SUMMARY
Bacteria sense their environment using receptors of the histidine sensor kinase family, but how kinase activity is regulated by ligand binding is not well understood. Autoinducer-2 (AI-2), a secreted signaling molecule originally identified in studies of the marine bacterium Vibrio harveyi, regulates quorum-sensing responses and allows communication between different bacterial species. AI-2 signal transduction in V. harveyi requires the integral membrane receptor LuxPQ, comprised of periplasmic binding protein (LuxP) and histidine sensor kinase (LuxQ) subunits. Combined X-ray crystallographic and functional studies show that AI-2 binding causes a major conformational change within LuxP, which in turn stabilizes a quaternary arrangement in which two LuxPQ monomers are asymmetrically associated. We propose that formation of this asymmetric quaternary structure is responsible for repressing the kinase activity of both LuxQ subunits and triggering the transition of V. harveyi into quorum-sensing mode.
INTRODUCTION
Quorum sensing is a bacterial cell-cell communication process driven by secreted signaling molecules called autoinducers (Waters and Bassler, 2005). At low cell density, in the absence of appreciable amounts of autoinducers, bacteria act as individuals. At high cell density, bacteria respond to the accumulation of autoinducers by synchronizing the gene expression of the community. Thus, quorum sensing allows groups of bacteria to act in unison. Typically, acylated homoserine lactones are used by Gram-negative bacteria, and modified oligopeptides are used by Gram-positive bacteria, as autoinducers. A quorum-sensing signal called autoinducer-2 (AI-2) is unusual both in its chemical structure (a furanosyl borate diester), and in that it is produced and detected by many different species of Gram-negative and Gram-positive bacteria, allowing interspecies cell-cell communication (Bassler et al., 1993; Chen et al., 2002; Miller et al., 2004; Xavier and Bassler, 2003, 2005).
AI-2 signaling has been most extensively studied in Vibrio harveyi (Pappas et al., 2004). V. harveyi is a bioluminescent marine bacterium that controls light production, as well as other behaviors, through quorum sensing (Bassler et al., 1993; Henke and Bassler, 2004a). The V. harveyi AI- 2 receptor is composed of two polypeptides, LuxP and LuxQ (Bassler et al., 1994). LuxP is a periplasmic binding protein that binds AI-2 by clamping it between two domains (Chen et al., 2002), whereas LuxQ is an integral membrane protein of the two-component sensor kinase family (Bassler et al., 1994). The periplasmic domain of LuxQ associates with LuxP (Neiditch et al., 2005). The cytoplasmic region of LuxQ includes both a histidine kinase domain and a receiver domain. Although not previously demonstrated, it is likely that LuxQ, similar to other two-component sensor kinases, is constitutively dimeric (Stock et al., 2000).
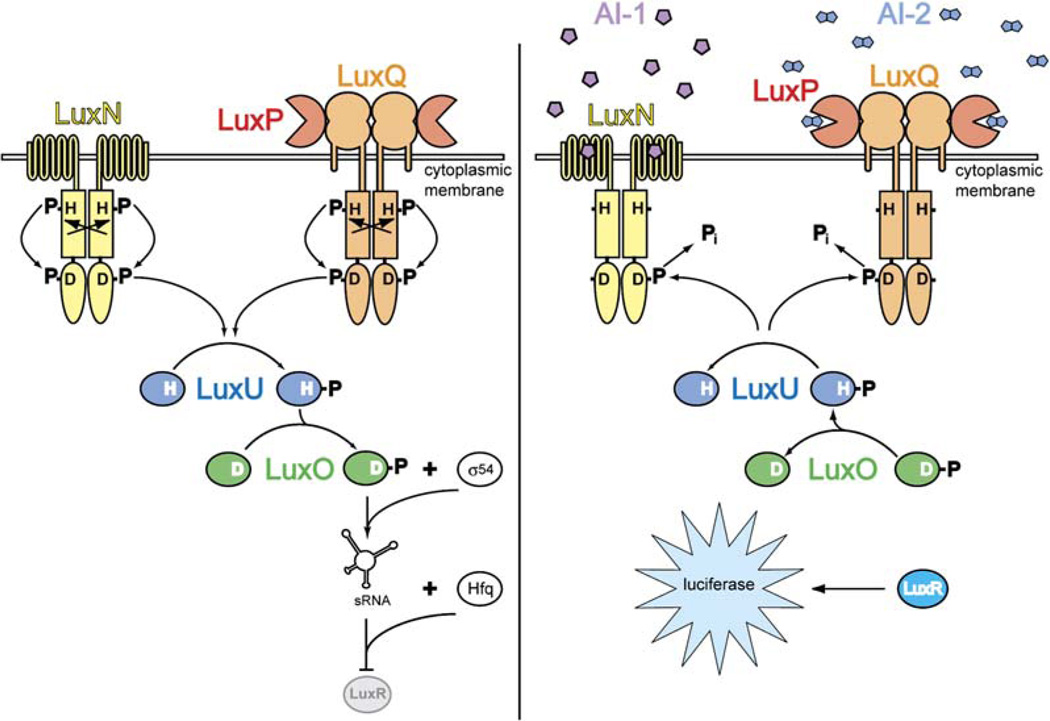
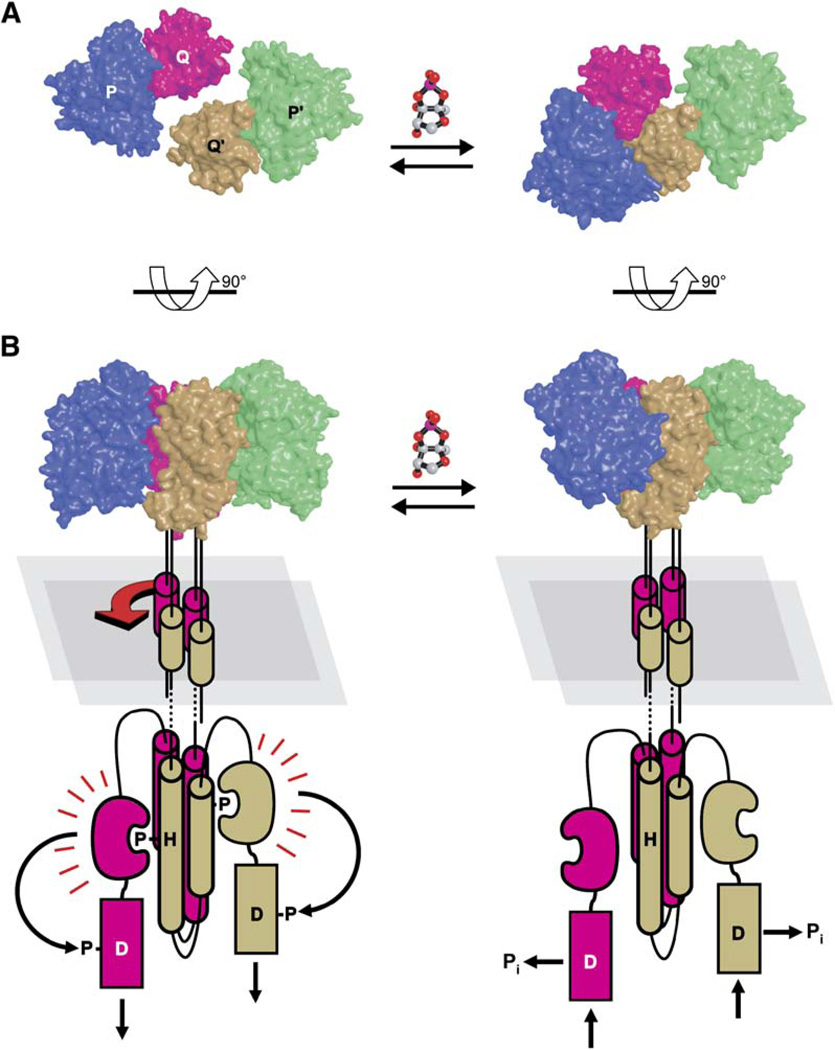
At low cell density, when AI-2 concentrations are low, LuxPQ acts as a kinase, autophosphorylating a conserved histidine that lies within the LuxQ histidine kinase domain (Figure 1, left). This phosphoryl group is shuttled sequentially from the histidine to an aspartic acid residue in the LuxQ receiver domain, then to a histidine in the phosphotransferase protein LuxU, and finally to an aspartic acid in the transcriptional regulator LuxO (Freeman and Bassler, 1999a, 1999b). LuxO phosphate represses quorum-sensing responses by repressing production of the transcription factor LuxR (Lenz et al., 2004). By contrast, when cell population density— and hence AI-2 concentration— is high, the binding of AI-2 to the LuxPQ receptor appears to switch it from a kinase to a phosphatase (Figure 1, right). As a consequence, the concentration of LuxO-phosphate declines and quorum-sensing responses are derepressed. Autoinducer-1 (AI-1), an acyl homoserine lactone that serves as a species-specific quorum-sensing signal, also regulates the levels of LuxO phosphate, but through a distinct two-component sensor kinase, LuxN (Bassler et al., 1993; Cao and Meighen, 1989; Freeman et al., 2000).
Figure 1. The Vibrio harveyi Quorum-Sensing Signal Transduction Circuit.
