Summary
IL-12 is a potent antitumor cytokine that exhibits significant clinical toxicities following systemic administration. We hypothesized that intratumoral (i.t.) administration of IL-12 coformulated with the biodegradable polysaccharide chitosan could enhance the antitumor activity of IL-12 while limiting its systemic toxicity. Noninvasive imaging studies monitored local retention of IL-12, with and without chitosan coformulation, following i.t. injection. Antitumor efficacy of IL-12 alone and IL-12 coformulated with chitosan (chitosan/IL-12) was assessed in mice bearing established colorectal (MC32a) and pancreatic (Panc02) tumors. Additional studies involving depletion of immune cell subsets, tumor rechallenge, and CTL activity were designed to elucidate mechanisms of regression and tumor-specific immunity. Coformulation with chitosan increased local IL-12 retention from 1 to 2 days to 5 to 6 days. Weekly i.t. injections of IL-12 alone eradicated ≤ 10% of established MC32a and Panc02 tumors, while i.t. chitosan/IL-12 immunotherapy caused complete tumor regression in 80% to 100% of mice. Depletion of CD4+ or Gr-1+ cells had no impact on chitosan/IL-12-mediated tumor regression. However, CD8+ or NK cell depletion completely abrogated antitumor activity. I.t. chitosan/IL-12 immunotherapy generated systemic tumor-specific immunity, as > 80% of mice cured with i.t. chitosan/IL-12 immunotherapy were at least partially protected from tumor rechallenge. Furthermore, CTLs from spleens of cured mice lysed MC32a and gp70 peptide-loaded targets. Chitosan/IL-12 immunotherapy increased local retention of IL-12 in the tumor microenvironment, eradicated established, aggressive murine tumors, and generated systemic tumor-specific protective immunity. Chitosan/IL-12 is a well-tolerated, effective immunotherapy with considerable potential for clinical translation.
Keywords: IL-12, paracrine delivery, immunotherapy, chitosan, intratumoral
INTRODUCTION
Interleukin (IL)-12 is a TH1-polarizing, proinflammatory cytokine that has demonstrated profound antitumor efficacy in numerous preclinical models. The multiple antitumor mechanisms of action of IL-12 are well documented and include: (a) activation and expansion of CD8+ T cells and natural killer (NK) cells; (b) increased production of interferon (IFN)-γ; (c) suppression of angiogenesis; (d) enhanced trafficking of T cells; and (e) enhanced activation of dendritic cells.1,2 Unfortunately, schedule-dependent toxicities in early clinical trials, including 2 on-study deaths,3,4 together with disappointing clinical responses in large phase II studies,5,6 have mitigated early enthusiasm for the use of systemic IL-12 therapy.
The clinical failures of IL-12 may be due in part to the inability of intravenous (i.v.) or subcutaneous (s.c.) administered IL-12 to achieve biologically relevant concentrations of IL-12 in the tumor microenvironment at the maximum tolerated dose in humans. Consequently, local/paracrine IL-12 delivery strategies are now under investigation. The goal of these strategies is to maximize IL-12 levels in the tumor microenvironment while limiting systemic exposure to IL-12 and resultant toxicity.
Cell-based, virus-based, and plasmid-based paracrine IL-12 delivery strategies have been reviewed elsewhere.7,8 Each of these delivery strategies has shown promise preclinically and each has distinct advantages and disadvantages. Strategies focusing on the paracrine delivery of recombinant IL-12 protein are the most direct and quantifiable in terms of ensuring the accuracy and reproducibility of a delivered dose. Several sustained, local release platforms, including IL-12 encapsulation in polymeric microspheres and IL-12 incorporation into gels9,10 and liposomes,11,12 are currently being explored.
Here, we investigate the potential of chitosan solutions to enhance the intratumoral delivery and antitumor efficacy of IL-12. Our previously published research using granulocyte/macrophage-colony stimulating factor (GM-CSF) demonstrated for the first time that viscous chitosan solutions can control the dissemination and enhance the immunoadjuvant properties of a recombinant cytokine.13 Our more recent study demonstrated that the mucoadhesive properties of chitosan solution were effective in enhancing the intravesical delivery of recombinant IL-12.21 Chitosan is a nontoxic (LD50 > 16 g/kg),14 biodegradable, natural polysaccharide derived from the exoskeletons of crustaceans. Chitosan is a widely used biomaterial with an established safety profile in humans. It is used as a pharmaceutical excipient,15 a weight-loss supplement,16 and an experimental mucosal adjuvant,17 and in an FDA-approved hemostatic dressing.18 High-molecular-weight chitosan (> 100 kDa), by virtue of its long polymer chains, forms highly viscous solutions in mild aqueous solvents. Viscous solutions have been widely used to control release of drugs and macromolecules in vivo, as they hinder the diffusion and dissemination of these molecules following injection.19,20 Finally, unlike many other biomaterials, chitosan does not require the use of organic solvents for formulation and has been shown to maintain the bioactivity of labile cytokines.13,21
In the present studies, we expand upon our previous findings to demonstrate that i.t. injection of a simple coformulation of chitosan solution and recombinant IL-12 can eradicate established tumors and generate tumor-specific memory. The ability of chitosan to provide local, sustained delivery of IL-12 abrogates the need for daily systemic injections, which have been shown to be toxic in both preclinical22,23 and clinical studies.3,4 Furthermore, the simplicity, versatility, and biocompatibility of local chitosan/IL-12 immunotherapy make it a suitable candidate for clinical translation.
