Abstract
Background
Dyslipidemia causes coronary heart disease in middle-aged and elderly adults, but the consequences of lipid exposure during young adulthood are unclear.
Objective
To assess whether exposure to non-optimal lipids during young adulthood causes atherosclerotic changes that persist into middle age
Design
We estimated time-averaged cumulative exposure to lipids between ages 20-35 years using repeated serum lipid measures collected over 20 years by the Coronary Artery Risk Development in Young Adults (CARDIA) Study, and related this to coronary calcium measured later in life (45±4 years).
Setting
Four US cities
Participants
Black and white men and women recruited at age 18-30 in 1985-6
Measurements
Low- and high-density lipoprotein cholesterol (LDL and HDL) and triglycerides; coronary calcium
Results
Of 3258 participants, 2824 (87%) were exposed to non-optimal levels of LDL (≥100 mg/dl), HDL (<60 mg/dl) or triglyceride (≥150 mg/dl) during young adulthood. Coronary calcium prevalence two decades later was 8% in participants who maintained optimal LDL levels <70 mg/dl, and 44% in participants with LDL >160 mg/dl (p<.001). The association was similar across race and gender, and strongly graded, with odds ratios for coronary calcium of 1.5 (95% confidence interval 0.7-3.3) for LDL 70-99 mg/dl, 2.4 (1.1-5.3) for 100-129, 3.3 (1.3-7.8) for 130-159 and 5.6 (2.0-16) for ≥160 compared with LDL <70 mg/dl after adjustment for lipid exposure after age 35 and other coronary risk factors. After excluding lipid-lowering medication users and participants with clinically abnormal lipids, both LDL and HDL were independently associated with coronary calcium.
Limitations
Coronary calcium, although a strong predictor of future coronary heart disease, is not a clinical outcome.
Conclusions
Non-optimal LDL and HDL cholesterol at commonly observed levels during young adulthood are independently associated with coronary atherosclerosis two decades later.
INTRODUCTION
Abnormal blood lipids are a major cause of coronary heart disease (CHD) in middle-aged and older adults1. Associations with CHD risk are consistent across a wide range of cholesterol levels, in men and women, and in persons as young as 40 years of age2, and lowering cholesterol reduces CHD risk in these age groups3.
It is unclear, however, whether cholesterol levels are important earlier in life when short-term CHD risk is low. Long-term follow-up studies demonstrate associations between total cholesterol measured once during young adulthood and CHD events later in life4-6, but this association could be wholly attributable to later-life lipid abnormalities, which are strongly associated with lipid levels earlier in life7. Whether early-life lipid levels themselves cause atherosclerotic damage during young adulthood that persists into middle age is unknown.
The Coronary Artery Risk Development in Young Adults (CARDIA) Study provides a unique opportunity to evaluate the consequences of lipid abnormalities during young adulthood. Using CARDIA’s repeated measurements of fasting lipids starting at the outset of adulthood and continuing over 20 years of follow-up, we estimated cumulative exposure to lipid abnormalities between age 20 and 35 years and observed associations with coronary calcium measured after age 35 with adjustment for lipid levels later in life.
METHODS
Study Design and Sample
CARDIA is an institutional review board-approved longitudinal cohort of 5115 black and white women and men recruited from 4 U.S. cities, aged 18-30 years and healthy at the time of enrollment in 19858. Consenting participants underwent a baseline examination and follow-up examinations at Years 2, 5, 7, 10, 15, and 20. For this report, we assessed all 3258 CARDIA participants who received a cardiac computed tomography scan for coronary calcium after the age of 35 in either the Year 15 or 20 follow-up examinations and had complete CHD risk factor data at the time of the scan, excluding the 375 participants with missing risk factor measurements. Excluded participants were slightly older (45.2 vs. 44.6 years), no different in sex, race, income or education, no different in cumulative exposure to LDL, but had slightly lower time-averaged HDL (52 vs. 54 mg/dl) and higher time-averaged triglyceride (75 vs. 68 mg/dl) exposure between ages 20-35. Inclusion of these participants in unadjusted analyses (and adjusted analyses, where possible), had no qualitative effect on results.
Lipid Measurements
Fasting blood samples were drawn at each CARDIA examination, and measurements on plasma stored at −70°C were carried out at the Northwest Lipid Research Lab, University of Washington. Total cholesterol and triglycerides were measured enzymatically, high density lipoprotein (HDL) cholesterol was determined by precipitation with dextran sulfate-magnesium chloride, and low density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation. These methods and our extensive quality control procedures have been described9.
Estimating lipid trajectories and cumulative exposure
We used mixed models to estimate trajectories (age-dependent within-person average values) for LDL cholesterol, HDL cholesterol and triglycerides for each participant from age 20 up to the time of his or her coronary calcium measurement, using methods previously described for blood pressure in CARDIA10. Triglyceride measurements were normally distributed after log-transformation, so log-transformed values were used for all statistical purposes and back-transformed to triglyceride levels for presentation. We assumed that trajectories for each participant had a constant slope within each decade of life (age 20-29, 30-39, 40-49), so we allowed each participant a different random intercept and 3 random slopes, modeled as deviations from race- and gender-specific mean trajectories (see Online Data Supplement and a previous publication for details10).
