Abstract
Background
Inflammation has been linked to depression and suicide risk. One inflammatory process that has been minimally investigated in this regard is cytokine-stimulated production of kynurenine (KYN) from tryptophan (TRP). Recent data suggest that KYN increases in cerebrospinal fluid (CSF) are associated with depressive symptoms secondary to immune activation. KYN may alter dopaminergic and glutamatergic tone, thereby contributing to increased arousal, agitation and impulsivity - important risk factors in suicide. We hypothesized that patients with Major Depressive Disorder (MDD) and a history of suicide attempt would have higher levels of KYN than depressed nonattempters, who in turn would have higher levels than healthy volunteers.
Methods
Plasma KYN, TRP, and neopterin were assayed by high performance liquid chromatography in three groups: healthy volunteers (n=31) and patients with MDD with (n=14) and without (n=16) history of suicide attempt. Analysis of variance tested for group differences in KYN levels.
Results
KYN levels differed across groups (F=4.03, df=(2,58), p=0.023): a priori planned contrasts showed that KYN was higher in the MDD suicide attempter subgroup compared with MDD non-attempters (t=2.105, df=58, p=0.040), who did not differ from healthy volunteers (t=0.418, df=58, p=0.677). In post-hoc testing, KYN but not TRP was associated with attempt status, and only suicide attempters exhibited a positive correlation of the cytokine activation marker neopterin with the KYN:TRP ratio, suggesting that KYN production may be influenced by inflammatory processes among suicide attempters.
Conclusions
These preliminary results suggest that KYN and related molecular pathways may be implicated in the pathophysiology of suicidal behavior.
Keywords: Suicide, Depression, Kynurenine, Serotonin, Tryptophan, Inflammation, Cytokines, Neopterin, Major Depressive Disorder
1. Introduction
Neuroimmune factors have been proposed as contributors to the pathogenesis of major depression (Dantzer, 2010; Maes, 2009). For instance, clinical depression can be reversibly caused by medical treatment with cytokines such as interferons, and depression is associated with higher levels of proinflammatory cytokines even in patients with no medical illness (reviewed in (Raison, 2006)).
One cascade that has been linked to depressive behaviors is inflammatory activation of the enzyme indoleamine-2,3-dioxygenase (IDO) by a variety of stimuli, including tumor necrosis factor-α and interferons (IFN) (Dantzer, 2008), lipopolysaccharide (Fu, 2010), and infection with bacillus Calmette Gueren (BCG) (O'Connor, 2009). The potential importance of IDO in depression is demonstrated in the latter study, in which knockout mice without IDO did not exhibit depressive-like behaviors despite immune activation (O'Connor, 2009). Induction of IDO catalyzes the formation of kynurenine (KYN) from its precursor, tryptophan (TRP), and thus potentially may deplete TRP, leading to reduced levels of the TRP metabolite, serotonin (5-HT). Both major depression and suicidal behavior have been linked to low 5-HT function (Mann, 2003). However, depression additionally has been more directly associated with cytokine-stimulated KYN in the absence of cerebrospinal fluid (CSF) TRP depletion (Raison, 2010). Therefore, we tested the hypothesis that peripheral KYN levels would be elevated in major depression, and would be highest in individuals with suicide attempt history. Secondary logistic regression analyses tested for confounding effects of other clinical and demographic factors on suicide attempt status. Additional exploratory analyses tested correlations between the KYN:TRP ratio, a measure of TRP degradation along the KYN pathway, and neopterin, a marker for cytokine activation.
2. Methods
2.1 Sample
All participants were adults who gave written informed consent to enroll in IRB-approved studies at the New York State Psychiatric Institute. Participants were healthy volunteers (n=31) or were acutely depressed patients (n=30) presenting for evaluation and treatment. All but 3 depressed participants were medication-free. Diagnosis of a major depressive episode in context of Major Depressive Disorder (MDD) was made by trained psychologists using the Structured Clinical Interview for DSM-IV (SCID) (First, 1997) and verified in consensus conference. Individuals with active major medical or neurologic illness were excluded from participation. Prior suicide attempters (n=14) were identified using the Columbia Suicide History Scale (Oquendo, 2003) as having made actual (as opposed to ambiguous, interrupted, or aborted) attempts. Depression severity was assessed using the clinician-administered 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960) and the Beck Depression Inventory (BDI) self-report (Beck, 1961).
2.2 Blood Collection
Blood samples were obtained after subjects gave informed consent for the procedures. Blood was collected in EDTA-containing tubes and maintained in an ice-water bath until refrigerated centrifugation for 10 min, followed by transfer to cryotubes and storage at −80° C until analysis.