Under conditions of low cell density (i.e., in the absence of appreciable concentrations of autoinducers; left), LuxN and LuxPQ act as kinases that catalyze histidine (H) phosphorylation, presumably across dimer pairs (arrows). Phosphate is subsequently transferred to a conserved aspartate (D) in the receiver domains of LuxN or LuxQ and then to histidine and aspartate residues on LuxU and LuxO, respectively. LuxO-phosphate, in conjunction with the sigma factor σ54, promotes transcription of genes encoding small regulatory RNAs (sRNAs). The sRNAs, together with the chaperone Hfq, destabilize the mRNA encoding the transcription factor LuxR. Under high cell density conditions (i.e., in the presence of high concentrations of autoinducers; right), LuxN and LuxPQ bind their respective autoinducer ligands (AI-1: pentagons; AI-2: double pentagons) and are converted from kinases to phosphatases. Phosphate is stripped from LuxO and LuxU and is hydrolyzed to inorganic phosphate (Pi). Because dephosphorylated LuxO is inactive, LuxR is produced and activates the expression of the luciferase operon. As a result, the bacteria produce light.
We previously determined the structures of LuxP bound to AI-2 and of a complex containing LuxP and the periplasmic domain of LuxQ (LuxPQp) without bound AI-2 (Chen et al., 2002; Neiditch et al., 2005). Here, we present crystal structures of the isolated periplasmic domain of LuxQ (LuxQp) and of LuxPQp bound to AI-2. These structures reveal ligand-induced conformational changes resulting in strikingly asymmetric (LuxPQp)2 dimers. To examine the functional significance of these structural observations, we performed extensive in vivo genetic analyses. Our results are consistent with a model in which alteration in the quaternary arrangement of LuxPQ periplasmic domains plays a pivotal role in determining the level of cytoplasmic kinase activity. These findings also provide a mechanism for coupling ligand binding by a periplasmic binding protein to two-component signal transduction across the bacterial membrane.
RESULTS
LuxQ Kinase Activity Requires Dimerization
Genetic, biochemical, and structural evidence from many two-component sensor kinases suggests that the members of this family function in vivo as dimers (Khorchid and Ikura, 2006; Stock et al., 2000). Although we have not been able to isolate full-length LuxQ protein, the histidine kinase dimerization domain itself is indeed dimeric, as judged by equilibrium analytical ultracentrifugation (R.C.K., B. Kokona, R. Fairman, and F.M.H., unpublished data). To determine whether LuxQ functions as a dimer in vivo, we attempted to “poison” wild-type LuxQ by introducing inactive LuxQ mutants, predicting a dominant negative effect if LuxQ, like other sensor kinases, functions by trans-phosphorylation within homodimers (Heermann et al., 1998; Malpica et al., 2004; Ninfa et al., 1993; Surette et al., 1996; Wang et al., 2001; Yang and Inouye, 1991).
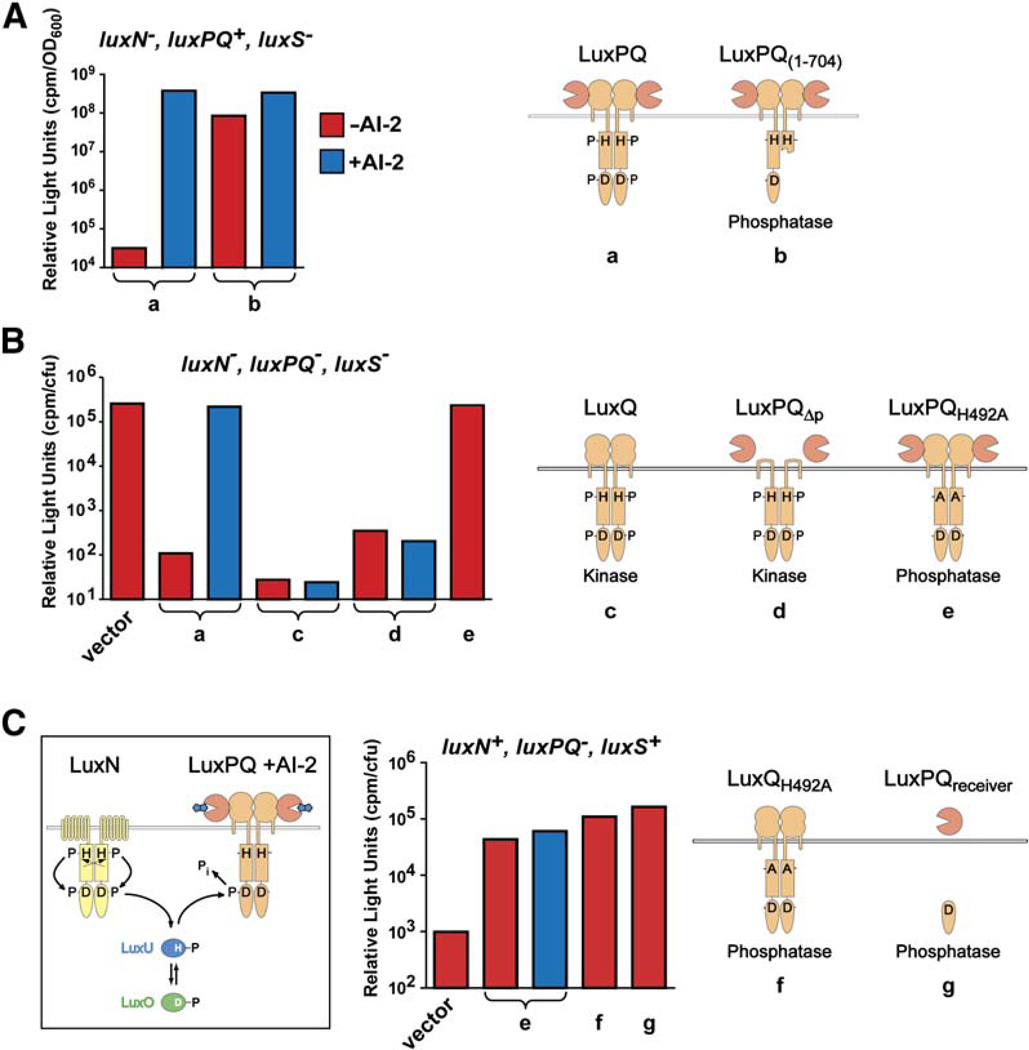
The canonical quorum-sensing readout in V. harveyi— bioluminescence— was used to evaluate LuxQ kinase activity. To achieve precise control over AI-2 concentration, we used a strain lacking the AI-2 synthase, LuxS. AI-2 was generated in situ by adding boric acid and synthetically prepared 4,5-dihydroxy-2,3-pentane dione (DPD), which combine to form V. harveyi AI-2 (Miller et al., 2004; Semmelhack et al., 2005). To avoid the confounding effects of AI-1 signaling, we deleted the gene encoding the AI-1 receptor LuxN (see Figure 1). The control experiment (Figure 2A[a], left bar) shows that the luxN−, luxPQ+, luxS− strain is dark in the absence of AI-2 because LuxQ functions as a kinase, repressing bioluminescence. In the presence of AI-2, the strain is bright because LuxQ kinase activity is inhibited, derepressing bioluminescence (Figure 2A[a], right bar). To assay dimerization, we introduced a truncated LuxQ (residues 1–704) lacking an intact histidine kinase catalytic domain as well as the receiver domain. LuxQ1–704 was dominant negative for kinase activity, conferring a bright (kinase-off) phenotype in the presence and absence of AI-2 (Figure 2A[b]). Thus, the kinase activity of wild-type LuxQ is poisoned by coexpression of truncated LuxQ, and we conclude that kinase activity requires dimers (or conceivably higher-order oligomers) of wild-type LuxQ. We observed a slight residual AI-2 responsiveness in Figure 2A[b], which we attribute to a small proportion of wild-type LuxQ dimers. We note that LuxN kinase activity is dominant over LuxQ1–704. Thus, the effect of LuxQ1–704 on bioluminescence is due to poisoned LuxQ dimers and not to nonspecific interactions with LuxN or other signaling components.
Figure 2. Dimerization and In Vivo Activities of LuxPQ.
Bioluminescence activity was measured in various genetic backgrounds (indicated above each graph); cartoons depict the LuxPQ proteins expressed in trans. The V. harveyi strains used were the following: (A) JMH610 (luxN−, luxPQ+, luxS−), (B) FED119 (luxN−, luxPQ−, luxS−), and (C) BB886 (luxN+, luxPQ−, luxS+). The luxPQ plasmids examined carried the following: (a) wild-type LuxPQ; (b) LuxPQ1–704; (c) LuxQ alone, generated by mutating luxP such that secretion into the periplasm was abolished (LuxPss; see Experimental Procedures); (d) LuxPQΔp (wild-type LuxP, together with LuxQ lacking its periplasmic domain, residues 45–265); (e) LuxPQH492A; (f) LuxQH492A (with an in-frame deletion of luxP); and (g) LuxPQreceiver. V. harveyi AI-2 was generated in situ in the specified cultures (blue bars) by adding 1 µM synthetic DPD in the presence of boric acid. Further details are given in the Experimental Procedures.