We then calculated the area under the curve for each lipid trajectory for each participant. These measurements estimate the cumulative exposure to each lipid, and when divided by the number of years of exposure, provide time-averaged lipid levels for the given time period. Time-averaged exposures from age 20-35 for each lipid were the primary predictors in this analysis. Cumulative exposure after age 35 and the lipid measurements made at the time of the coronary calcium scan for each participant were used as covariates in the multivariable analysis in order to isolate the putative effects of lipid exposure during young adulthood. We also examined exposure before age 35 with finer stratification by age (20-25, 25-30, 30-35 years) to evaluate for interaction by recency of exposure.
Participants were categorized by their average exposure to each lipid from age 20-35, and also categorized as having “Normal”, “Borderline” or “Abnormal” lipid exposure from age 20-35 based on cutoffs derived from National Cholesterol Education Program guidelines1 (see Table footnotes for details). Use of cholesterol-lowering medications was self-reported; participants reporting use of a lipid-lowering medication at any exam (n=302, 9%) were included in the primary analysis, and then excluded in a secondary analysis.
Coronary calcium
Consenting CARDIA participants underwent cardiac computed tomography scanning at Year 15 and Year 20 to measure coronary calcium. We obtained two sequential scans using either an electron beam or multi-detector electrocardiographically-gated cardiac computed tomography scanner, with a standard phantom for calibration. Image analysts blinded to participant characteristics and paired scan results calculated a total coronary calcium score using a modified Agatston method11, with select overreading by an expert physician in cardiovascular imaging. Accuracy, comparability, and reproducibility of these methods has been published12, 13. We used the scan from Year 20, if available (n=2792); otherwise the scan from Year 15 (n=466).
Other measurements
We ascertained gender, ethnicity, date of birth, serum cotinine levels, and family history of premature CHD (defined by a mother or father with myocardial infarction before age 60) at baseline. We used measurements of blood pressure (systolic and diastolic) and smoking habits from all CARDIA examinations. Other covariates, including educational grade attained and income, fasting glucose, diabetes history and medication use, body mass index and waist circumference, alcohol use, and physical activity (self-reported on a scale of 1-5) were taken from the time of the coronary calcium scan9. We estimated total cumulative exposure to systolic and diastolic blood pressure using the same method as was used for lipids, as previously described10.
Statistical analysis
We described persons with normal, borderline, and abnormal lipid exposure during young adulthood, and compared characteristics using Spearman rank correlation (for continuous variables) and chi-square tests of trend (for dichotomous variables). We then described coronary calcium presence and extent by level of exposure to each lipid and by race/sex subgroups, using chi-squared tests of trend.
We used logistic regression to analyze adjusted associations between average lipid exposures and presence/absence of coronary calcium. We fit models for each lipid predictor, with full adjustment for potential confounders, flexibly modeled using linear splines with knots where suggested by Lowess plots (see Online Data Supplement for details). Fully adjusted models for LDL cholesterol, for example, included adjustment for current and cumulative exposure to HDL and triglycerides from age 20 on; for cumulative exposure to systolic and diastolic blood pressure from age 20 on, measured in mmHg-years, as described in our prior publication on this topic10, and for current levels as well; for current levels and cumulative exposure to LDL after age 35; for demographics (age, sex, race); and for socioeconomic status (income and education), body mass index and waist circumference, self-reported physical activity, alcohol use and other CHD risk factors including diabetes, pack-years of tobacco exposure, and family history of premature CHD obtained at the time of the coronary calcium scan, as well as serum cotinine at baseline. We also fit adjusted models after excluding participants with clinically abnormal lipids (LDL cholesterol ≥160 mg/dl, HDL cholesterol <40 mg/dl, or triglycerides ≥200 mg/dl) or self-reported use of a lipid-lowering medication at any CARDIA examination.
Role of the Funding Source
CARDIA is funded by the National Heart, Lung and Blood Institute, which had input into the overall design and conduct of the study and was represented on the Publications Committee that approved this manuscript.
RESULTS
Coronary calcium and CHD risk factor measurements were available for 3258 CARDIA participants. About half were black (47%), half were female (56%), and the average age was 45±4 years at the time of the coronary calcium scan. Coronary calcium was present in 17% of participants and the coronary calcium score was over 100 in 4%.
Only 13% of participants (n=434) maintained average lipid levels throughout young adulthood that would be considered normal or optimal per National Cholesterol Education Program (ATPIII) guidelines (LDL cholesterol < 100 mg/dl, HDL cholesterol > 60 mg/dl and triglycerides < 150 mg/dl); 12% (n=381) had at least one lipid that averaged in the abnormal range (LDL cholesterol ≥ 160 mg/dl, HDL cholesterol < 40 mg/dl, or triglycerides ≥ 200 mg/dl) during young adulthood; the remainder, representing a majority of the sample (n=2443, 75%), had borderline/non-optimal lipids. Abnormal lipid levels were associated with male sex, white race/ethnicity, higher income, family history of premature CHD, low self-reported activity levels and low levels of alcohol consumption, high BMI and waist circumference, diabetes, and higher blood pressure. Lipid levels during young adulthood were also strongly related to lipid levels measured at the time of the coronary calcium scan (Table 1).