2.3 Kynurenine, Tryptophan, and Neopterin Determinations
TRP and KYN concentrations were measured by high performance liquid chromatography (HPLC) using 3-nitro-L-tyrosine as internal standard (Widner, 1997). For separation, reversed-phase cartridges LiChroCART RP18 columns, 55 mm length, were used. TRP was detected by a fluorescence detector at an excitation wavelength of 285 nm and an emission wavelength of 365 nm. A Shimadzu SPD-6A UV-detector in flow stream series connection was used for detection of both KYN and nitrotyrosine at a wavelength of 360 nm. The elution buffer was a degassed potassium phosphate solution (0.015 mol/L, pH 6.4) containing 5% methanol. Analyses were carried out at a flow rate of 0.8 ml/min and a temperature of 25 °C. Frozen serum specimens were thawed at room temperature, 200 ml of serum were diluted with 200 μL of potassium phosphate buffer (0.05 mol/L, pH 6.0) containing the internal calibrator 3-nitro-L-tyrosine (100 μmol/L). Protein was precipitated with 50 μL of trichloroacetic acid (2 mol/L). The capped tubes with the precipitate were immediately vortex-mixed and centrifuged for 10 min at 13 000g. One-hundred-fifty microliters of the supernatants were transferred into microvials and placed into the autosampling device. The external calibrator was prepared from freshly thawed stock solutions of TRP and KYN (1 mmol/L in bidistilled water, stored at −20 °C) and albumin (70 g/L, which corresponds to the average physiological protein content in human serum). Fifty microliters of TRP, 10 μL of KYN, and 940 μL of albumin stock solution were mixed together. Aliquots of 200 μL of this external calibrator preparation were run through the entire analytic procedure in parallel with the serum specimens. Neither degradation of TRP nor production of KYN were observed in the calibration preparations, and our standards confirmed the stability of the KYN and TRP when compared with protein-free standard preparations measured without any precipitation step. Concentrations of neopterin, a marker for activation of the cellular immune system (Murr, 2002), were measured by enzyme-linked immunosorbent assay (BRAHMS GmbH, Berlin, Germany) (Mayersbach, 2010).
2.4 Statistical Analyses
Diagnostic groups (patients with MDD vs. healthy volunteers) and subgroups (depressed suicide attempters vs. nonattempters) were characterized with respect to clinical and demographic factors. The primary outcome measure was KYN levels. We hypothesized that depressed patients with a history of suicide attempt would have higher levels of KYN than depressed nonattempters, and that healthy volunteers would have the lowest KYN levels. A one-way ANOVA was performed with KYN as the dependent variable and three predictor groups: MDD attempters, MDD nonattempters, and healthy volunteers. Two a priori planned contrasts were performed: MDD attempters vs. MDD nonattempters, and MDD non-attempters vs. healthy volunteers.
Logistic regression analyses testing main effect of KYN on suicide attempt were also performed with and without three MDD subjects who were on medications. Secondary analyses were performed to investigate other possible confounders, testing whether TRP levels, sex, body-mass index (BMI), educational level, smoking status, or age were correlated with KYN levels (see Table 2). Significantly associated variables were then tested for possible additive effects on suicide attempt, by entering them as covariates in separate binary logistic regression analyses with KYN as predictor. As age and smoking are known to be implicated in suicide risk but were not correlated with KYN (and thus would not have any additive effects in a linear model) main effects and interactions were also tested for these two selected variables.
Table 2.
Associations between kynurenine levels and characteristics of the study population.
| Characteristic | Mean KYN levels (SD) by characteristic | t-score | df | p-value | |
|---|---|---|---|---|---|
|
| |||||
| Sex (male / female) | 1.60 (0.30) | 1.27 (0.34) | 3.95 | 59 | <0.001 |
| Race (white / nonwhite) | 1.50 (0.31) | 1.3 (0.39) | 2.145 | 58 | 0.036 |
| Smoker (yes/no) | 1.37 (0.38) | 1.42 (0.36) | − 0.362 | 59 | 0.719 |
|
| |||||
| Correlation with KYN Pearson's r | F-score | df | p-value | ||
|
| |||||
| Age (yrs) | 0.04 | 0.105 | 1, 59 | 0.747 | |
| BMI (kg*m−2) | 0.28 | 4.766 | 1, 58 | 0.033* | |
| Education (yrs) | 0.07 | 0.314 | 1, 59 | 0.578 | |
| HDRS-17a | 0.16 | 0.762 | 1, 28 | 0.390 | |
| BDIa | 0.09 | 0.209 | 1, 28 | 0.651 | |
| TRP | 0.40 | 11.266 | 1, 59 | 0.001* | |
|
| |||||
| TRP correlation with KYN among subgroups | |||||
|
| |||||
| in MDD | 0.46 | 7.535 | 1, 29 | 0.01* | |
| In healthy volunteers | 0.40 | 5.576 | 1, 29 | 0.025* | |
| in MDD nonattempters | 0.73 | 15.490 | 1, 15 | 0.001* | |
| in MDD attempters | 0.05 | 0.167 | 1, 13 | 0.87 | |
Abbreviations: BDI, Beck Depression Inventory; BMI, body-mass index; HDRS-17, Hamilton Depression Rating Scale (17-item); KYN, kynurenine; MDD, Major Depressive Disorder; SD, standard deviation; TRP, tryptophan.
Test performed in depressed subgroup only.
As differential rates of production of KYN from TRP were of interest, we tested for correlations between KYN and TRP among the various subgroups. The KYN to TRP (KYN:TRP) ratio (Schrocksnadel, 2006) was also entered as predictor into a binary regression analysis with suicide attempt as outcome measure. To test for differential involvement of the cellular immune system (Murr, 2002), correlations were studied between the KYN:TRP ratio and levels of neopterin within the different subgroups (MDD attempter, MDD nonattempter, healthy volunteers).