Regulation of Kinase Activity by the LuxPQ Periplasmic Domain
LuxQ functions in conjunction with the periplasmic AI-2 binding protein LuxP. Previously, we showed that LuxP and LuxQ are constitutively associated (Neiditch et al., 2005). Here, we demonstrate that the entire periplasmic domain of the LuxPQ receptor is dispensible for kinase activity. For these experiments, we engineered a V. harveyi strain lacking the AI-1 receptor (LuxN), the AI-2 receptor (LuxPQ), and the AI-2 synthase (LuxS). This strain (FED119), because it lacks both LuxN and LuxQ kinases, is bright (Figure 2B, vector). Introduction of wild-type LuxPQ on a plasmid restores AI-2 responsiveness (Figure 2B[a]). Introduction of LuxQ only, without LuxP, produces a dark (kinase-on) phenotype (Figure 2B[c]), confirming the earlier finding that LuxQ is intrinsically a kinase (Neiditch et al., 2005). Introduction of LuxP in combination with LuxQΔp, a LuxQ deletion lacking the entire periplasmic domain, also generates a dark (kinase-on) phenotype (Figure 2B[d]). Thus, removing either LuxP or the periplasmic domain of LuxQ results in a constitutively active LuxQ kinase, demonstrating that no part of the periplasmic region is needed for kinase activity. Taken together, these results strongly imply that the function of the periplasmic region of LuxPQ is to shut off kinase activity in response to AI-2.
We also tested whether LuxQ displays phosphatase activity and, if so, whether this activity is regulated by AI-2. To facilitate examination of LuxQ phosphatase activity, we eliminated its kinase function by changing His-492, the presumed target of the kinase reaction, to alanine. As expected, cells bearing LuxQH492A were bright (kinase-off; Figure 2B[e]). This construct enabled us to test whether LuxQH492A possesses phosphatase activity because we could exploit the unusual circuitry of the AI-1/AI-2 pathways, driving phosphoryl groups in with LuxN and testing whether various LuxQ constructs could pull them out (Figure 2C, left panel). Introduction of LuxQH492A into a luxN+, luxPQ−, luxS+ strain caused an approximately 40-fold increase in light production (Figure 2C, compare vector and [e]), demonstrating that LuxQH492A is capable of acting as a phosphatase and counteracting the AI-1-pathway-driven accumulation of LuxO phosphate. The LuxQH492A phosphatase activity is insensitive to the presence or absence of AI-2 or LuxP (Figure 2[e and f]). Therefore, the phosphatase activity of LuxQ does not appear to be regulated by AI-2. This phosphatase activity resides within the receiver domain of LuxQ since expression of the receiver domain alone (Figure 2C[g] had essentially the same effect as expression of full-length LuxQH492A. Overall, these findings reinforce the conclusion that the periplasmic domain of LuxPQ functions to bind AI-2 and shut off kinase activity.
Our results indicate that the functional unit for LuxPQ is the (LuxPQ)2 dimer. The periplasmic domains cannot be essential for dimerization, however, because they are dispensible for kinase activity, which requires dimerization (Figure 2B). Indeed, no evidence of an interaction between LuxPQ periplasmic domains has been reported. To investigate further, we performed dynamic light-scattering experiments on the purified periplasmic domain of LuxPQ (LuxPQp) in the presence and absence of AI-2. To our surprise, these measurements suggested that AI-2 binding promotes dimerization of the periplasmic domain (i.e., formation of [LuxPQp]2). The hydrodynamic radius (RH), a measure of molecular size and shape, was 3.4 ± 0.1 nm in the absence of AI-2 but increased to 3.8 ± 0.1 nm in the presence of AI-2. While the RH of unliganded LuxPQp is in excellent agreement with calculations based on the crystal structure (Neiditch et al., 2005), the RH after AI-2 addition is significantly larger. The measured value (3.8 nm) is intermediate between those predicted for LuxPQp monomers (3.4 nm) and (LuxPQp)2 dimers (~4.3 nm) and probably reflects an equilibrium mixture of monomers and dimers. After deleting five residues from the N terminus of LuxP, a modification shown to stabilize the AI-2 bound state in vivo (Neiditch et al., 2005), liganded LuxPQp displayed a hydrodynamic radius of 4.0 ± 0.1 nm. These experiments, while preliminary, provided the first indication that AI-2 binding promotes interaction between the periplasmic regions of a (LuxPQ)2 dimer. We note that the interaction between AI-2 bound periplasmic domains could be much stronger in vivo if, as seems likely, the domains were held in proximity and oriented relative to one another by dimeric interactions occurring elsewhere in LuxQ.
Structures of LuxQp and LuxPQp Bound to AI-2
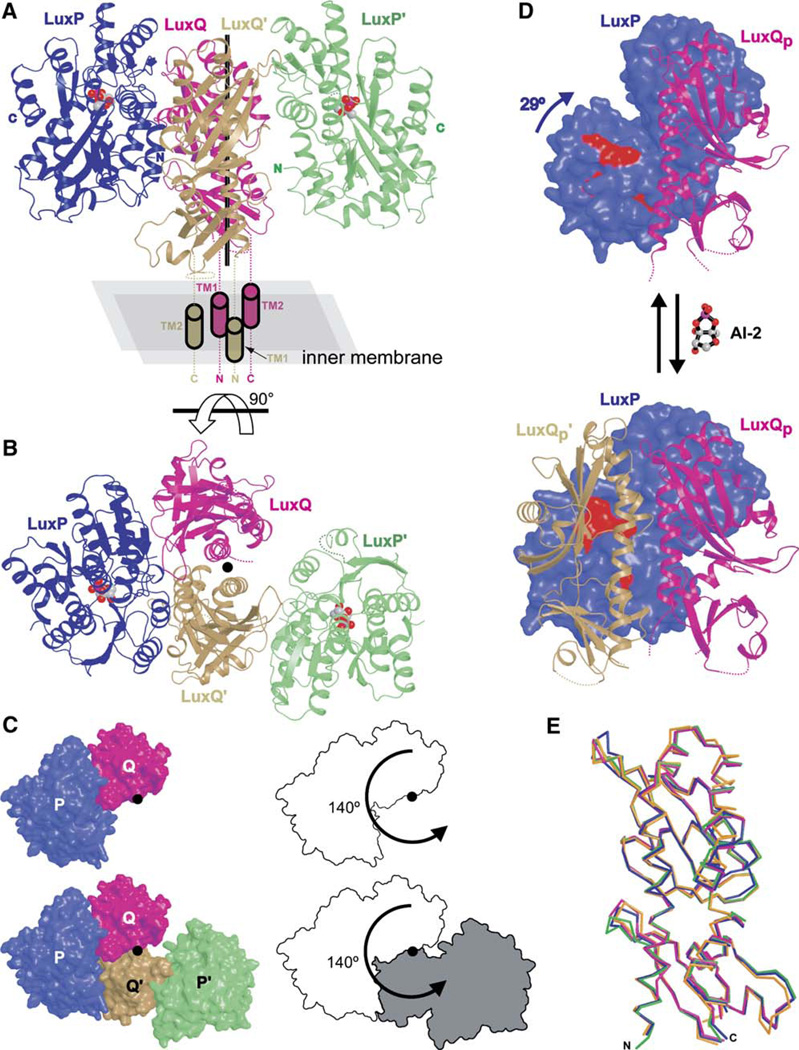
If indeed the periplasmic domains of LuxPQ dimerize upon AI-2 binding, it is of interest both to determine the structure of these dimers and to evaluate their physiological significance. As a first step, we crystallized AI-2 bound LuxPQp, after first deleting five residues from the N terminus of LuxP (see above). Crystals were improved by deleting, from the termini of LuxQp, those residues that were poorly ordered in the previously determined AI-2-free LuxPQp crystal structure (Neiditch et al., 2005). The 2.3Å resolution structure (Figures 3A–3C) was determined using phases obtained by molecular replacement (see Experimental Procedures, as well as Table S1 in the Supplemental Data available with this article online). The structure indeed contains two similar LuxPQp complexes, as discussed below. We also crystallized LuxQp alone and determined its structure to 1.7 Å resolution (Table S1).
Figure 3. Crystal Structure of AI-2 Bound LuxPQp.
(A) Two LuxPQp monomers are observed in the crystal structure of AI-2 bound LuxPQp. One monomer is colored blue (LuxP) and magenta (LuxQ), while the other monomer is colored green (LuxP′) and tan (LuxQp′). AI-2 (space-filling models) is observed bound to both LuxP and LuxP′. The two LuxPQp monomers can be approximately superimposed by rotating one complex 140° around an axis normal to the inner membrane (vertical black line). Cylinders denote the inferred positions of LuxQp and LuxQp′ transmembrane helices. Dashed lines denote other regions that are missing from the crystallized receptor or are apparently disordered in the crystal structure (see Experimental Procedures for details).
(B) AI-2 bound LuxPQp viewed from “above” looking toward the bacterial inner membrane down the rotation axis (filled circle). To obtain this view, the structure illustrated in panel (A) was rotated 90° in the direction indicated by the arrow. In this and all succeeding figures, the four polypeptides are colored as in panel (A).
(C) Surface representations of the AI-2 bound LuxPQp receptor (left panels), viewed in the same orientation as in panel (B). A corresponding outline representation, emphasizing the relationship between the two LuxPQp receptors, is also pictured (right panels). The top panels show LuxPQp only, whereas the bottom panels include LuxPQp and LuxP′Qp′.
(D) AI-2 binding to LuxPQp induces a conformational change in LuxP (blue; compare open LuxP in top panel to closed LuxP in bottom panel). This conformational change creates space for binding of LuxQp′ (tan). LuxP residues Thr138, Arg139, Phe143, and Trp167 are indicated in red (see also Figure 4A). For clarity, LuxP′ (green in Figures 2A–C) is omitted. The AI-2-free LuxPQp structure was determined previously (Neiditch et al., 2005).
