Abstract
Identifying well-tolerated, oral medications that enhance adult neurogenesis is of great clinical interest. In this issue of Cell Stem Cell, Wang et al. (2012), demonstrate that the diabetes medication metformin enhances spatial learning in mice by activating the atypical PKC/CBP pathway in adult neural stem cells.
For organs and tissues that maintain stem cells throughout life, one strategy for the treatment of injury or disease is to enhance the therapeutic potential of such endogenous stem cell populations. The adult hippocampus – a brain structure critical for learning and memory – harbors a population of neural stem cells (NSCs) that produce new neurons throughout life (Ming and Song, 2011), and this adult neurogenesis is important for certain forms of memory (Deng et al., 2010). Adult brain NSCs respond to a wide range of neurological disorders including stroke, seizure, and Alzheimer’s disease; thus, the prospect of enhancing their regenerative potential is tantalizing (Emsley et al., 2005). In this issue of Cell Stem Cell, Miller and colleagues (Wang et al., 2012) make advances in this translational direction with their study of metformin, a commonly used diabetes medication, describing its pro-neurogenic effects on NSCs and enhancement of one form of hippocampal-dependent memory in mice.
Metformin was first synthesized in the 1920s, however it was not until 1957 that its use for the treatment of diabetes was clinically appreciated. Metformin ameliorates high blood sugar without stimulating insulin secretion or causing low blood sugar levels, and its long history of efficacy and safety have made this small molecule drug the most commonly prescribed medication for type II diabetes worldwide. Though its therapeutic mechanisms have not been fully elucidated, metformin activates AMP-activated kinase (AMPK), which reduces the production of glucose in the liver. In hepatocytes, metformin-activated AMPK phosphorylates atypical protein kinase C (aPKC), which stimulates phosphorylation of CREB binding protein (CBP), resulting in decreased gene expression for hepatic gluconeogenesis (He et al., 2009, Fig. 1A).
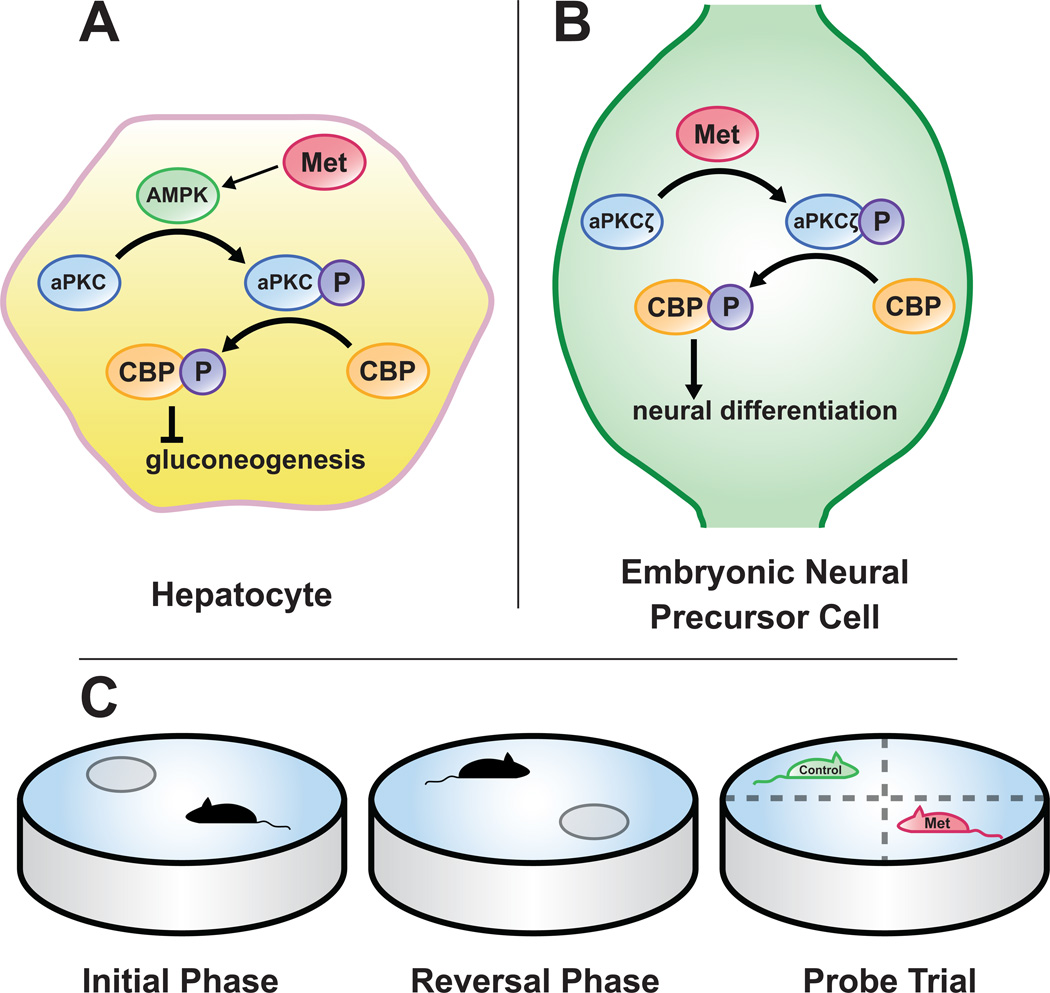
Figure 1. Metformin induces neurogenesis via CBP activation and enhances spatial memory.