For correlation analyses with neopterin, the nonparametric Spearman's rho (rs) was used, due to a highly skewed distribution for this measure.
Statistical analyses were performed using PASW Statistics v. 18.0.0 (SPSS, Inc., 2009, Chicago, IL) for Mac.
3. Results
3.1 Sample characteristics
Participants (n=61), ages 18–73, were healthy volunteers (n=31) or were moderately depressed (mean score ± SD: HDRS-17, 20.13 ± 3.40; BDI, 25.87 ± 8.22) patients with a DSM-IV diagnosis of MDD (n=31) seeking evaluation and treatment, with (n=14) or without (n=16) a prior history of suicide attempt. There were no differences between depressed and healthy groups with respect to demographic characteristics, and suicide attempters differed from nonattempters only in having a lower educational level (see Table 1). Of note, depression severity as measured by either clinician rating (HDRS-17) or self-report (BDI) did not distinguish between attempters and nonattempters. Depressed subjects were not on psychotropic medications except for two suicide attempters, who were taking (1) hypericum perforatum and (2) eszoplicone and zolpidem; and one nonattempter who was taking duloxetine.
Table 1.
Demographic characteristics of the study population across diagnostic groups.
| Characteristic | Healthy volunteers n=31 | Total Depressed n=30 | χ 2 | df | p | Suicide Attempters n=14 | Suicide Non-Attempters n=16 | χ 2 | df | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex (% male) | 32.3% | 53.3% | 2.769 | 1 | 0.096 | 64.3% | 43.8% | 1.265 | 1 | 0.261 |
| Race (% white) | 48.4% | 63.3% | 1.380 | 1 | 0.240 | 57.1% | 68.8% | 0.433 | 1 | 0.510 |
| Smoker (% yes) | 6.5% | 23.3% | 3.455 | 1 | 0.063 | 35.7% | 12.5% | 2.249 | 1 | 0.134 |
| Mean values | t-score | df | p | t-score | df | p | ||||
|
| ||||||||||
| Age (yrs) | 35.68(13.92) | 37.80 (13.08) | 0.613 | 59 | 0.613 | 34.07 (13.52) | 41.06 (12.20) | −1.489 | 28 | 0.148 |
| BMI (kg*m−2) | 24.25 (5.7) | 25.30 (5.46) | 0.724 | 57 | 0.472 | 26.23 (6.76) | 24.43 (3.90) | 0.887 | 27 | 0.383 |
| Education (yrs) | 16.10 (3.22) | 14.79 (2.54) | −1.734 | 58 | 0.088 | 13.69 (2.17) | 15.69 (2.52) | −2.251 | 27 | 0.033* |
| HDRS-17 | N/A | 20.13 (3.40) | N/A | N/A | N/A | 19.14 (3.11) | 21.00 (3.50) | −1.526 | 28 | 0.138 |
| BDI | N/A | 25.87 (8.22) | N/A | N/A | N/A | 25.5 (9.04) | 26.19 (7.70) | −0.225 | 28 | 0.824 |
| KYNb (μmol/L) | 1.33 (0.36) | 1.50 (0.34) | 1.853 | 59 | 0.069 | 1.64 (0.33) | 1.37 (0.30) | 2.263 | 28 | 0.032* |
| TRP (μmol/L) | 60.21 (7.7) | 59.15(10.4) | −0.455 | 59 | 0.651 | 63.18 (10.6) | 55.61 (9.15) | 2.099 | 28 | 0.045* |
| KYN:TRP ratio | 22.18 (5.5) | 25.62 (5.7) | 2.385 | 59 | 0.020* | 26.60 (7.14) | 24.76 (4.22) | 0.873 | 28 | 0.390 |
Abbreviations: BDI, Beck Depression Inventory, BMI, body-mass index; HDRS-17, Hamilton Depression Rating Scale (17-item); KYN, kynurenine; MDD, Major Depressive Disorder; SD, standard deviation; TRP, tryptophan.
Test performed was a one-way Analysis of Variance with contrasts.
p < 0.05.
3.2 Associations between kynurenine levels and suicide attempt status
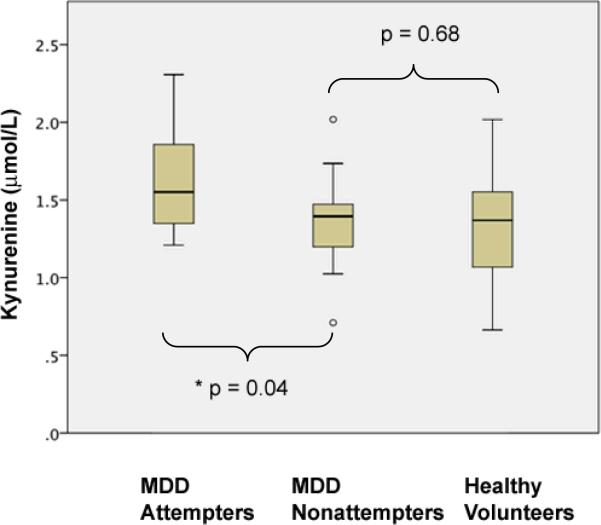
There was a significant effect of KYN levels on suicide attempt status across the three groups: MDD attempter, MDD nonattempter, and healthy volunteers (F (2,58) = 4.03, p = 0.023). We tested two a priori planned contrasts and found that mean plasma KYN levels were higher in MDD suicide attempters compared with MDD nonattempters (t (58) = 2.10, p = 0.040), but MDD nonattempters did not differ from healthy volunteers (t (58) = 0.418, p = 0.677) (see Figure 1). In binary logistic regression analysis, higher KYN predicted suicide attempt (χ2(1) = 6.354, p = 0.012). Results were unchanged when the three subjects on medications were omitted (data not shown).