Comparing AI-2-free and AI-2 bound structures of the LuxPQp receptor reveals that the LuxP subunit, as anticipated, undergoes a major conformation change upon ligand binding (Figure 3D). The observed domain closure is similar to that observed for other periplasmic binding proteins binding to their ligands (Bjorkman and Mowbray, 1998; Miller et al., 2004). In contrast to the large movements within LuxP, the LuxQp subunit undergoes only minor conformational changes upon AI-2 binding (Figure 3E). Indeed, in three separate crystal structures—LuxQp alone, LuxQp complexed with unliganded LuxP, and LuxQp complexed with ligand bound LuxP—LuxQp displayed almost identical tertiary structures (rms d for Cα carbons = 0.7–1.0 Å for all pairwise comparisons). Thus, although LuxP undergoes a major conformational change upon binding AI-2, this change is apparently not transmitted to the periplasmic domain of the histidine sensor kinase subunit LuxQ. Furthermore, within each LuxPQp receptor, the subunit interface between LuxP and LuxQp is relatively unchanged.
AI-2-Induced Formation of Asymmetric Dimers
The most striking effect of AI-2 binding observed in the crystal structures is the induction of LuxPQp-LuxPQp dimerization (Figures 3A–3C). Crystals grown in the presence of AI-2 contain two LuxPQp monomers per asymmetric unit, denoted LuxPQp and LuxP′Qp′. LuxPQp and LuxP′Qp′ are related to one another by an approximately 140° rotation around an axis between them (Figure 3C). Nearest the rotation axis lie the two LuxQp subunits, interacting with one another, while the two AI-2 bound LuxP subunits are situated more peripherally. Importantly, the four LuxQp termini, each of which would be attached to a transmembrane anchor in vivo, are located close to one another on the same face of the LuxPQp-LuxP′Qp′ dimer (Figure 3A). This observation suggests that the LuxPQp-LuxP′Qp′ dimer observed crystallographically is plausible topologically and that, in vivo, the face containing all four LuxQp termini would be oriented toward the bacterial inner membrane.
Formation of LuxPQp-LuxP′Qp′ dimers coincides with the formation of a large, asymmetric interface between LuxPQp and LuxP′Qp′ (Figures 3B and 3C). When LuxPQp and LuxP′Qp′ interact, 4176 Å2 of previously solvent accessible surface area is buried; 60% of this total is attributable to a new interface between LuxP and LuxQp′. The new LuxP-LuxQp′ interaction appears to be triggered by the closure of the LuxP subunit around AI-2, which creates a new surface to which LuxQp′ binds (Figure 3D). As a result, LuxP interacts with both LuxQp and LuxQp′. Alanine-scanning mutagenesis experiments (below) identified a set of four LuxP residues important for LuxQp′ binding: Thr138, Arg139, Phe143, and Trp167 (see Figure 4A, lower left panel). All four residues (red in Figure 3D) lie in the LuxP domain that rotates 29° (relative to the remainder of LuxPQp) upon AI-2 binding. This rotation is essential to make room for LuxQp′; without it, LuxQp′ would clash sterically with LuxQp and therefore be unable to bind LuxP.
Figure 4. Alanine-Scanning Mutagenesis of the LuxPQp-LuxP′Qp′ Interface.
(A) Dissected (LuxPQp)2 dimer displaying the location of alanine-substituted interfacial residues in LuxPQp (left panel) and LuxP′Qp′ (right panel). Residues colored red, gray, and green exhibited dark, wild-type, and bright phenotypes, respectively, when substituted with alanine ([B ] and Table 1).
(B) LuxQ activity measured as a function of AI-2 concentration in V. harveyi strain FED119 (luxN−, luxPQ−, luxS−). Only alanine substitutions that caused a significant change in AI-2 sensitivity compared to wild-type are shown. In Figures 4 and 5, AI-2 was created in situ by adding synthetic DPD at the given concentrations in the presence of boric acid, as described in the Experimental Procedures. Error bars represent the standard deviations for three independent experiments.
The AI-2 bound (LuxPQp)2 dimer is strongly asymmetric: LuxPQp and LuxP′Qp′ are related by a 140° rotation (Figure 3C). In a symmetric complex, LuxPQp and LuxP′Qp′ would be related by a 180° rotation, so the magnitude of the observed asymmetry can be thought of as 40°. Because of this asymmetry, LuxP′ and LuxQp are separated by approximately 10 Å and make no direct contact with one another (Figure 3C, bottom left). The large asymmetry in the AI-2 bound configuration is likely important in the inhibition of kinase activity.
Activation of Quorum Sensing via LuxPQp Dimerization
To test whether the LuxPQp-LuxP′Qp′ interface observed crystallographically in the presence of AI-2 is important for signal transduction in vivo, we systematically substituted interfacial residues with alanine. Nine alanine substitutions were made (one at a time) in LuxP, and 20 alanine substitutions were made in LuxQ (Table 1). The genes encoding each mutant AI-2 receptor were introduced into V. harveyi FED119, and, in all cases, normal levels of the mutant LuxP and LuxQ subunits were confirmed by Western blotting (data not shown). To evaluate the in vivo activity of the mutant receptors, bioluminescence was measured as a function of autoinducer concentration. Although 17 of the alanine substitutions (gray in Figure 4A) did not cause a significant change in AI-2 responsiveness, all but one of the remaining 12 alanine substitutions (red in Figure 4A) desensitized the cells to AI-2 (Figure 4B). The single sensitizing mutation (L59A; green in Figure 4A) is discussed below. The proportion of alanine substitutions causing a significant phenotype (about 40%) is typical for large protein:protein interfaces (see, for example, Bogan and Thorn, 1998).
Table 1.
LuxP and LuxQ Mutant Phenotypes
| Allelea | EC50b | S.D.c | Residue Location by Subunit Interfaced | |||||
|---|---|---|---|---|---|---|---|---|
| AI-2 Bound (LuxPQp)2e | AI-2-Free LuxPQpf | |||||||
| P-Q′ | Q-Q′ (Q)g | Q-Q′ (Q′)h | P-Q | P′-Q′ | P-Q | |||
| Wild-Type | 21 | 4 | ||||||
| LuxPQ-LuxP′Q′ Interfacei | ||||||||
| LuxP | ||||||||
| K114A | −j | + | ||||||
| S117A | − | + | ||||||
| M121A | − | + | ||||||
| T138A | 220 | 130 | + | |||||
| R139A | 190 | 50 | + | |||||
| F143A | 140 | 30 | + | |||||
| W167A | 2070 | 70 | + | |||||
| H170A | − | + | ||||||
| K238A | − | + | ||||||
| LuxQ | ||||||||
| L59A | constitutive bright | + | + | |||||
| L59F | constitutive bright | + | + | |||||
| N62A | − | + | + | |||||
| S66A | 74 | 25 | + | + | ||||
| I73A | − | + | + | |||||
| K80A | − | + | ||||||
| S81R | 250 | 40 | + | |||||
| E82A | − | + | + | |||||
| R85A | − | + | ||||||
| F98A | 43 | 6 | + | + | ||||
| L101A | − | + | ||||||
| S102A | 34 | 11 | + | + | ||||
| Q105A | 78 | 16 | + | + | ||||
| S106A | − | + | ||||||
| H131A | − | + | ||||||
| F132A | 2540 | 10 | + | |||||
| F132L | 1940 | 150 | + | |||||
| Y133A | 1940 | 690 | + | |||||
| I162A | − | + | ||||||
| F196A | 360 | 10 | + | |||||
| M199V | constitutive bright | (+)k | ||||||
| E200A | − | + | ||||||
| K203R | constitutive bright | (+) | ||||||
| E205A | − | + | ||||||
| A221P | constitutive dark | (+) | ||||||
| I225A | − | + | ||||||
| LuxP-LuxQ Interfacel | ||||||||
| LuxP | ||||||||
| Δ22–26 | constitutive bright | −m | −m | +m | ||||
| E230A | − | + | + | |||||
| Y235A | 15 | 5 | + | + | + | |||
| LuxQ | ||||||||
| R146A | − | +n | ||||||
| F149A | − | + | + | + | ||||
| S150A | − | + | + | + | ||||
| N152A | constitutive bright | +n | ||||||
| W153A | constitutive bright | +n | ||||||
| N258A | − | + | + | + | ||||
Bold and bold italic text indicate significantly higher and lower EC50 values than wild-type, respectively.
EC50 values are given in concentrations of AI-2 (nM).
S.D. indicates standard deviation from three independent EC50 measurements.
Plus signs indicate that residue is located in the indicated subunit interface in the corresponding crystal structure, as defined by a reduction in buried surface area upon removal of the interaction partner.
AI-2 bound LuxPQp (this work).
AI-2-free LuxPQp (1ZHH; Neiditch et al., 2005).
LuxQp or
LuxQp′ residues located in the Q-Q′ interface. The two columns differ because the Q-Q′ interface is asymmetric.
A dash indicates that the EC50 value does not differ significantly from wild-type.
Parentheses indicate that the residue is near, but not in, the indicated subunit interface (see Figure 5B).
See also Figure S1.
LuxP residues 22–26 are present in the ligand-free LuxPQp structure, where they nestle into a deep groove between the two PAS domains of LuxQp (see Figure 3B of Neiditch et al., 2005). These residues are absent from AI-2 bound (LuxPQp)2.
These LuxQ residues contact residues 22–26 of LuxP in the ligand-free LuxPQp structure (see Figure S1; Neiditch et al., 2005).