Table 1. Characteristics of participants with normal, borderline, or clinically abnormal lipids during young adulthood.
| Characteristics at the time of the coronary calcium measurement* |
Average lipid levels from age 20-35† | P-value‡ | ||
|---|---|---|---|---|
| Normal N=434 |
Borderline N=2443 |
Abnormal N=381 |
||
| Age, years, mean +/− SD | 44.7 ± 3.9 | 44.6 ± 3.9 | 44.1 ± 4.1 | .04 |
| Sex, % male | 26% | 43% | 70% | <.001 |
| Race/ethnicity, % Black | 48% | 48% | 38% | .004 |
| Never went to college, % | 28% | 22% | 31% | .44 |
| Income < $25,000/year, % | 21% | 14% | 16% | .04 |
| Family history of premature coronary disease, % | 10% | 12% | 15% | .02 |
| Ever smoked, % | 51% | 42% | 51% | .80 |
| Baseline cotinine level, ng/ml | 67 ± 134 | 58 ± 121 | 94 ± 157 | .32 |
| Low self-reported physical activity§, % | 18% | 21% | 24% | .038 |
| Alcohol use, ml per day**, mean +/− SD | 16 +/− 25 | 11 +/− 22 | 8 +/− 22 | <.001 |
| Body mass index ≥ 30 kg/m2, % | 23% | 37% | 52% | <.001 |
| Waist circumference, cm, mean +/− SD | 83 +/− 13 | 91 +/− 14 | 100 +/− 14 | <.001 |
| Diabetes, % | 5% | 8% | 14% | <.001 |
| Blood pressure exposure, % | ||||
| - Normotensive throughout follow-up | 70% | 63% | 50% | <.001 |
| - Prehypertensive¶ after age 35 | 15% | 17% | 16% | |
| - Prehypertensive¶ before age 35 | 10% | 13% | 23% | |
| - Hypertensive (anytime) | 5% | 7% | 11% | |
| Current systolic blood pressure, mean +/− SD | 114 ± 16 | 116 ± 15 | 120 ± 16 | <.001 |
| Current LDL cholesterol, mg/dl, mean +/− SD | 86 ± 23 | 113 ± 29 | 125 ± 42 | <.001 |
| Current HDL cholesterol, mg/dl, mean +/− SD | 73 ± 17 | 53 ± 14 | 39 ± 11 | <.001 |
| Current triglycerides∥, mg/dl, mean +/− SD | 67 ± 1.6 | 89 ± 1.6 | 140 ± 1.6 | <.001 |
Characteristics measured at the time of the coronary calcium scan (Year 20, or Year 15 if a scan at Year 20 is not available).
Per NCEP guidelines, lipid levels defined as normal/borderline/abnormal are LDL cholesterol <100/100-159/≥160 mg/dl, HDL cholesterol ≥60/40-59/<40 mg/dl, or Triglycerides <150/150-199/≥200 mg/dl; participants categorized according to the most abnormal lipid.
Using chi-squared tests of trend (dichotomous) or spearman rank correlation (continuous)
Low self-reported physical activity is defined by an answer of 1 or 2 on a scale of 1-5.
Log-transformed values of triglycerides were used for all estimation procedures; log(triglyceride) values have been back-transformed to natural triglyceride levels for presentation purposes.
Prehypertension is defined as a systolic blood pressure trajectory over 120 mg/dl or a diastolic blood pressure trajectory over 80 mg/dl; hypertension is defined by a systolic blood pressure trajectory over 140 mg/dl or a diastolic blood pressure trajectory over 90 mg/dl. Trajectories estimated via mixed modeling10.
Usual weekly alcohol consumption was queried separately for beer, wine and spirits, and then converted to ml of alcohol consumption per day.
SD – Standard deviation; LDL – Low-density lipoprotein; HDL – High-density lipoprotein; NCEP – National Cholesterol Education Program
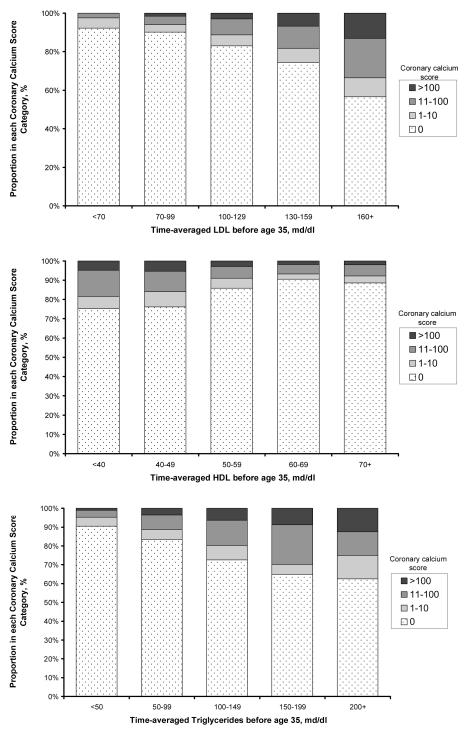
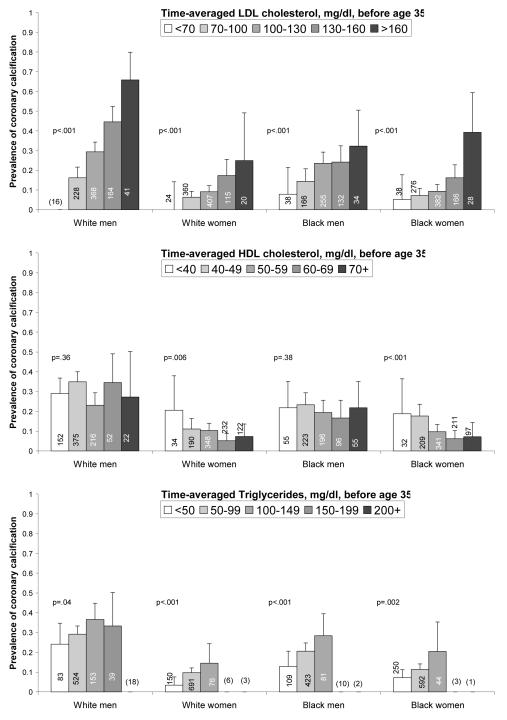
Presence and extent of coronary calcification were strongly associated with lipid exposure before age 35 (Figure 1). Nearly one half (44%) of persons with time-averaged LDL cholesterol levels over 160 mg/dl during young adulthood had coronary calcium compared with only 8% of persons with optimal levels less than 70 mg/dl. Extensive coronary calcification (a coronary calcium score > 100) was completely absent in the latter group (prevalence = 0%; 95% CI: 0-3%). Similar trends were present for HDL (inverse) and for triglycerides (Figure 1). These trends were generally consistent across the four race-sex subgroups (Figure 2).