Figure 1.
Plasma kynurenine levels are elevated in suicide attempters compared with nonattempters or healthy volunteers. Abbreviations: MDD, Major Depressive Disorder; *, p < 0.05.
3.3 Post-hoc analyses of the effects of other clinical and demographic factors on suicide attempt status
KYN levels did not correlate with age, educational level, smoking status, or HDRS-17 or BDI scores, but positively correlated with BMI and TRP in the total sample (see Table 2). Mean KYN levels were higher in males (1.60 ± 0.30 vs. 1.27 ± 0.33 μmol/L, t (59) = 3.95, p < 0.001). When the KYN-associated covariates (BMI, TRP, sex) were included in separate logistic regression models, only KYN was a significant predictor of suicide attempt status in all models (data not shown).
Since neither age nor smoking status correlated significantly with KYN levels (see Table 2), including them in the regression model would have a negligible additive effect on the association between KYN and suicide attempt status. However, both age (Shah, 2011) and smoking status (Hughes, 2008) have been associated with suicide risk, so we tested for main effects and interactions between each of these variables and KYN, with regard to suicide attempt. In this sample, age was not associated with attempts (Wald statistic = 0.669, df = 1, p = 0.414) and the KYN by age interaction term was not significant (Wald statistic = 0.464, df = 1, p = 0.496). Within depressed individuals, smoking status was not significantly different between attempters and nonattempters (see Table 1). However, when smoking and KYN levels were included in a regression model with all participants, both were associated with attempt (KYN: Wald statistic = 7.404, df = 1, p = 0.007; smoking status: Wald statistic = 6.6, df = 1, p = 0.010). The interaction term was not significant (Wald statistic = 0.875, df = 1, p = 0.350), indicating that KYN levels were associated with suicide attempt in both smokers and non-smokers.
3.4 Relationships between tryptophan and kynurenine
The KYN:TRP ratio estimates the rate of TRP metabolism. When entered as a predictor in a logistic regression model in the entire sample with suicide attempter status as outcome measure, the KYN:TRP ratio showed a strong trend toward predicting suicide attempt (χ2 (1) = 3.70, p = 0.056). Average TRP was higher in suicide attempters than in nonattempters (see Table 1). However, when included as covariate in a logistic regression with KYN as predictor, KYN levels predicted suicide attempt status (Wald statistic = 4.573, df = 1, p = 0.032) but TRP levels did not (Wald statistic = 0.414, df = 1, p = 0.520). Subgroup correlational analyses revealed that TRP correlated positively with KYN in the total sample and in all subgroups except among suicide attempters, where there was a clear lack of association (see Table 2).
3.4 Neopterin levels as an indicator of immune system activation
In correlation analyses, the KYN:TRP ratio correlated positively with neopterin levels, a marker of a cell-mediated immune response, in suicide attempters (rs = 0.55, p = 0.043) but there was only a nonsignificant trend for a positive correlation in depressed nonattempters (rs = 0.44, p = 0.09) and the association was again weaker in the controls (rs = 0.29, p = 0.11).
4. Discussion
4.1 Association of kynurenine with history of suicide attempts
This is the first report of an association between KYN levels and history of suicide attempts among individuals with MDD. Miller et al. (Miller, 2008) studied postmortem levels of KYN pathway components in schizophrenia, bipolar disorder, and unipolar depression, and found a significant relationship of increased nictonamide, a downstream metabolite of KYN, with suicide in the bipolar sample only. However, the sample size was only 8 subjects each in the bipolar and unipolar samples and 3 subjects in the schizophrenia sample. Altered levels of cytokines known to stimulate KYN have been found in postmortem brains of suicide victims (Tonelli, 2008), and in cerebrospinal fluid (CSF) (Lindqvist, 2009), and blood (Janelidze, 2011; Kim, 2008) of suicide attempters compared with depressed non-attempters (Janelidze, 2011; Kim, 2008) and normal controls (Janelidze, 2011; Kim, 2008; Lindqvist, 2009). Replicated findings were elevated levels of IL-6 (Janelidze, 2011; Lindqvist, 2009) and lower levels of IL-2 (Janelidze, 2011; Kim, 2008) in suicide attempters. Another marker of neuroinflammation, microgliosis, has also found to be associated with suicide attempters postmortem, irrespective of diagnosis (schizophrenia or affective disorders), compared with normal controls (Steiner, 2008). Additionally, suicide risk is elevated among patients being treated with cytokines for medical problems, as seen in individual case histories (Baron, 1993; Janssen, 1994); in a case series of patients with multiple sclerosis who developed suicidal ideation (n=11) or made suicide attempts (n=4), and whose suicidality remitted after withdrawal of the interferon (Fragoso, 2010); and in a prospective study of patients with hepatitis C who experienced increased suicidal ideation over 8 weeks (Dieperink, 2004). KYN may be involved in other types of violent behavior apart from suicide attempts, according to two different research groups who have observed increased KYN plasma levels in antisocial violent offenders (Tiihonen, 2001) and in alcoholics with a history of blacked-out violent impulsive behaviors (Vignau, 2010).