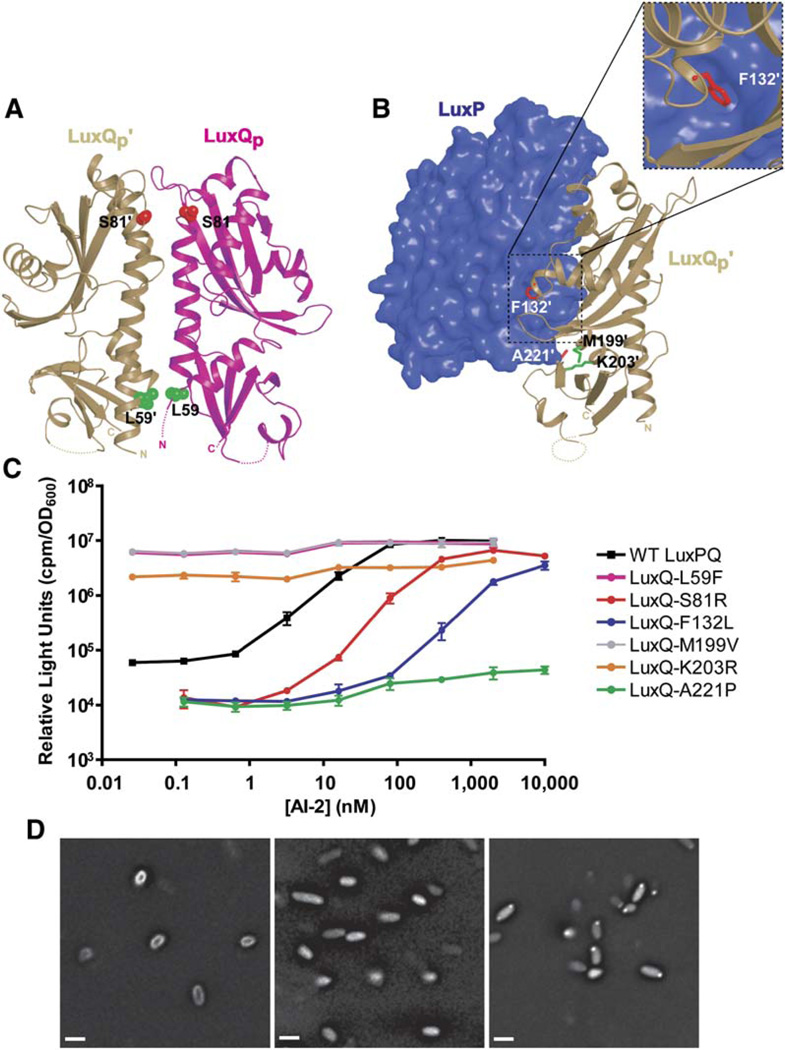
The most strongly desensitizing mutations invariably involved aromatic residues in the LuxPQp-LuxP′Qp′ interface (LuxP W167A; and LuxQ F132A, Y133A, and F196A). In several cases, the concentration of AI-2 required to activate the expression of bioluminescence was increased more than 100-fold (Table 1 and Figure 4B). The results provide strong confirmation that the crystallographic LuxPQp-LuxP′Qp′ interface is physiologically relevant. We infer that destabilizing this interface destabilizes the kinase-off (i.e., high cell density) state of the LuxPQ receptor. Because the kinase-off state is rendered less stable relative to the kinase-on state, more AI-2 is required to convert the mutant LuxPQ receptors to their kinase-off states. To confirm that the substitutions destabilize the LuxPQp-LuxP′Qp′ interface, we used light scattering to test whether two of the mutant proteins (LuxP W167A and LuxQ F132A), when paired with wild-type partners, were capable of forming (LuxPQp)2 dimers. As expected, neither mutant LuxPQp displayed significant dimerization in the presence of AI-2 (RH = 3.5 ± 0.1 nm for LuxP W167A; RH = 3.4 ± 0.1 nm for LuxQ F132A).
To extend this analysis, random mutagenesis of the periplasmic domain of LuxQ was carried out, followed by screens for mutations conferring altered AI-2 signaling phenotypes (see Experimental Procedures). These screens identified six mutations; all six mutant strains produced wild-type levels of LuxQ as judged by Western blotting (data not shown). Similar to the alanine substitutions discussed above, LuxQ S81R, F132L, and A221P (red in Figures 5A and 5B) desensitized V. harveyi to AI-2 (Figure 5C and Table 1). These three residues lie in or near the LuxPQp-LuxP′Qp′ interface, presumably functioning— like the engineered alanine substitutions—to destabilize the interaction. The finding that three random mutations—isolated in a genetic screen because they desensitize cells to AI-2—map to the LuxPQp-LuxP′Qp′ dimer interface provides additional support for the in vivo significance of this interface. By contrast, three other mutations (L59F, M199V, and K203R; green in Figures 5A and 5B) were identified that conferred a constitutively bright (kinase-off) phenotype to V. harveyi; these mutations allow V. harveyi to produce light in the absence of AI-2 (Figure 5C and Table 1). The alanine substitution L59A, like L59F, also conferred a constitutively bright phenotype (see Figure 4). The bright phenotypes were, however, largely LuxP independent (data not shown). Therefore, these substitutions appear to inactivate LuxQ kinase activity by an allosteric mechanism unrelated to AI-2 signaling.
Figure 5. V. harveyi Mutations Displaying Dark or Bright Phenotypes.
(A and B) Random mutations mapping to the LuxQp-LuxQp′ (A) and LuxP-LuxQp′ (B) interfaces were identified in screens for dark (red) or bright (green) phenotypes. The inset displays a close-up view highlighting the interaction of LuxQp′F132 with LuxP.
(C) LuxQ activity measured as in Figure 4B.
(D) LuxPQ does not form protein clusters. YFP was fused to the C terminus of full-length LuxQ (left, LuxQ-YFP) or to an N-terminal truncation of LuxQ that is not localized to the membrane (middle, LuxQ319–860-YFP), or GFP was fused to V. harveyi CheW (right, CheW-GFP). Scale bar is 1 µm.
LuxPQ Oligomeric State In Vivo
Bacterial chemotaxis receptors, which have served as a paradigm for bacterial sensory transduction, form large arrays of membrane-spanning dimers (Maddock and Shapiro, 1993). Clustering is proposed to be important for enabling small changes in attractant and repellent concentrations to generate dramatic changes in swimming behavior (Bray et al., 1998; Keymer et al., 2006; Sourjik and Berg, 2004). To test whether LuxPQ forms large clusters in vivo, we tagged the C terminus of full-length LuxQ with yellow fluorescent protein (YFP) and examined its cellular localization (Figure 5D, left panel). The LuxQ-YFP fusion retained wild-type signaling function (data not shown). LuxQ-YFP localized homogenously over the entire cell membrane, and addition of AI-2 did not noticeably alter this pattern (Figure 5D, left panel, and data not shown). By contrast, LuxQcyto-YFP, containing only the cytoplasmic portion of LuxQ, displayed diffuse fluorescence throughout the cytoplasm (Figure 5D, middle panel). As a control, we fused the V. harveyi chemotaxis protein CheW to GFP. CheW-GFP localized to punctate membrane arrays (Figure 5D, right panel). We conclude that LuxPQ, unlike the chemotaxis receptors, does not form detectable clusters either in the presence or absence of ligand.
DISCUSSION
Two-component histidine sensor kinases play a central role in signal transduction in microorganisms, yet how ligand binding controls receptor activity is not well understood (Stock et al., 2000; Wolanin and Stock, 2003). For most sensor kinases, the relevant ligands have not been identified, while for those kinases whose ligands are known, structural data comparing ligand-free and ligand bound states of the kinases have not, in most cases, been available. A notable exception is the chemotaxis aspartate receptor Tar, which, though not itself a kinase, complexes with the CheA kinase and has served as a model for transmembrane signal transduction (Falke and Hazelbauer, 2001; Müller-Dieckmann and Kim, 2003). The crystal structures of the Tar periplasmic domain with and without bound aspartate ligand reveal relatively subtle conformational changes (Milburn et al., 1991; Yeh et al., 1996). These conformational changes create, in the aspartate bound conformation, a slight asymmetry that shuts off the associated CheA kinase. The question naturally arises as to how relatively small conformational changes are transduced into large changes in activity. A related question is whether subtle changes are the rule or the exception.
Conformational Changes Caused by AI-2 Binding to LuxPQp
To address such questions, we used X-ray crystallography to define ligand-induced structural changes in the two-component sensor kinase complex LuxPQ. LuxPQ is required for detection of a quorum-sensing signal, AI-2, produced and detected by diverse bacteria and proposed to foster interspecies bacterial communication. Comparison of the ligand-free and ligand bound structures of the periplasmic region of LuxPQ reveals that large conformational changes accompany ligand binding. Specifically, the two domains of the LuxP subunit move relative to one another as they clamp down on AI-2 (Figure 3D). Because both LuxP domains contact the periplasmic region of the kinase subunit LuxQ, we initially supposed that the LuxP conformational change might drive a corresponding conformational change in LuxQp. This does not, however, appear to be the case: crystal structures of LuxQp by itself, or in complex with either unliganded or liganded LuxP, reveal only slight differences in LuxQp conformation (Figure 3E). Rather, upon ligand binding, the periplasmic regions of two LuxPQ receptors interact to form an asymmetric (LuxPQp)2 dimer (Figures 3A–3C). In the crystal structure, LuxP′Qp′ is rotated relative to LuxPQp by 140° around an axis normal to the bacterial inner membrane. Extensive mutagenesis results support the argument that the asymmetric interface between LuxPQp and LuxP′Qp′ is biologically relevant and that its role is to stabilize a conformational state in which kinase activity is shut off (Figures 4 and 5).