Figure 1. Coronary calcium score distribution in middle age with increasing exposure to lipids before age 35 years.
Cumulative exposure to each lipid (LDL cholesterol, HDL cholesterol and triglycerides) is estimated via integrated lipid trajectory analysis (see Methods), and expressed as a time-averaged value. The association with coronary calcium score category is significant in all cases (p<.001). Ten persons with a positive coronary calcium scan but an unknown coronary calcium score were excluded from this analysis.
Figure 2. Prevalence of coronary calcium by lipid exposure before age 35 years, stratified by race and sex.
Cumulative exposure to each lipid (LDL cholesterol, HDL cholesterol and triglycerides) is estimated via integrated lipid trajectory analysis (see Methods), and expressed as a time-averaged value. Numbers in bars are subgroup sample sizes; no bar with fewer than 20 participants is shown (n is shown in parentheses for these). P-values represent trend for increasing odds of calcification by category.
After adjustment for lipid exposure after age 35 and other CHD risk factors, LDL cholesterol levels during young adulthood remained strongly associated with coronary calcium later in life (Table 2). Compared with optimal levels <70 mg/dl, the odds of coronary calcium in persons with abnormal LDL ≥160 mg/dl were more than five times higher (adjusted odds ratio (OR) 5.6; 95%CI: 2.0-16), and even participants with levels as low as 100-129 mg/dl had more than double the odds of coronary calcium (adjusted OR 2.4; 95%CI: 1.1-5.3). Associations for HDL cholesterol and triglyceride were weakened and not statistically significant in the adjusted analysis. We found no statistical evidence that the association of coronary calcium with LDL varied by age (20-35 vs. >35 years, interaction p=0.29) or within sub-intervals of age during young adulthood (20-25, 25-30, and 30-35 years, interaction trend p=0.17).
Table 2. Average exposure to lipids before age 35 and coronary calcium.
| Average exposure to lipids from age 20-35* |
N | Proportion with coronary calcium |
Coronary calcium odds ratio | ||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for current lipids and other risk factors† |
||||||
| OR (95%CI) | p-value‡ | OR (95%CI) | p-value‡ | %¶ | |||
| Overall | 3258 | 17% | -- | N/A | -- | N/A | 17% |
| Lipid exposure category§ | |||||||
| Normal | 434 | 7% | 1 (Reference) | <.001 | 1 (ref) | .031 | 7% |
| Borderline | 2443 | 17% | 2.6 (1.8-3.9) | 1.6 (1.0-2.5) | 11% | ||
| Abnormal | 381 | 30% | 5.7 (3.7-8.7) | 1.9 (1.1-3.3) | 13% | ||
| Time-averaged LDL, mg/dl | |||||||
| <70 | 116 | 8% | 1 (Reference) | <.001 | 1 (ref) | <.001 | 8% |
| 70-99 | 1030 | 10% | 1.3 (0.7-2.7) | 1.5 (0.7-3.3) | 12% | ||
| 100-129 | 1412 | 17% | 2.4 (1.2-4.9) | 2.4 (1.1-5.3) | 17% | ||
| 130-159 | 577 | 26% | 4.2 (2.1-8.6) | 3.3 (1.3-7.8) | 22% | ||
| ≥160 | 123 | 44% | 9.3 (4.3-20) | 5.6 (2.0-16) | 33% | ||
| Time-averaged HDL, mg/dl | |||||||
| <40 | 273 | 25% | 2.6 (1.7-4.1) | <.001 | 1.4 (0.6-3.0) | .25 | 24% |
| 40-49 | 997 | 24% | 2.5 (1.7-3.6) | 1.5 (0.8-2.9) | 24% | ||
| 50-59 | 1101 | 14% | 1.3 (0.9-1.9) | 1.1 (0.6-1.9) | 14% | ||
| 60-69 | 591 | 10% | 0.9 (0.5-1.3) | 1.0 (0.6-1.6) | 10% | ||
| ≥70 | 296 | 11% | 1 (Reference) | 1 (ref) | 11% | ||
| Time-averaged triglycerides∥, mg/dl |
|||||||
| <50 | 592 | 10% | 1 (Reference) | <.001 | 1 (ref) | .48 | 10% |
| 50-99 | 2230 | 17% | 1.9 (1.4-2.5) | 1.1 (0.8-1.6) | 11% | ||
| 100-149 | 354 | 28% | 3.6 (2.5-5.2) | 1.2 (0.7-2.0) | 12% | ||
| 150-199 | 58 | 36% | 5.3 (2.9-10) | 1.4 (0.6-3.1) | 13% | ||
| ≥200 | 24 | 38% | 5.6 (2.4-13) | 1.4 (0.5-4.3) | 13% | ||
Cumulative exposure to each lipid is estimated via integrated lipid trajectory analysis (see Methods), and expressed as a time-averaged value for each type of lipid.