Since most (90%) patients were not on psychotropic medications at the time of enrollment and KYN predicted suicide attempt status equally well when patients on medications were excluded, the correlation of suicide attempt status with KYN levels does not appear to be an artifact of medication or medication withdrawal. Nor was the KYN effect attributable to the clinical or demographic factors examined. However, one possible clinical confounder not tested for was pyridoxine (vitamin B6) deficiency, which can inhibit the breakdown of KYN (Linkswiler, 1967).
Elevated KYN associated with suicide attempts might be just a marker for severity of mood `state'. However, KYN did not correlate with HDRS-17 or BDI scores; nor were MDD attempters distinguished from MDD nonattempters by depression severity on either scale. Moreover, depressed non-attempters did not differ from healthy volunteers with respect to KYN, providing evidence that elevated KYN levels also were not explained by presence of the MDD `trait'. Thus we hypothesize that elevated KYN may be a marker of suicide attempt risk, independent of depression severity, related to the diathesis for suicidal behavior (Mann, 1999) and not to the associated mood disorder, unless MDD with suicidal behavior comprises a distinct biological subtype of MDD. If true, it is possible that studies finding elevated KYN associated with depressed state did not control for the effects of suicide history.
4.2 Possible mechanisms of pathogenesis
Two mechanisms have been suggested through which the degradation of TRP to KYN might impact depression and suicidality (Wichers, 2005). (The relevant biochemical pathways are depicted schematically in Figure 2.) In one model, cytokine activation of IDO causes TRP depletion and thereby reduces 5-HT synthesis (Maes, 1993). This putative pathway has face value, since hypofunction of the 5-HT system has been associated with recurrent depression and independently with suicide attempts (reviewed in (Mann, 2003)). Lower peripheral levels of TRP also have been associated with suicidal behaviors in some (Almeida-Montes, 2000; Clark, 2003; Pfeffer, 1998) but not all (Roggenbach, 2007) studies of susceptible individuals.
Figure 2.
Hypothetical pathways whereby increased levels of plasma kynurenine (KYN) could mediate suicide attempts. We found that a history of suicide attempt(s) was associated with increased KYN rather than tryptophan (TRP) depletion, and evidence that increased KYN in suicide attempters may be due to cytokine-stimulated indoleamine 2,3-dioxygenase (IDO). In brain, KYN can give rise to either quinolinic acid (QA) or kynurenic acid (KA), which bind to N-methyl D-aspartate receptor (NMDAR) sites and act as agonist and antagonist, respectively. (TDO, tryptophan 2,3-dioxygenase; KMO, kynurenine 3-monooxygenase; KAT I&II, kynurenine aminotransferases I and II.)
As an alternative mechanism to TRP depletion, we posit that increased KYN and/or its downstream metabolites may more directly influence the brain. This hypothesis is also supported by a murine model linking a high KYN:TRP ratio with increased, not decreased, levels of 5-HT and its metabolic product 5-hydroxyindoleacetic acid (5-HIAA), and with decreased levels of KA in specific brain regions (Laugeray, 2011). Additionally, a study of interferon-α induced depression found that elevated peripheral KYN was associated with elevated CSF levels of KYN and its metabolites quinolinic acid (QA) and kynurenic acid (KA), while peripherally depleted TRP did not translate into lower CSF TRP concentrations (Raison, 2010).
KYN is known to move across the blood-brain barrier (Fukui, 1991), and can be metabolized in the brain through two different pathways. The major, rate-limiting pathway, in microglial cells, is via kynurenine 3-monooxygenase (KMO) to downstream product QA (Wonodi and Schwarcz, 2010). The secondary metabolic pathway, via kynurenine aminotransferases I and II (KAT) in astrocytes, leads to production of KA (Wonodi and Schwarcz, 2010). As QA and KA have opposing effects on N-methyl-D-aspartate (NMDA) receptors (Muller and Schwarz, 2007), the relative production of QA to KA has important functional consequences. For instance, in two independent studies of patients with hepatitis C treated with IFN-α, elevated CSF levels of KYN were associated with increases in CSF KA and QA, while a positive correlation was seen between plasma and CSF QA levels (Raison, 2010); and depression severity correlated positively with the KYN to KA plasma ratio (Wichers, 2005), suggesting an association of depression with a relatively greater production of QA. We note that, apart from increased IDO activity, decreased enzymatic degradation of KYN theoretically could also result in elevated KYN levels. The data obtained in this study did not include measurement of KA or QA levels, and thus additional studies are required to test for the relevance of these pathways in depression and suicidality.