Metformin is a commonly-prescribed oral medication for type II diabetes and activates AMP-kinase (AMPK). In hepatocytes, activation of AMPK results in phophorylation of activated protein kinase C (aPKC), which in turn phosphorylates CREB-binding protein (CBP), leading to decreased production of glucose from the liver (A). In embryonic precursor cells, metformin activates aPKC isoform ζ (aPKCζ) to enhance neuronal differentiation with CBP serving as a downstream effector (B). This occurs presumably through metformin’s effect on AMPK. Administration of metformin also results in improved spatial memory as tested in the Morris water maze. Mice were treated with metformin for 38 days during which time they were trained to find a submerged platform (C, initial phase). The platform was then moved to the opposite quadrant (C, reversal phase) and the mice were once again trained to find it. There were no differences among metformin-treated and control groups in these testing phases. Finally, the platform was removed and the amount of time mice spent within each quadrant was recorded (C, probe trial). Metformin-treated mice spent a proportionately larger amount of time in the quadrant that contained the platform during the reversal phase while control mice spent more time in the opposite quadrant, suggesting that metformin enhances the ability to update spatial memory.
Abbreviations: Met, metformin; P, phosphorylation.
CBP is a ubiquitously expressed histone acetyltransferase and transcriptional coactivator. Recently, Miller and colleagues studied the role of CBP in the developing murine cortex, showing that CBP haploinsufficiency results in cognitive deficits (Wang et al., 2010). Given that phosphorylation of CBP by aPKC isoform ζ (aPKCζ) is required for CBP-mediated differentiation of cortical precursors, the authors hypothesized that metformin might activate aPKCs in NSCs as well, thereby increasing neurogenesis.
In their report, Wang et al. first demonstrate that the aPKC-CBP pathway regulates neuronal differentiation from embryonic neural precursor cells. While shRNA knockdown of aPKCζ or aPKCι isoforms both reduced the number of βIII-tubulin-positive neurons, co-transfection of a plasmid encoding an activated form of CBP with a phosphomimic mutation (serine to aspartic acid) at the aPKC site rescued aPKCζ knockdown, but not aPKCι knockdown. Taken together, these data suggest that CBP is downstream of aPKCζ (Fig. 1B).
Next, the authors investigated whether metformin can activate the aPKC-CBP pathway in NSCs and promote neurogenesis. aPKC phosphorylation at threonine 403 is important for its kinase activity, and metformin treatment appeared to modestly increase this modification of aPKC in NSCs. More importantly, metformin increased the number of βIII-tubulin-positive cells in culture by up to 50% while decreasing the proportion of Pax6- and Sox2-positive precursor cells. This pro-neurogenic effect was blocked by aPKCζ/ι shRNAs as well as CBP siRNA. When administered to wild-type adult mice, metformin increased the number of neurons generated from NSCs in the dentate gyrus of the hippocampus; however, when administered to mice haploinsufficient for CBP, metformin did not stimulate neurogenesis, suggesting that this effect of metformin is CBP-dependent.