Adjusted for current levels and cumulative exposure to all three lipids after age 35, and cumulative exposure from age 20-35 for the other lipids in the table, as well as for age, sex, race, socioeconomic status (income and education), body mass index, waist circumference, self-reported physical activity, systolic and diastolic blood pressure (current and cumulative exposure), tobacco exposure (cumulative pack-years and baseline cotinine level), alcohol use, diabetes, and family history of premature CHD.
P-values refer to a test of trend in the fully adjusted model.
Per NCEP guidelines, lipid levels defined as normal/borderline/abnormal are LDL cholesterol <100/100-159/≥160 mg/dl, HDL cholesterol ≥60/40-59/<40 mg/dl, or Triglycerides <150/150-199/≥200 mg/dl; participants categorized according to the most abnormal lipid.
Log-transformed values of triglycerides were used for all estimation procedures; log(triglyceride) values have been back-transformed to natural triglyceride levels for presentation purposes.
Represents adjusted prevalence %, assuming prevalence in the reference category is fixed. For example, if the reference prevalence is 10% (odds = .111), an odds ratio of 2 would represent a prevalence of 18% (odds = .222). The equivalent prevalence ratio can also be calculated from these adjusted prevalence estimates; in this example, the prevalence ratio would be 18%/10% = 1.8.
LDL – Low-density lipoprotein cholesterol; HDL – High-density lipoprotein cholesterol; NCEP – National Cholesterol Education Program
In the subset of participants without clinically abnormal lipids and who never reported use of a lipid-lowering medication, the association with LDL cholesterol remained strong (Table 3). Coronary calcium was nearly absent with optimal LDL cholesterol levels (prevalence 4%), and even modest elevations in LDL of 130-159 mg/dl were associated with a more than 4-fold higher prevalence of coronary calcium. In this subset, lower HDL cholesterol before age 35 was also associated with coronary calcium later in life, with odds of coronary calcium nearly three times higher when participants were exposed to time-averaged HDL cholesterol levels of 40-49 mg/dl compared with ≥70 mg/dl (adjusted OR 2.8; 95%CI: 1.1-6.8) after adjustment for LDL cholesterol, triglyceride, HDL cholesterol after age 35, and other CHD risk factors. After multivariate adjustment, there was no association between triglyceride levels and coronary calcium.
Table 3. Average exposure to lipids before age 35 and coronary calcium in persons without significant lipid abnormalities or medications for dyslipidemia.
| Average exposure to lipids from age 20-35* |
N† | Proportion with coronary calcium |
Coronary calcium odds ratio | ||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for current lipids and other risk factors† |
||||||
| OR (95%CI) | p-value‡ | OR (95%CI) | p-value‡ | %¶ | |||
| Overall | 1854 | 11% | -- | -- | N/A | 11% | |
| Lipid exposure category∥ | |||||||
| Normal | 421 | 7% | 1 (Reference) | .008 | 1 (ref) | .41 | 7% |
| Borderline | 1433 | 12% | 1.7 (1.2-2.6) | 1.2 (0.7-2.1) | 8% | ||
| Time-averaged LDL, mg/dl | |||||||
| <70 | 80 | 4% | 1 (Reference) | .003 | 1 (ref) | .04 | 4% |
| 70-99 | 723 | 8% | 2.2 (0.7-7.2) | 2.8 (0.8-10) | 10% | ||
| 100-129 | 838 | 13% | 3.8 (1.2-12) | 3.8 (1.0-15) | 14% | ||
| 130-159 | 213 | 17% | 5.2 (1.6-17) | 4.8 (1.1-22) | 17% | ||
| Time-averaged HDL, mg/dl | |||||||
| 40-49 | 232 | 19% | 2.5 (1.4-4.2) | <.001 | 2.8 (1.1-6.8) | .03 | 20% |
| 50-59 | 803 | 11% | 1.3 (0.8-2.1) | 1.7 (0.8-3.6) | 13% | ||
| 60-69 | 547 | 9% | 1.1 (0.7-1.9) | 1.5 (0.8-2.8) | 12% | ||
| ≥70 | 272 | 8% | 1 (Reference) | 1 (ref) | 8% | ||
| Time-averaged triglycerides**, mg/dl |
|||||||
| <50 | 494 | 8% | 1 (Reference) | .023 | 1 (ref) | .84 | 8% |
| 50-99 | 1287 | 12% | 1.5 (1.0-2.2) | 1.1 (0.7-1.7) | 9% | ||
| 100-149 | 73 | 16% | 2.2 (1.1-4.5) | 1.1 (0.4-2.9) | 9% | ||
| 150-199 | 0 | -- | -- | -- | |||
Cumulative exposure to each lipid is estimated via integrated lipid trajectory analysis (see Methods), and expressed as a time-averaged value for each type of lipid.
This analysis excludes persons with an LDL, HDL or triglyceride trajectory that ever exceeds “abnormal” levels∥ or who reported being on a lipid-lowering medication at a CARDIA examination (n=1417).
Adjusted for current levels and cumulative exposure to all three lipids after age 35, and cumulative exposure from age 20-35 for the other lipids in the table, as well as for age, sex, race, socioeconomic status (income and education), body mass index, waist circumference, self-reported physical activity, systolic and diastolic blood pressure (current and cumulative exposure), tobacco exposure (cumulative pack-years and baseline cotinine level), alcohol use, diabetes, and family history of premature CHD.
P-values refer to a test of trend in the fully adjusted model.