Deleting the periplasmic domain of LuxQ yields a constitutively active kinase, demonstrating that the periplasmic regions— and, by extension, any interaction between them— are dispensible for kinase activity (Figure 2B). Likewise, in the absence of AI-2 (corresponding to the kinaseon state), we have been unable to detect any interaction between two LuxPQ periplasmic domains using crystallographic or biochemical approaches. We conclude that the kinase-on state— unlike the kinase-off state— is not stabilized by interactions occurring within the periplasm. Rather, the kinase-on state is apparently defined primarily by the transmembrane and cytoplasmic regions of LuxQ. A recent X-ray structure shows that the intact cytoplasmic region of the histidine sensor kinase Thermotoga maritima HK853 is dimeric and symmetric (Marina et al., 2005). If this is also the case for LuxQ, then the kinase-on to kinase-off transition would involve a symmetry-breaking 40° rotation of one LuxPQ receptor relative to the other. Rotation of LuxPQp relative to LuxP′Qp′ presumably repositions the LuxQ transmembrane helices (Figure 6). We hypothesize that this repositioning destabilizes or rearranges the association between the two LuxQ cytoplasmic domains, disabling trans-phosphorylation. Testing this model will require future structural studies that include cytoplasmic and transmembrane regions of LuxQ.
Figure 6. Model of AI-2-Regulated LuxPQ Receptor Activity.
(A and B) Structures are displayed from “above,” looking toward the bacterial inner membrane (A), or from the “side” (B). The relative disposition of the two AI-2-free LuxPQ receptors (left panels) is unknown, but the orientation shown here assumes that the transmembrane and histidine kinase domains contain four-helix bundles, as observed crystallographically for the transmembrane domain of Natronomonas pharaonis NpHtrII protein (Moukhametzianov et al., 2006) and for several histidine kinase domains (reviewed in Tomomori et al., 2003). At low cell density (left panels), in the absence of AI-2, we propose that the LuxQ cytoplasmic dimerization domains form symmetrical four-helix bundles, favoring autophosphorylation in trans. The phosphate is subsequently transferred from His492 to a conserved aspartate (Asp785) in the receiver domain, ultimately resulting in the formation of LuxO-phosphate (see Figure 1). At high cell density (right panels), AI-2 binding induces asymmetric interaction of the LuxPQ periplasmic regions, causing changes in the transmembrane helices (indicated schematically; red arrow). These changes are transmitted to the LuxQ cytoplasmic domains, inhibiting kinase activity. In the kinase-off state, the receiver domain hydrolyzes its aspartyl-phosphate into inorganic phosphate (Pi), thus reversing the flow of phosphate along the signal transduction pathway and reducing the concentration of LuxO-phosphate.
A Clasp Preventing Dimerization
In the previously determined crystal structure of AI-2-free LuxPQp monomers (Neiditch et al., 2005), the N terminus of LuxP was observed to bind in a deep groove between the two domains of LuxQ (Figure S1A). Unexpectedly, progressive truncation of the N terminus was found to sensitize V. harveyi to AI-2 (Neiditch et al., 2005). Additional alanine-scanning mutagenesis within the LuxP-LuxQp interface confirmed this finding: disrupting the interaction between the LuxP N terminus and the LuxQ groove (specifically, by changing LuxQ residues Asn152 or Trp153 to Ala) lowered the EC50 for AI-2 (Table 1, Figure S1). This observation can be rationalized in light of our new crystallographic results. Specifically, we hypothesize that the intimate interaction between the LuxP N terminus and the LuxQ groove serves as a clasp, locking LuxPQp monomers into a conformation unable to form stable (LuxPQp)2 dimers. AI-2 binding causes LuxP to transition from an open conformation to a closed one, simultaneously releasing the clasp. According to our model, these changes would allow the periplasmic domains of two LuxPQ monomers to dimerize, shutting off kinase activity. Importantly, mutations that weaken the clasp would permit dimerization at reduced AI-2 concentrations. Therefore, these mutants would display increased sensitivity to AI-2, as observed (Table 1, Figure S1; Neiditch et al., 2005).
Biochemical evidence provides additional support for the unclasping model: truncating the N terminus of LuxP increased the propensity of AI-2 bound LuxPQp to dimerize as judged by dynamic light scattering, and the same truncation was crucial for generating AI-2 bound LuxPQp crystals. Taken together, our results lead us to propose that AI-2-induced receptor inactivation proceeds by a two-step mechanism: a first step in which AI-2 binds and the LuxP-LuxQp clasp becomes unfastened, and a second step in which LuxPQp and LuxP′Qp′ associate asymmetrically. Although we have not characterized the unclasped intermediate directly, it seems plausible that releasing the clasp might permit the modest reorientations (3–7°) about the LuxP-LuxQp and LuxP′-LuxQp′ interfaces observed in the liganded structure.
Receptor Clustering and Biological Function
In the X-ray crystal structure, both LuxP and LuxP′ are bound to AI-2 ligands. Nonetheless, the LuxPQp-LuxP′Qp′ complex is asymmetric. This asymmetry arises because only one of the two LuxP subunits is able to bind both LuxQp and LuxQp′ simultaneously (Figure 3C). The other LuxP (P′ in Figure 3C) is separated from LuxQp by approximately 10 Å, so it does not make contact with LuxQp. Nonetheless, the close apposition of the two binding surfaces would block the formation of higher-order oligomers.
Indeed, we do not observe higher-order complexes of (LuxPQ)2 receptors (Figure 5D). This finding contrasts with elegant analyses demonstrating that chemotaxis receptors form large arrays (Maddock and Shapiro, 1993; Parkinson et al., 2005). We suspect that the fundamentally different biological decisions that are elicited by the chemotaxis and the quorum-sensing circuits underpin the striking difference in their respective receptor organizations. In chemotaxis, the receptors detect extremely small fluctuations in chemoattractant concentrations and amplify this sensory information into large alterations in motility. Furthermore, signaling is rapidly reversible, allowing the bacteria to alter swimming direction frequently. For this reason, organization of the chemotaxis receptors into cooperative arrays is proposed to be critical for rapid, high-gain signaling. Importantly, no changes in gene expression occur in chemotaxis signaling. By contrast, the receptors that mediate the quorum-sensing response measure the relatively gradual accumulation of autoinducer signals over time. This sensory information is used to invert the patterns of expression of hundreds of genes. Thus, the quorum-sensing transition is a slow all-or-none biological decision that is not readily reversed. We presume that receptor clustering, which potentially amplifies small signal changes, might have undesirable consequences in quorum sensing by rendering bacteria vulnerable to minor fluctuations in signal concentrations. Thus, at least for AI-2 signaling, the architecture of the LuxPQ receptor is such that clustering— and therefore high-order cooperativity— is precluded, and this ensures that the cells avoid the deleterious consequences that would be incurred from mounting a community-wide response to minor changes in signal concentrations.
EXPERIMENTAL PROCEDURES
Protein Production
V. harveyi LuxQ (residues 53–271; LuxQp) was overproduced as a glutathione S-transferase (GST) fusion protein using the expression vector pGEX-4T1 (GE Healthcare). This LuxQ fragment corresponds to the predicted periplasmic region, except for 14 N-terminal and 6 C-terminal residues, which were disordered in the X-ray crystal structure of the AI-2-free periplasmic portion of LuxPQ (Neiditch et al., 2005). V. harveyi LuxP (residues 27–365) was overproduced using the expression vector pBB75. This LuxP fragment corresponds to the entire mature protein (residues 22–365 after signal-sequence cleavage), lacking only the N-terminal five residues previously shown to stabilize the AI-2-free state of the LuxPQ complex (Neiditch et al., 2005). GST-LuxQp was expressed in E. coli strain BL21; GST-LuxQp and LuxP were coexpressed in E. coli strain BL21 (DE3). Proteins were purified from cell lysates by glutathione agarose affinity column chromatography followed by thrombin cleavage of the GST tags. LuxQp contains two heterologous N-terminal residues (Gly-Ser) derived from the thrombin cleavage signal. Proteins were further purified by anion exchange (Source 15Q; GE Healthcare) and size exclusion (S200; GE Healthcare) chromatography.
Dynamic light scattering experiments were performed using a Wyatt Technologies MiniDAWN instrument at 23°C. All samples contained 30 µM LuxPQp in 14 mM Tris-HCl (pH 7.5), 105 mM NaCl, 4 mM ammonium acetate, and 2 mM boric acid; AI-2 bound protein samples were supplemented with 40 µM DPD, prepared as described (Schauder et al., 2001). LuxPQp containing full-length LuxP (residues 22–365 after signal-sequence cleavage) was prepared as described (Neiditch et al., 2005). Theoretical hydrodynamic radii were calculated from crystal structures using HYDROPRO (Garcia De La Torre et al., 2000).