Intriguingly, Wang et al. show that metformin also improves the ability to update new spatial memories, a task related to hippocampal neurogenesis (Deng et al., 2010). Mice were given daily metformin injections for 38 days and were evaluated in the Morris water maze to assess their learning and spatial memory (Fig. 1C). Metformin-treated and control mice learned and remembered the initial position of a submerged platform in the water maze equally well. When the platform was moved to the opposite quadrant, both groups were also able to learn the second position with equal proficiency. The metformin-treated mice, however, were better able to remember the new platform position compared to controls and therefore spent more time in the quadrant with the new platform; this finding suggests that metformin enhanced the ability of mice to update their spatial memory. In mice treated with temozolomide, a potent DNA alkylating agent that kills dividing cells, neurogenesis was reduced, and metformin administration did not enhance spatial memory.
Although our understanding of the functional role of adult neurogenesis is limited, the notion of modulating this endogenous process with oral medications for the treatment of neurological disorders has garnered much interest. McKnight and colleagues recently screened 1000 small molecules for in vivo enhancement of murine dentate gyrus neurogenesis and identified one candidate, P7C3, with significant efficacy and favorable pharmacological properties (Pieper et al., 2010). It would be interesting to compare the therapeutic efficacy of metformin with that of P7C3 in animal models of Alzheimer’s disease, aging, and other neurological disorders. Compared to a novel compound such as P7C3, clinical trials with metformin would be relatively easy to establish, as this diabetes medication has been FDA-approved since 1995. Such repurposing of drugs has recently been announced as a major initiative by the National Center for Advancing Translational Sciences as it provides a speedy and relatively low-cost strategy for developing new therapies to treat human disease.
How else might metformin enhance spatial learning and have therapeutic potential for the treatment of cognitive problems? While the data in this report support a mechanism that involves hippocampal neurogenesis, it is also possible that other mechanisms contribute. For instance, activated AMPK increases glucose uptake by neurons (Amato and Man, 2011); thus, metformin may affect neural metabolism more globally. CBP is a histone acetyltransferase, and as such, its activation by metformin may facilitate memory formation via synapse plasticity in a manner similar to the clinically approved histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA). Intriguingly, SAHA rescues certain memory deficits observed in CBP haploinsufficient mice (Alarcón et al., 2004). Furthermore, HDAC inhibitors enhance neurogenesis from adult NSC populations (Hsieh et al., 2004). Perhaps metformin in combination with SAHA will have additive or even synergistic effects on hippocampal-dependent learning.
These studies by Wang et al. demonstrate that drug repurposing may be an effective strategy for translational research aimed at treating diseases of the brain. There are relatively few drugs developed specifically for neurological conditions and even fewer engineered for stem cell-based regenerative medicine. Perhaps it will be well worth opening up the medicine cabinet of the past to look for unexpected cellular effects and potential therapeutic benefits using the modern tools and lessons learned from stem cell biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcón JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Amato S, Man H-Y. Bioenergy sensing in the brain: the role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle. 2011;10:3452–3460. doi: 10.4161/cc.10.20.17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Publishing Group. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog. Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- He L, Sabet A, Djedjos S, Miller R, Sun X, Hussain MA, Radovick S, Wondisford FE. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci U.S.a. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G-L, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieper AA, Xie S, Capota E, Estill SJ, Zhong J, Long JM, Becker GL, Huntington P, Goldman SE, Shen C-H, et al. Discovery of a proneurogenic, neuroprotective chemical. Cell. 2010;142:39–51. doi: 10.1016/j.cell.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gallagher D, Devito LM, Cancino GI, Tsui D, He L, Keller GM, Frankland PW, Kaplan DR, Miller FD. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and spatial memory formation. Cell Stem Cell. 2012 doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Wang J, Weaver ICG, Gauthier-Fisher A, Wang H, He L, Yeomans J, Wondisford F, Kaplan DR, Miller FD. CBP histone acetyltransferase activity regulates embryonic neural differentiation in the normal and Rubinstein-Taybi syndrome brain. Developmental Cell. 2010;18:114–125. doi: 10.1016/j.devcel.2009.10.023. [DOI] [PubMed] [Google Scholar]