Per NCEP guidelines, lipid levels defined as normal/borderline/abnormal are LDL cholesterol <100/100-159/≥160 mg/dl, HDL cholesterol ≥60/40-59/<40 mg/dl, or Triglycerides <150/150-199/≥200 mg/dl; participants categorized according to the most abnormal lipid.
Represents adjusted prevalence %, assuming prevalence in the reference category is fixed. For example, if the reference prevalence is 10% (odds = .111), an odds ratio of 2 would represent a prevalence of 18% (odds = .222). The equivalent prevalence ratio can also be calculated from these adjusted prevalence estimates; in this example, the prevalence ratio would be 18%/10% = 1.8.
Log-transformed values of triglycerides were used for all estimation procedures; log(triglyceride) values have been back-transformed to natural triglyceride levels for presentation purposes.
LDL – Low-density lipoprotein cholesterol; HDL – High-density lipoprotein cholesterol; NCEP – National Cholesterol Education Program
DISCUSSION
Exposure to non-optimal levels of LDL cholesterol during young adulthood is a strong risk factor for coronary calcification later in life. LDL cholesterol levels during young adulthood are correlated with lipid levels later in life, but accounting for later-life lipid exposure did not explain the association of young adult LDL cholesterol levels with calcification. After removing the potentially obscuring influences of medication use and clinically abnormal levels of other lipids, the graded association between non-optimal LDL cholesterol and coronary calcium remained, and we also observed an association with HDL cholesterol (in the opposite direction), but not with triglyceride. Our results suggest that atherosclerotic changes begin during young adulthood as a result of non-optimal lipids, that these changes persist into middle age, and that maintaining optimal levels of lipids (particularly LDL cholesterol) throughout young adulthood could provide substantial benefits in terms of lifetime CHD prevention.
Associations between cholesterol and CHD events in middle-aged and elderly adults have been described in many studies since the 1950’s14, 15. Descriptions of the effects of lipid levels during young adulthood, which require long-term follow-up and/or measurement of subclinical disease, are less complete. Several long-term follow-up studies have demonstrated associations between total serum cholesterol measured once during young adulthood with CHD events later in life4-6, but lipid elevations later in life could explain these associations. Follow-up studies in middle-aged16 and elderly adults17 suggest that vascular damage from lipid abnormalities may persist for many years, but it is unclear if damage accumulates as early as young adulthood from borderline and modestly abnormal lipid levels typical of this age. A previous CARDIA analysis found that baseline lipids were stronger predictors of coronary calcium than current lipids, but no attempt was made to measure cumulative time-averaged exposure or to isolate exposure during a particular age range (e.g., young adulthood)18. Other studies among children and young adults show associations between lipid levels and atherosclerosis, demonstrated by autopsy19-22, carotid intima-media thickness23-28 and coronary calcium29, but no prior study has provided adequate sample size, repeated measurements of the three major lipids, and follow-up long enough to isolate the association of lipid levels during young adulthood with atherosclerosis during middle-age while controlling for confounding from other risk factors including lipid levels later in life. Our analysis is large enough (overall and for the four race-gender subgroups) and uses repeated measures of the three major lipids over a long enough period (two decades before measuring coronary calcium during middle age) to provide these answers.
Even the moderate lipid levels seen in the majority of young adults were associated with coronary calcium later in life, and the smoothly graded association with LDL cholesterol appeared to extend to very low levels. CARDIA participants maintaining optimal LDL cholesterol levels below 70 mg/dl during young adulthood without lipid-lowering medications had a very low prevalence of coronary calcium in middle age, and none had evidence of extensive atherosclerosis. These findings are consistent with cohort studies in middle-aged and older adults showing associations with CHD events down to a total cholesterol level of 135 mg/dl2 and randomized trials showing benefits from very aggressive lipid-lowering30 in both primary31 and secondary32-34 prevention studies. Moderate elevations in LDL cholesterol (and other lipids) are commonly ignored by both patients and physicians during young adulthood35.
Our findings – that LDL cholesterol, HDL cholesterol, and triglycerides all predicted coronary calcium in unadjusted analyses, but that only LDL and HDL cholesterol did so in adjusted analysis of participants without clinically abnormal lipids or lipid treatment – mirror many previous epidemiologic studies of older populations that have found a similar pattern of associations for lipid predictors of coronary heart disease (CHD)36, 37. The strong clinical trial evidence that LDL cholesterol-lowering agents reduce CHD incidence and mortality1 leaves little doubt about the causal basis of the association between LDL cholesterol and CHD. The evidence that low HDL cholesterol is causally related to CHD is less certain, though supported by epidemiologic and pathophysiologic evidence1, and by the Veteran’s Affairs HDL cholesterol Intervention Trial (HIT)38. The causal relationships supported by these lines of evidence suggest that some of the atherosclerotic changes occurring during young adulthood may be preventable with optimization of LDL and HDL levels.
Several limitations are worth noting. Despite standardized approaches to measuring lipids at seven examinations over 20 years, our findings are susceptible to measurement error and within-individual variation in lipid levels between examinations. Our mixed modeling approach should minimize these problems, but random error in the primary lipid predictor could result in underestimation of true associations with coronary calcium (regression dilution bias39). On the other hand, measurement error in covariates (including later life lipid exposure, self-reported factors like alcohol use, etc) or unmeasured factors could lead to residual confounding. We rely on a subclinical endpoint (coronary calcium) because our cohort is still too young to have suffered many myocardial infarctions or deaths from CHD; coronary calcium, however, is a strong, independent predictor of these CHD events40-42, and the complete absence of coronary calcium is a strongly protective factor43, 44.