Interestingly, KA also acts as an antagonist of the α7 nicotinic acetylcholine receptor (Hilmas, 2001). Thus KYN metabolites can influence glutamatergic (Muller and Schwarz, 2007), dopaminergic (Amori, 2009; Poeggeler, 2007), and cholinergic (Schwarcz and Pellicciari, 2002) tone. Elevated KA causes impairments of spatial working memory (Chess, 2007) and contextual learning (Chess, 2009) in animals and could plausibly play a role in executive functioning deficits associated with suicidal behavior (Keilp, 2001; Marzuk, 2005). Therefore, KYN metabolites may contribute to the aggression/impulsivity and neurocognitive deficits proposed as endophenotypes associated with suicidal behavior (Mann, 2009). A possible relationship between KYN and impulsivity is also suggested by two studies finding that KYN was strongly elevated (7-fold) in patients with a history of violent impulsive behaviors and alcohol-related blackouts (Vignau, 2010). Relationships between KYN, its metabolites, and behavioral phenotypes were not assessed in this study but should be tested in future studies.
A role for increased inflammation among suicide attempters was further suggested by subgroup differences in the KYN:TRP ratio. TRP and KYN were positively correlated in controls and depressed nonattempters, while among suicide attempters, TRP and KYN were uncorrelated (see Table 2). The relative levels of this substrate/metabolite pair are controlled in large part by the differences between Km values for the two main enzymes that degrade TRP (Hayaishi, 1976; Pfefferkorn, 1986; Ren, 1996; Uchida, 1992). We speculate that the positively correlated TRP and KYN levels were due to the activity of the default hepatic tryptophan 2,3-dioxygenase (TDO) pathway, wherein dietary intake of TRP generates proportional amounts of KYN; but that among suicide attempters, cytokine-mediated IDO activation, which results in lower relative levels of TRP, may have occurred sufficient to uncouple the TRP and KYN levels, but not enough to produce a negative correlation and TRP depletion. Other factors may also influence the KYN to TRP balance in this complex system, including parallel utilization of TRP for other processes such as protein biosynthesis, and catabolism of KYN by liver enzymes. Our hypothesis is consistent with the finding that only in the attempter subgroup did the KYN:TRP ratio correlate with neopterin, an independent indicator of cytokine activation of the Th1 inflammatory response (which induces IDO) that has been linked with depressive symptoms in chronic illness (Widner, 2002). It is noteworthy that this study utilized a sample of depressed patients who were otherwise medically healthy.
In animal models, increased KYN levels also have been shown to be triggered by glucocorticoid stimulation of TDO (Comings, 1995; Laugeray, 2010). In depressed humans, abnormalities of reactivity of the hypothalamic-pituitary-adrenal (HPA) axis have been associated with suicide risk, although there is some controversy over which direction the abnormality may manifest (Mann, 2006; McGirr). Thus, differences in corticotropin releasing factor (CRF) and cortisol among patients with a history of suicide attempt—information not available for this sample—may result in potential effects on the brain via TDO-mediated increases in KYN.
4.3 Limitations
Our discussion of putative mechanisms of KYN elevation in suicide attempters is largely theoretical, as this study did not measure inflammatory indices apart from KYN, TRP, and neopterin levels. Moreover, the measures used were peripheral, although KYN is known to cross the blood-brain barrier (Fukui, 1991). This pilot study should be replicated in a larger sample that would control for nutritional parameters, such as pyridoxine levels and TRP intake, and for relevant neuroinflammatory markers and neurotransmitters, including C-reactive protein, which would give more information about Th2 activation, interferon-γ, QA, KA, 5-HT, and cortisol levels. This study used logistic regression, a technique that is regularly used in case control studies, like this one, to test association between an exposure and an outcome, both of which occurred in the past. Its utility is that it allows for the inclusion of multiple predictor variables in order to tease out which ones are truly associated with the outcome measure. However, we note that on the basis of this study, KYN levels cannot be said to “predict” suicide risk, given the reversed temporal relationship between KYN measurement and suicide attempt status. Conclusions also cannot be drawn from this study of depressed, unmedicated individuals, concerning associations between KYN levels and suicide risk in other types of psychiatric or medical populations in which KYN may be elevated. Finally, we note that a number of post-hoc tests were performed which must be considered exploratory given the small sample size.
4.4 Conclusions
Results of this preliminary study suggest that inflammatory processes may be differentially activated in depressed individuals who are at risk for suicide, as higher plasma KYN concentrations were associated with a history of suicide attempt, correlations between KYN and TRP were markedly different in suicide attempters, and the KYN:TRP ratio correlated positively with neopterin in attempters. The association between KYN and suicide attempt status does not appear to be a function of depression severity, MDD trait, or TRP depletion. Future, larger studies of the role of KYN in suicide risk should include additional markers such as inflammatory cytokines, QA, and KA. Our findings also raise the possibility that pharmacologic manipulation of KYN levels might reduce suicide risk.