Crystallization and Diffraction Data Collection
Preliminary AI-2 bound LuxPQp crystallization conditions were identified by high-throughput screening using a variant of LuxQp containing residues 39–278. High-throughput screening was performed by the Hauptman-Woodward Medical Research Institute using the microbatch-under-oil technique at room temperature (Luft et al., 2003). Following protein engineering to remove the LuxQp N and C termini and optimization of the crystallization conditions, X-ray diffraction quality LuxPQp crystals were produced by vapor diffusion at 23°C using a 1:1 mixture of protein (20 mg/ml in 20 mM Tris-HCl [pH 7.5], 150 mM NaCl) and well solution (27.5% [w/v] PEG 400, 100 mM potassium nitrate, 100 mM 2-morpholinoethanesulfonic acid monohydrate (pH 6.0), 100 µM DPD, and 1 mM boric acid). DPD was prepared as described (Schauder et al., 2001). LuxQp was crystallized in a similar manner using a 1:3 mixture of protein (10 mg/ml in 10 mM Tris-HCl, 150 mM NaCl, pH 7.5) and well solution (8% [w/v] PEG 2000 monomethyl ether [MME], 10 mM Tris-HCl [pH 7.0], and 10 mM NiCl2). LuxQp crystals were cryoprotected by a brief soak in mother liquor solutions containing 25% (w/v) PEG 2000 MME and 25% glycerol; LuxPQp crystals did not require cryoprotection prior to freezing. Data were collected on nitrogen-cooled crystals [Table S1] at NSLS beamline X25. Diffraction data were processed using the HKL software package (Otwinowski and Minor, 1997).
Structure Determination and Refinement
The AI-2 bound LuxPQp structure was determined by molecular replacement with PHASER (Storoni et al., 2004) using AI-2 bound LuxP (1JX6) and LuxQp from AI-2-free LuxPQp (1ZHH) as search models. AI-2 was not included in the LuxP search model to ensure that any density observed in the LuxP ligand binding pocket was uninfluenced by phase bias. AI-2 was built into this density during subsequent rounds of refinement and was found to be identical to the structure of AI-2 in LuxP crystallized in the absence of LuxQp. The LuxQp structure was determined by molecular replacement with MOLREP (Vagin and Teplyakov, 1997) using LuxQp′ from the LuxPQp structure as a search model. Iterative cycles of building in O (Jones et al., 1991) and refinement in CNS (Brunger et al., 1998) or REFMAC5 (Murshudov, 1997) were then performed.
The final LuxPQp model lacks LuxQ residues 53–57 (N terminus), 238–247, and 271 (C terminus); LuxQ′ residues 239–247 and 271 (C terminus); and LuxP′ residues 109–118. The model includes one heterologous Ser residue, derived from thrombin cleavage, at the N terminus of LuxQ′. The LuxPQp and LuxQp structures have excellent geometry with only one residue (Asn216) falling outside of the additional allowed regions of the Ramachandran plot as determined by PROCHECK (Laskowski et al., 1993) both in uncomplexed LuxQp and in LuxQp′ of the LuxPQp structure (Table S1). The building of four Ni2+ ions into the electron density of uncomplexed LuxQp was justified by their hexameric coordination state, their binding to histidine and aspartic acid residues, and the presence of 10 mM NiCl2 in the crystallization conditions.
Structure-based sequence alignment was guided by DALI (Holm and Sander, 1993) and PyMOL (DeLano, 2002). Analysis of protein domain motion was carried out using DynDom (Hayward and Lee, 2002; Lee et al., 2003), LSQMAN (Kleywegt, 1996), and PEK2 (P.D.J., unpublished data). Buried surface and contacts were analyzed in CNS. Molecular graphics were produced using PyMOL.
V. harveyi Strain Construction
To make the luxN, luxPQ, luxS mutant V. harveyi strain FED119, the luxS gene of V. harveyi BB170 (luxN::Tn5) (Bassler et al., 1993) was deleted using plasmid pKAS46-ΔluxS as described (Bassler et al., 1993; Henke and Bassler, 2004b), generating strain JMH610. Cosmid pBB713 (Bassler et al., 1994) carrying luxPQ was modified to delete the DNA specifying amino acids 34–366 and 1–843 of LuxP and LuxQ, respectively. This plasmid, pDLS100, was introduced into JMH610, and the deletion transferred to the chromosome to generate V. harveyi strain FED119.
Mutagenesis of luxPQ
The luxPQ operon was amplified from wild-type V. harveyi by PCR and inserted into pEVS143 (Dunn et al., 2006) at the SphI and BamHI sites, making pFED367. A chloramphenicol resistance cassette from pHP45Ω-Cm (Fellay et al., 1987) was inserted into pFED367 at a SmaI site located in the kanamycin resistance gene, generating pFED368. Site-directed mutagenesis was conducted on pFED368 using complementary PCR primers containing targeted mutations in luxP and luxQ. The entire plasmid was amplified using PfuUltra high-fidelity polymerase (Stratagene).
To generate random amino acid substitutions in the periplasmic domain of LuxQ, luxQp was amplified with Mutazyme DNA polymerase (Stratagene). A library of mutant fragments was subcloned into pFED132 (Neiditch et al., 2005) to regenerate luxPQ, and these constructs were introduced into V. harveyi strains. To identify luxPQs conferring a bright phenotype to V. harveyi in the absence of AI-2, the library was introduced into V. harveyi lacking luxN and luxS (JMH610). To identify luxPQ mutations conferring a dark phenotype in the presence of AI-2, the mutant library was introduced into a V. harveyi strain lacking luxN (BB170). In both cases, luxPQ genes from plasmids that altered the V. harveyi Lux phenotype were collected and sequenced.
Additional site-specific mutations were engineered in luxP and luxQ to examine kinase and phosphatase activities. The LuxPss secretion mutant, containing substitutions L9R and I10K in the signal sequence (Neiditch et al., 2005), and the LuxQH492A mutant were constructed by site-directed mutagenesis as above. The LuxQΔp allele was constructed by deleting amino acids 45–265. The in-frame deletion of luxP has the entire open reading frame of luxP replaced with an NheI site and maintains the native ribosome binding site and first codon of luxQ. To express the receiver domain of LuxQ, DNA encoding LuxQ amino acids 708–859 (LuxQreceiver) was amplified by PCR and cloned into pEVS143 at the NheI/BamHI restriction sites (Dunn et al., 2006), generating plasmid pFED553. Expression from pFED553 was driven by the Ptac promoter and induction with 10 µM IPTG.
Luciferase Bioassays
AI-2 responses were measured from V. harveyi FED119 carrying pFED368 or plasmids harboring mutant luxPQ alleles. Strains were grown overnight in AB medium (Greenberg et al., 1979) containing 10 µg/ml chloramphenicol. Cultures were diluted 1/10,000 into identical medium supplemented with 0.5 mM boric acid and dispensed into microtiter plates containing from 0.1–10,000 nM synthetically prepared DPD (Semmelhack et al., 2005). The plates were incubated at 30°C for 16–20 hr. EC50 values are defined as the concentration of DPD required to produce 50% maximal bioluminescence and were obtained by curve fitting using Prism (GraphPad Software). Bioluminescence assays were also performed on strains of V. harveyi that endogenously produce AI-2 (luxS+). For these assays, overnight cultures were diluted 1/5,000, and the relative light production (counts per minute per colony-forming unit × 1,000) was monitored throughout growth. Bioluminescence values reported in bar graphs show the point in growth at which time wild-type V. harveyi produces the lowest level of light, typically at 1 × 106 cfu/ml.
Construction and Visualization of YFP Fusions
The complete luxQ ORF and a region encoding only the cytoplasmic domain of luxQ (nucleotides 1114–2577; luxQcyto) were each fused to the A206K allele of eyfp (Zacharias et al., 2002) via a flexible Gly-Gly-Gly-Ala linker. luxQ-eyfp was cloned in place of luxQ on pFED368, and luxQcyto-eyfp was cloned under the Ptac promoter of pEVS143 (Dunn et al., 2006), to generate plasmids pFED376 and pFED537, respectively. egfp was fused to the C terminus of the V. harveyi cheW gene by inserting the cheW open reading frame into pEVS143 at an NheI site. All fusions were visualized using a Nikon Eclipse TE200 microscope with a 100× objective equipped with a Photometrics Cool- SNAPHQ CCD camera. Images were deconvolved using SoftWoRx v. 2.5 (Creative Softworx).
Immunoblotting
V. harveyi whole-cell extracts were sonicated, electrophoresed on SDS-PAGE gels, and blotted to nitrocellulose for immunostaining with anti-LuxP or anti-LuxQ rabbit antisera followed by anti-rabbit IgG HRP conjugated antibody. Protein was detected using Chemi Glow substrate by a FluorChem imaging system (Alpha Innotech).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge George DeTitta, Joseph Luft, and the staff of the High-Throughput Crystallization Laboratory of the Hauptman-Woodward Medical Research Institute for assistance in crystallization; Michael Becker and the staff of the National Synchrotron Light Source X25 beamline for assistance with X-ray data collection; Shawn Campagna and Martin Semmelhack for chemically synthesized DPD; Bashkim Kokona and Robert Fairman for equilibrium analytical ultracentrifugation; Yi Ren and Yufang Wang for additional experimental contributions; and Yigong Shi, Tom Silhavy, Ann Stock, Daniel Ungar, and Ned Wingreen for critical review of the manuscript and, together with members of our laboratories, for advice and discussion. This work was supported by NIH grants AI-054442 and GM-065859, HHMI, and NIH postdoctoral fellowships (to M.B.N. and M.J.F.).
Footnotes
Supplemental Data
Supplemental Data include one figure and one table and can be found with this article online at http://www.cell.com/cgi/content/full/126/6/1095/DC1/.
Accession Numbers
Atomic coordinates and structure factors for LuxQp and AI-2 bound LuxPQp have been deposited in the Protein Data Bank under accession codes 2HJE and 2HJ9, respectively.