Our findings may have implications for the prevention of CHD. Average LDL cholesterol levels in young adults (114 mg/dl for 20-29 year-olds and 126 mg/dl in 30-39 year-olds35) are well above what is considered optimal1. Our results suggest that non-optimal lipids, particularly LDL cholesterol, are not just a cause of short-term CHD event rates during middle age, as is well known, but cause atherosclerotic changes even during young adulthood that persist into middle-age, and probably contribute to higher CHD event rates through subsequent years of life. These findings reinforce the importance of a heart-healthy diet, exercise, and maintenance of normal weight beginning at least as early as young adulthood.
Whether to screen and/or treat adults for sub-optimal lipids before middle age is less clear1, 45, 46. The benefits of lipid-lowering do not appear to be any smaller at younger ages3, but previous trials have not tested effectiveness in adults younger than 40 years of age, and safety concerns rightly magnify when contemplating treatment starting early in life. Our observational study cannot provide evidence for effectiveness or safety of initiating pharmacological lipid-lowering during young adulthood, but does suggest that this could be an area for further investigation. Meanwhile, young adults and their physicians should realize that what they eat and how much they exercise matters even early in life when short-term CHD risk is extremely low, and that healthy behavior modifications should not be deferred until middle-age.
Supplementary Material
ACKNOWLEDGEMENTS
None.
FUNDING SOURCES
Work on this manuscript was partially supported by: University of Alabama at Birmingham, Coordinating Center, N01-HC-95095; University of Alabama at Birmingham, Field Center, N01-HC-48047; University of Minnesota, Field Center and Diet Reading Center (Year 20 Exam), N01-HC-48048; Northwestern University, Field Center, N01-HC-48049; Kaiser Foundation Research Institute, N01-HC-48050; University of California, Irvine, Echocardiography Reading Center (Year 5 & 10), N01-HC-45134; Harbor-UCLA Research Education Institute, Computed Tomography Reading Center (Year 15 Exam), N01-HC-05187; Wake Forest University (Year 20 Exam), N01-HC-45205; New England Medical Center (Year 20 Exam), N01-HC-45204 from the National Heart, Lung and Blood Institute.
Footnotes
DISCLOSURES
None
REPRODUCIBLE RESEARCH STATEMENTS
CARDIA Protocol/Methods: Available at http://www.cardia.dopm.uab.edu/
Statistical Code: Available to interested readers by contacting Dr. Pletcher (mpletcher@epi.ucsf.edu)
Data: A limited access dataset is available through NHLBI at http://www.nhlbi.nih.gov/resources/deca/descriptions/cardia.htm
REFERENCES
- 1.Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) National Cholesterol Education Program, National Heart, Lung, and Blood Institute. Available at: http://www.nhlbi.nih.gov/guidelines/cholesterol/ Accessed 2/28/05, 2005.
- 2.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists’ Collaborators Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality. 30 years of follow-up from the Framingham study. JAMA. 1987;257(16):2176–2180. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 5.Klag MJ, Ford DE, Mead LA, He J, Whelton PK, Liang KY, Levine DM. Serum cholesterol in young men and subsequent cardiovascular disease. N Engl J Med. 1993;328(5):313–318. doi: 10.1056/NEJM199302043280504. [DOI] [PubMed] [Google Scholar]
- 6.Stamler J, Daviglus ML, Garside DB, Dyer AR, Greenland P, Neaton JD. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284(3):311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 7.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Usefulness of childhood low-density lipoprotein cholesterol level in predicting adult dyslipidemia and other cardiovascular risks. The Bogalusa Heart Study. Arch Intern Med. 1996;156(12):1315–1320. [PubMed] [Google Scholar]
- 8.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr., Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Jacobs DR, Liu K, Williams OD, Hilner JE, Perkins LL, Marcovina SM, Hulley SB. Seven-year trends in plasma low-density-lipoprotein-cholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6(3):235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 10.Pletcher MJ, Bibbins-Domingo K, Lewis CE, Wei GS, Sidney S, Carr JJ, Vittinghoff E, McCulloch CE, Hulley SB. Prehypertension during young adulthood and coronary calcium later in life. Ann Intern Med. 2008;149(2):91–99. doi: 10.7326/0003-4819-149-2-200807150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 12.Carr JJ, Crouse JR, 3rd, Goff DC, Jr., D’Agostino RB, Jr., Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol. 2000;174(4):915–921. doi: 10.2214/ajr.174.4.1740915. [DOI] [PubMed] [Google Scholar]
- 13.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility--MESA study. Radiology. 2005;236(2):477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 14.Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47(4 Pt 2):4–24. doi: 10.2105/ajph.47.4_pt_2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J., 3rd Factors of risk in the development of coronary heart disease--six year follow-up experience. The Framingham Study. Ann Intern Med. 1961;55:33–50. doi: 10.7326/0003-4819-55-1-33. [DOI] [PubMed] [Google Scholar]
- 16.Nieto FJ, Diez-Roux A, Szklo M, Comstock GW, Sharrett AR. Short- and long-term prediction of clinical and subclinical atherosclerosis by traditional risk factors. J Clin Epidemiol. 1999;52(6):559–567. doi: 10.1016/s0895-4356(99)00030-x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, Hoeg JM, D’Agostino RB, Silbershatz H, Belanger AM, Poehlmann H, O’Leary D, Wolf PA. Cumulative effects of high cholesterol levels, high blood pressure, and cigarette smoking on carotid stenosis. N Engl J Med. 1997;337(8):516–522. doi: 10.1056/NEJM199708213370802. [DOI] [PubMed] [Google Scholar]