Acknowledgements
This work was supported by NIMH MH079033 and NARSAD Young Investigator Award (Sublette); MH074068 (Galfalvy); NARSAD Young Investigator Award and MH076049 (Grunebaum); The Moody Foundation (Oquendo); MH040695 and MH062185 (Mann); MH075891 and MH074891, NARSAD Independent Investigator Award, and American Foundation for Suicide Prevention (Postolache).
We thank Dr. Robert Schwarcz for his comments on the manuscript.
Footnotes
Conflicts of Interest: Dr. Sublette is the recipient from Unicity, International of a grant of fish oil supplements used in an unrelated study. In the past 3 years, Dr. Oquendo has received unrestricted educational grants from Astra Zeneca, Janssen, Bristol Myers Squibb, Pfizer, Eli Lilly, and Shire. Her family owns stock in Bristol Myers Squibb. Dr. Mann is the recipient of unrelated brain imaging grants from Novartis and GSK. The remaining authors have no conflicts of interest to report.
References
- Almeida-Montes LG, Valles-Sanchez V, Moreno-Aguilar J, Chavez-Balderas RA, Garcia-Marin JA, Cortes Sotres JF, Hheinze-Martin G. Relation of serum cholesterol, lipid, serotonin and tryptophan levels to severity of depression and to suicide attempts. J. Psychiatry Neurosci. 2000;25:371–7. [PMC free article] [PubMed] [Google Scholar]
- Amori L, Wu HQ, Marinozzi M, Pellicciari R, Guidetti P, Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron DA, Hardie T, Baron SH. Possible association of interleukin-2 treatment with depression and suicide. J. Am. Osteopath. Assoc. 1993;93:799–800. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav. Brain Res. 2009;201:325–31. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ. Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr. Bull. 2007;33:797–804. doi: 10.1093/schbul/sbl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB. Serum tryptophan ratio and suicidal behavior in adolescents: a prospective study. Psychiatry Res. 2003;119:199–204. doi: 10.1016/s0165-1781(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Dietz G, Sherman M, Forest GL. Sequence of human tryptophan 2,3-dioxygenase (TDO2): presence of a glucocorticoid response-like element composed of a GTT repeat and an intronic CCCCT repeat. Genomics. 1995;29:390–6. doi: 10.1006/geno.1995.9990. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor J, Freund G, Johnson R, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieperink E, Ho SB, Tetrick L, Thuras P, Dua K, Willenbring ML. Suicidal ideation during interferon-alpha2b and ribavirin treatment of patients with chronic hepatitis C. Gen. Hosp. Psychiatry. 2004;26:237–40. doi: 10.1016/j.genhosppsych.2004.01.003. [DOI] [PubMed] [Google Scholar]
- First M, Williams J, Spitzer R, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Publishing, Inc.; Washington D.C.: 1997. [Google Scholar]
- Fragoso YD, Frota ER, Lopes JS, Noal JS, Giacomo MC, Gomes S, Goncalves MV, da Gama PD, Finkelsztejn A. Severe depression, suicide attempts, and ideation during the use of interferon beta by patients with multiple sclerosis. Clin. Neuropharmacol. 2010;33:312–6. doi: 10.1097/WNF.0b013e3181f8d513. [DOI] [PubMed] [Google Scholar]
- Fu X, Zunich SM, O'Connor JC, Kavelaars A, Dantzer R, Kelley KW. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56:2007–17. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaishi O. Properties and function of indoleamine 2,3-dioxygenase. J Biochem. 1976;79:13P–21P. doi: 10.1093/oxfordjournals.jbchem.a131115. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J. Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Smoking and suicide: a brief overview. Drug Alcohol Depend. 2008;98:169–78. doi: 10.1016/j.drugalcdep.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain. Behav. Immun. 2011;25:335–9. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Janssen HL, Brouwer JT, van der Mast RC, Schalm SW. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. J. Hepatol. 1994;21:241–3. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Brodsky BS, Oquendo MA, Malone KM, Mann JJ. Neuropsychological dysfunction in depressed suicide attempters. Am. J. Psychiatry. 2001;158:735–41. doi: 10.1176/appi.ajp.158.5.735. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee SW, Kim SH, Shim SH, Han SW, Choi SH, Lee BH. Differences in cytokines between non-suicidal patients and suicidal patients in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:356–61. doi: 10.1016/j.pnpbp.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav. Brain Res. 2010;210:84–91. doi: 10.1016/j.bbr.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, Callebert J, Surget A, Belzung C, Barone PR. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: focus on individual differences. Pharmacol. Biochem. Behav. 2011;98:161–8. doi: 10.1016/j.pbb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Björkqvist M, Träskman-Bendz L, Brundin L. Interleukin-6 Is Elevated in the Cerebrospinal Fluid of Suicide Attempters and Related to Symptom Severity. Biol. Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Linkswiler H. Biochemical and physiological changes in vitamin B6 deficiency. Am. J. Clin. Nutr. 1967;20:547–61. doi: 10.1093/ajcn/20.6.547. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Scharpe S, Bosmans E, Suy E, De Meester I, Calabrese J, Cosyns P. Relationships between lower plasma L-tryptophan levels and immune-inflammatory variables in depression. Psychiatry Res. 1993;49:151–65. doi: 10.1016/0165-1781(93)90102-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Mann J. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–28. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Mann J, Currier D, Stanley B, Oquendo M, Amsel LV, Ellis SP. Can biological tests assist prediction of suicide in mood disorders? Int J Neuropsychopharmacol. 2006;9:465–74. doi: 10.1017/S1461145705005687. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Arango VA, Avenevoli S, Brent DA, Champagne FA, Clayton P, Currier D, Dougherty DM, Haghighi F, Hodge SE, Kleinman J, Lehner T, McMahon F, Moscicki EK, Oquendo MA, Pandey GN, Pearson J, Stanley B, Terwilliger J, Wenzel A. Candidate endophenotypes for genetic studies of suicidal behavior. Biol. Psychiatry. 2009;65:556–63. doi: 10.1016/j.biopsych.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. Am. J. Psychiatry. 1999;156:181–9. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Marzuk PM, Hartwell N, Leon AC, Portera L. Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr. Scand. 2005;112:294–301. doi: 10.1111/j.1600-0447.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- Mayersbach P, Fuchs D, Schennach H. Performance of a fully automated quantitative neopterin measurement assay in a routine voluntary blood donation setting. Clin. Chem. Lab. Med. 2010;48:373–7. doi: 10.1515/CCLM.2010.072. [DOI] [PubMed] [Google Scholar]
- McGirr A, Diaconu G, Berlim MT, Turecki G. Personal and family history of suicidal behaviour is associated with lower peripheral cortisol in depressed outpatients. J. Affect. Disord. doi: 10.1016/j.jad.2010.10.050. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Cwik M, Walkup J, Weis S. Alterations in kynurenine precursor and product levels in schizophrenia and bipolar disorder. Neurochem. Int. 2008;52:1297–303. doi: 10.1016/j.neuint.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–87. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J. Immunol. 2009;182:3202–12. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: utility and limitations of research instruments. In: First MB, editor. Standardized Evaluation in Clinical Practice. APPI Press; Washington, D.C.: 2003. pp. 103–30. [Google Scholar]
- Pfeffer CR, McBride PA, Anderson GM, Kakuma T, Fensterheim L, Khait V. Peripheral serotonin measures in prepubertal psychiatric inpatients and normal children: associations with suicidal behavior and its risk factors. Biol. Psychiatry. 1998;44:568–77. doi: 10.1016/s0006-3223(98)00020-1. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn ER, Rebhun S, Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J. Interferon Res. 1986;6:267–79. doi: 10.1089/jir.1986.6.267. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Rassoulpour A, Wu HQ, Guidetti P, Roberts RC, Schwarcz R. Dopamine receptor activation reveals a novel, kynurenate-sensitive component of striatal N-methyl-D-aspartate neurotoxicity. Neuroscience. 2007;148:188–97. doi: 10.1016/j.neuroscience.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Liu H, Licad E, Correia MA. Expression of rat liver tryptophan 2,3-dioxygenase in Escherichia coli: structural and functional characterization of the purified enzyme. Arch. Biochem. Biophys. 1996;333:96–102. doi: 10.1006/abbi.1996.0368. [DOI] [PubMed] [Google Scholar]
- Roggenbach J, Muller-Oerlinghausen B, Franke L, Uebelhack R, Blank S, Ahrens B. Peripheral serotonergic markers in acutely suicidal patients. 1. Comparison of serotonergic platelet measures between suicidal individuals, nonsuicidal patients with major depression and healthy subjects. J. Neural Transm. 2007;114:479–87. doi: 10.1007/s00702-006-0555-x. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J. Pharmacol. Exp. Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- Shah A. Suicide rates: age-associated trends and their correlates. Journal of Injury & Violence Research. 2012;4:79–86. doi: 10.5249/jivr.v4i2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008;42:151–7. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Virkkunen M, Rasanen P, Pennanen S, Sainio EL, Callaway J, Halonen P, Liesivuori J. Free L-tryptophan plasma levels in antisocial violent offenders. Psychopharmacology (Berl) 2001;157:395–400. doi: 10.1007/s002130100842. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Möller HJ, Chen HH, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatr. Scand. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Usami M, Bandow H, Harada I. Characteristics of substrates and inhibitors in binding to rat liver L-tryptophan 2,3-dioxygenase: a Fourier transform infrared and kinetic study. Biochim. Biophys. Acta. 1992;1121:153–9. doi: 10.1016/0167-4838(92)90348-h. [DOI] [PubMed] [Google Scholar]
- Vignau J, Soichot M, Imbenotte M, Jacquemont MC, Danel T, Vandamme M, Lhermitte M, Allorge D. Impact of tryptophan metabolism on the vulnerability to alcohol-related blackouts and violent impulsive behaviours. Alcohol Alcohol. 2010;45:79–88. doi: 10.1093/alcalc/agp044. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry. 2005;10:538–44. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain. Behav. Immun. 2002;16:590–5. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997;43:2424–6. [PubMed] [Google Scholar]
- Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr. Bull. 2010;36:211–8. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]