REFERENCES
- Bassler BL, Wright M, Showalter RE, Silverman MR. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 1993;9:773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Bassler BL, Wright M, Silverman MR. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 1994;13:273–286. doi: 10.1111/j.1365-2958.1994.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Bjorkman AJ, Mowbray SL. Multiple open forms of ribose-binding protein trace the path of its conformational change. J. Mol. Biol. 1998;279:651–664. doi: 10.1006/jmbi.1998.1785. [DOI] [PubMed] [Google Scholar]
- Bogan AA, Thorn KS. Anatomy of hot spots in protein interfaces. J. Mol. Biol. 1998;280:1–9. doi: 10.1006/jmbi.1998.1843. [DOI] [PubMed] [Google Scholar]
- Bray D, Levin MD, Morton-Firth CJ. Receptor clustering as a cellular mechanism to control sensitivity. Nature. 1998;393:85–88. doi: 10.1038/30018. [DOI] [PubMed] [Google Scholar]
- Brunger AT, Adams PD, Clore GM, Delano WL, Gros P, Grosse-Kunstleve RW, Jiang J-S, Kuszewski J, Nilges N, Pannu NS, et al. Crystallography & NMR System (CNS): a new software system for macromolecular structure determination. Acta. CrystallogrD. Biol. Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- Cao JG, Meighen EA. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J. Biol. Chem. 1989;264:21670–21676. [PubMed] [Google Scholar]
- Chen X, Schauder S, Potier N, Van Dorsselaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA: DeLano Scientific; 2002. [Google Scholar]
- Dunn AK, Millikan DS, Adin DM, Bose JL, Stabb EV. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 2006;72:802–810. doi: 10.1128/AEM.72.1.802-810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke JJ, Hazelbauer GL. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 2001;26:257–265. doi: 10.1016/s0968-0004(00)01770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Bassler BL. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 1999a;31:665–677. doi: 10.1046/j.1365-2958.1999.01208.x. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Bassler BL. Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol. 1999b;181:899–906. doi: 10.1128/jb.181.3.899-906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JA, Lilley BN, Bassler BL. A genetic analysis of the functions of LuxN: a two-component hybrid sensor kinase that regulates quorum sensing in Vibrio harveyi. Mol. Microbiol. 2000;35:139–149. doi: 10.1046/j.1365-2958.2000.01684.x. [DOI] [PubMed] [Google Scholar]
- Garcia De La Torre J, Huertas ML, Carrasco B. Calculation of hydrodynamic properties of globular proteins from their atomic-level structure. Biophys. J. 2000;78:719–730. doi: 10.1016/S0006-3495(00)76630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg EP, Hastings JW, Ulitzer S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch. Microbiol. 1979;120:87–91. [Google Scholar]
- Hayward S, Lee RA. Improvements in the analysis of domain motions in proteins from conformational change: DynDom version 1.50. J. Mol. Graph. Model. 2002;21:181–183. doi: 10.1016/s1093-3263(02)00140-7. [DOI] [PubMed] [Google Scholar]
- Heermann R, Altendorf K, Jung K. The turgor sensor KdpD of Escherichia coli is a homodimer. Biochim. Biophys. Acta. 1998;1415:114–124. doi: 10.1016/s0005-2736(98)00181-3. [DOI] [PubMed] [Google Scholar]
- Henke JM, Bassler BL. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 2004a;186:3794–3805. doi: 10.1128/JB.186.12.3794-3805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke JM, Bassler BL. Three parallel quorum-sensing systems regulate gene expression in Vibrio harveyi. J. Bacteriol. 2004b;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J. Mol. Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou J-Y, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Keymer JE, Endres RG, Skoge M, Meir Y, Wingreen NS. Chemosensing in Escherichia coli: two regimes of two-state receptors. Proc. Natl. Acad. Sci. USA. 2006;103:1786–1791. doi: 10.1073/pnas.0507438103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorchid A, Ikura M. Bacterial histidine kinase as signal sensor and transducer. Int. J. Biochem. Cell Biol. 2006;38:307–312. doi: 10.1016/j.biocel.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Kleywegt GJ. Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr. D Biol. Crystallogr. 1996;52:842–857. doi: 10.1107/S0907444995016477. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–291. [Google Scholar]
- Lee RA, Razaz M, Hayward S. The DynDom database of protein domain motions. Bioinformatics. 2003;19:1290–1291. doi: 10.1093/bioinformatics/btg137. [DOI] [PubMed] [Google Scholar]
- Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Luft JR, Collins RJ, Fehrman NA, Lauricella AM, Veatch CK, DeTitta GT. A deliberate approach to screening for initial crystallization conditions of biological molecules. J. Struct. Biol. 2003;142:170–179. doi: 10.1016/s1047-8477(03)00048-0. [DOI] [PubMed] [Google Scholar]
- Maddock JR, Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993;259:1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- Malpica R, Franco B, Rodriguez C, Kwon O, Georgellis D. Identification of a quinone-sensitive redox switch in the ArcB sensor kinase. Proc. Natl. Acad. Sci. USA. 2004;101:13318–13323. doi: 10.1073/pnas.0403064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina A, Waldburger CD, Hendrickson WA. Structure of the entire cytoplasmic portion of a sensor histidine-kinase protein. EMBO J. 2005;24:4247–4259. doi: 10.1038/sj.emboj.7600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn MV, Prive GG, Milligan DL, Scott WG, Yeh J, Jancarik J, Koshland DE, Jr, Kim SH. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991;254:1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol. Cell. 2004;15:677–687. doi: 10.1016/j.molcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Moukhametzianov R, Klare JP, Efremov R, Baeken C, Goppner A, Labahn J, Engelhard M, Buldt G, Gordeliy VI. Development of the signal in sensory rhodopsin and its transfer to the cognate transducer. Nature. 2006;440:115–119. doi: 10.1038/nature04520. [DOI] [PubMed] [Google Scholar]
- Müller-Dieckmann HJ, Kim SH. Structure-function relationships: chemotaxis and ethylene receptors. In: Inouye M, editor. Histidine Kinases in Signal Transduction. San Diego: Academic Press; 2003. pp. 123–141. [Google Scholar]
- Murshudov GN. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol. Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Ninfa EG, Atkinson MR, Kamberov ES, Ninfa AJ. Mechanism of autophosphorylation of Escherichia coli nitrogen regulator II (NRII or NtrB): trans-phosphorylation between subunits. J. Bacteriol. 1993;175:7024–7032. doi: 10.1128/jb.175.21.7024-7032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pappas KM, Weingart CL, Winans SC. Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signalling. Mol. Microbiol. 2004;53:755–769. doi: 10.1111/j.1365-2958.2004.04212.x. [DOI] [PubMed] [Google Scholar]
- Parkinson JS, Ames P, Studdert CA. Collaborative signaling by bacterial chemoreceptors. Curr. Opin. Microbiol. 2005;8:116–121. doi: 10.1016/j.mib.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum sensing signal molecule. Mol. Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- Semmelhack MF, Campagna SR, Federle MJ, Bassler BL. An expeditious synthesis of DPD and boron binding studies. Org. Lett. 2005;7:569–572. doi: 10.1021/ol047695j. [DOI] [PubMed] [Google Scholar]
- Sourjik V, Berg HC. Functional interactions between receptors in bacterial chemotaxis. Nature. 2004;428:437–441. doi: 10.1038/nature02406. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu. Rev. Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr. D Biol. Crystallogr. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- Surette MG, Levit M, Liu Y, Lukat G, Ninfa EG, Ninfa A, Stock JB. Dimerization is required for the activity of the protein histidine kinase CheA that mediates signal transduction in bacterial chemotaxis. J. Biol. Chem. 1996;271:939–945. doi: 10.1074/jbc.271.2.939. [DOI] [PubMed] [Google Scholar]
- Tomomori C, Kurokawa H, Ikura M. The histidine kinase family: structures of essential building blocks. In: Inouye M, editor. Histidine Kinases in Signal Transduction. San Diego: Academic Press; 2003. pp. 11–24. [Google Scholar]
- Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J. Appl. Crystallogr. 1997;30:1022–1025. [Google Scholar]
- Wang L, Fabret C, Kanamaru K, Stephenson K, Dartois V, Perego M, Hoch JA. Dissection of the functional and structural domains of phosphorelay histidine kinase A of Bacillus subtilis. J. Bacteriol. 2001;183:2795–2802. doi: 10.1128/JB.183.9.2795-2802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Wolanin PM, Stock JB. Transmembrane signaling and the regulation of histidine kinase activity. In: Inouye M, editor. Histidine Kinases in Signal Transduction. San Diego: Academic Press; 2003. pp. 73–122. [Google Scholar]
- Xavier KB, Bassler BL. LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 2003;6:191–197. doi: 10.1016/s1369-5274(03)00028-6. [DOI] [PubMed] [Google Scholar]
- Xavier KB, Bassler BL. Interference with AI-2-mediated bacterial cell-cell communication. Nature. 2005;437:750–753. doi: 10.1038/nature03960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Inouye M. Intermolecular complementation between two defective mutant signal-transducing receptors of Escherichia coli. Proc. Natl. Acad. Sci. USA. 1991;88:11057–11061. doi: 10.1073/pnas.88.24.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JI, Biemann HP, Prive GG, Pandit J, Koshland DE, Jr, Kim SH. High-resolution structures of the ligand binding domain of the wild-type bacterial aspartate receptor. J. Mol. Biol. 1996;262:186–201. doi: 10.1006/jmbi.1996.0507. [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.