- 18.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, Williams OD, Bild DE, Detrano R. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49(20):2013–2020. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Newman WP, 3rd, Freedman DS, Voors AW, Gard PD, Srinivasan SR, Cresanta JL, Williamson GD, Webber LS, Berenson GS. Relation of serum lipoprotein levels and systolic blood pressure to early atherosclerosis. The Bogalusa Heart Study. N Engl J Med. 1986;314(3):138–144. doi: 10.1056/NEJM198601163140302. [DOI] [PubMed] [Google Scholar]
- 20.A preliminary report from the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group Relationship of atherosclerosis in young men to serum lipoprotein cholesterol concentrations and smoking. JAMA. 1990;264(23):3018–3024. doi: 10.1001/jama.1990.03450230054029. [DOI] [PubMed] [Google Scholar]
- 21.McGill HC, Jr., McMahan CA, Malcom GT, Oalmann MC, Strong JP, The PDAY Research Group. Pathobiological Determinants of Atherosclerosis in Youth Effects of serum lipoproteins and smoking on atherosclerosis in young men and women. Arterioscler Thromb Vasc Biol. 1997;17(1):95–106. doi: 10.1161/01.atv.17.1.95. [DOI] [PubMed] [Google Scholar]
- 22.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 23.Tonstad S, Joakimsen O, Stensland-Bugge E, Leren TP, Ose L, Russell D, Bonaa KH. Risk factors related to carotid intima-media thickness and plaque in children with familial hypercholesterolemia and control subjects. Arterioscler Thromb Vasc Biol. 1996;16(8):984–991. doi: 10.1161/01.atv.16.8.984. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez A, Barth JD, Zhang L. The carotid artery wall thickness in teenagers is related to their diet and the typical risk factors of heart disease among adults. Atherosclerosis. 2000;152(1):265–266. doi: 10.1016/s0021-9150(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 25.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: The Muscatine Study. Circulation. 2001;104(23):2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 26.Knoflach M, Kiechl S, Kind M, Said M, Sief R, Gisinger M, van der Zee R, Gaston H, Jarosch E, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young males: ARMY study (Atherosclerosis Risk-Factors in Male Youngsters) Circulation. 2003;108(9):1064–1069. doi: 10.1161/01.CIR.0000085996.95532.FF. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 28.Raitakari OT, Juonala M, Kahonen M, Taittonen L, Laitinen T, Maki-Torkko N, Jarvisalo MJ, Uhari M, Jokinen E, Ronnemaa T, Akerblom HK, Viikari JS. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290(17):2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 29.Mahoney LT, Burns TL, Stanford W, Thompson BH, Witt JD, Rost CA, Lauer RM. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcificaction in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27(2):277–284. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr., Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr., Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 32.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 33.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 34.Chhatriwalla AK, Nicholls SJ, Wang TH, Wolski K, Sipahi I, Crowe T, Schoenhagen P, Kapadia S, Tuzcu EM, Nissen SE. Low levels of low-density lipoprotein cholesterol and blood pressure and progression of coronary atherosclerosis. J Am Coll Cardiol. 2009;53(13):1110–1115. doi: 10.1016/j.jacc.2008.09.065. [DOI] [PubMed] [Google Scholar]
- 35.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, Grundy SM, Johnson CL. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA. 2005;294(14):1773–1781. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 36.Hulley SB, Avins AL. Asymptomatic hypertriglyceridaemia. BMJ. 1992;304(6824):394–396. doi: 10.1136/bmj.304.6824.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hulley SB, Rosenman RH, Bawol RD, Brand RJ. Epidemiology as a guide to clinical decisions. The association between triglyceride and coronary heart disease. N Engl J Med. 1980;302(25):1383–1389. doi: 10.1056/NEJM198006193022503. [DOI] [PubMed] [Google Scholar]
- 38.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J, Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 39.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335(8692):765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 40.Pletcher MJ, Tice JA, Pignone M, Browner WS. Using the coronary artery calcium score to predict coronary heart disease events: a systematic review and meta-analysis. Arch Intern Med. 2004;164(12):1285–1292. doi: 10.1001/archinte.164.12.1285. [DOI] [PubMed] [Google Scholar]
- 41.Greenland P, Bonow RO, Brundage BH, Budoff MJ, Eisenberg MJ, Grundy SM, Lauer MS, Post WS, Raggi P, Redberg RF, Rodgers GP, Shaw LJ, Taylor AJ, Weintraub WS, Harrington RA, Abrams J, Anderson JL, Bates ER, Grines CL, Hlatky MA, Lichtenberg RC, Lindner JR, Pohost GM, Schofield RS, Shubrooks SJ, Jr., Stein JH, Tracy CM, Vogel RA, Wesley DJ. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Circulation. 2007;115(3):402–426. doi: 10.1161/CIRCULATIONAHA..107.181425. [DOI] [PubMed] [Google Scholar]
- 42.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 43.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Sarwar A, Shaw LJ, Shapiro MD, Blankstein R, Hoffman U, Cury RC, Abbara S, Brady TJ, Budoff MJ, Blumenthal RS, Nasir K. Diagnostic and prognostic value of absence of coronary artery calcification. JACC Cardiovasc Imaging. 2009;2(6):675–688. doi: 10.1016/j.jcmg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- 46.US Preventive Services Task Force [Accessed April 20, 2010];Screening for Lipid Disorders in Adults. Agency for Healthcare Research and Quality. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipidrs.htm. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.