 10.1177/019459989010300516. [DOI] [PubMed] [Google Scholar]
- 34.Betts NJ, Miloro M. Modification of the sinus lift procedure for septa in the maxillary antrum. J Oral Maxillofac Surg. 1994;52:332–333. doi: 10.1016/0278-2391(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 35.Jones NS, Strobl A, Holland A. A study of the CT findings in 100 patients with rhinosinusitis and 100 controls. Clin Otolaryngol. 1997;22:47–51. doi: 10.1046/j.1365-2273.1997.00862.x. [DOI] [PubMed] [Google Scholar]
- 36.Meloni F, Mini R, Rovasio S, et al. Anatomic variations of surgical importance in ethmoid labyrinth and sphenoid sinus. A study of radiological anatomy. Surg Radiol Anat. 1992;14:65–70. doi: 10.1007/BF01628046. [DOI] [PubMed] [Google Scholar]
- 37.Stackpole SA, Edelstein DR. The anatomic relevance of the Haller cell in sinusitis. Am J Rhinol. 1997;11:219–223. doi: 10.2500/105065897781751910. [DOI] [PubMed] [Google Scholar]
- 38.Alyea OE. Ethmoid labyrinth anatomic study, with consideration of the clinical significance of its structural characteristics. Arch Otolaryngol. 1939;29:881–901. [Google Scholar]
- 39.Tonai A, Baba S. Anatomic variations of the bone in sinusal CT. Acta Otolaryngol Suppl (Stockh) 1996;525:9–13. [PubMed] [Google Scholar]
- 40.Weinberger D, Anand V, Al-Rawi M, et al. Surgical anatomy and variations of the onodi cell. Am J Rhinol. 1996;10:365–370. [Google Scholar]
- 41.Keros P. Uber die praktische Bedeutung der Niveauunterschiede der Lamina cribrosa des Ethmoids. Laryngol Rhinol Otol (Stuttg) 1965;41:808–813. [Google Scholar]
- 42.Bent JP, Cuilty-Siller C, Kuhn FA, et al. The frontal cell as a cause of frontal sinus obstruction. Am J Rhinol. 1994;8:185–191. [Google Scholar]