MATERIALS AND METHODS
Mice transgenic for human carcinoembryonic antigen (CEA) were obtained from a breeding pair generously provided by Dr. John Shively (The Beckman Research Institute of the City of Hope, City of Hope National Medical Center, Duarte, CA). The generation and characterization of CEA-transgenic (CEA.Tg) mice has been previously described.24 C57BL/6 mice were purchased from Taconic Farms (Germantown, NY). All mice were housed and maintained under pathogen-free conditions in microisolator cages. Animal care was in compliance with recommendations of The Guide for Care and Use of Laboratory Animals (National Research Council).
Murine colon carcinoma MC38 cells expressing human CEA (designated MC32a) were generated by retroviral transduction with CEA cDNA. The murine pancreatic adenocarcinoma cell line Panc02 was generously provided by Dr. Michael A. Hollingsworth (University of Nebraska Medical Center, Omaha, NE). The Panc02 cell line was established through induction of pancreatic tumors with 3-methyl-cholanthrene and serial s.c. transplantation in C57BL/6 mice.25 The EL-4 thymoma cell line was purchased from American Type Culture Collection (Manassas, VA).
Chitosan glutamate (Protosan G 213) was purchased from NovaMatrix (Sandvika, Norway). Recombinant murine IL-12 and GM-CSF were purchased from PeproTech (Rocky Hill, NJ). Recombinant murine IFN-γ was purchased from PBL Laboratories, Inc. (Piscataway, NJ).
Noninvasive Fluorescence Imaging of IL-12 Administrations
CEA.Tg mice were inoculated s.c. in the shaved flank with 3×105 MC32a cells. Seven days later, 2 µg IL-12, labeled with Alexa Fluor 660 (AF660) (Invitrogen; Carlsbad, CA) according to the manufacturer’s instructions, were injected i.t. in a total volume of 50 µL in DPBS (n = 5) or 1.5% (w/v) chitosan solution (n = 5). Fluorescence and photographic images of treated mice were acquired over an 8-day period with a Lumina In Vitro Imaging System (Caliper Life Sciences; Alameda, CA). Prior to all imaging sessions, anesthesia was induced in an enclosed chamber with 4% to 5% isoflurane delivered by a gas mixture of oxygen, nitrogen, and medical air. Once mice were unconscious and unresponsive to toe pinch, anesthesia was maintained with 1% to 2% isoflurane administered via nosecone. Following each imaging session, mice were recovered on a circulating warm-water pad. The fluorescence intensity of a region of interest drawn around the injection site was calculated at each time point with Living Image software (Caliper Life Sciences) and used as a surrogate for IL-12 concentration. Background/autofluorescence from noninjected control mice was subtracted. Fluorescence data for each mouse were normalized by the initial measurement, which was taken immediately after injection, for each mouse. A separate cohort of mice was injected i.t. with decreasing doses of AF660-IL-12 to determine that the lower limit of detection was between 16 and 31 ng.
Intratumoral Immunotherapy with Chitosan/Cytokine Formulations
CEA.Tg and C57BL/6 mice were inoculated s.c. in the shaved flank with 3×105 MC32a cells and 4×106 Panc02 cells, respectively. Beginning 7 days later, mice were given 2 to 3 weekly i.t. injections of one of the following: (a) DPBS; (b) 1.5% chitosan solution; (c) 1 µg IL-12; (d) 40 µg GM-CSF; (e) 25,000 IU IFN-γ; (f) 1 µg IL-12 in 1.5% chitosan solution (chitosan/IL-12); (g) 40 µg GM-CSF in 1.5% chitosan solution (chitosan/GM-CSF); or (h) 25,000 IU IFN-γ in 1.5% chitosan solution (chitosan/IFN-γ). All formulations were prepared immediately prior to treatment. Tumor volumes were measured twice/week.
Tumor Rechallenge of Cured Mice
CEA.Tg mice that were designated tumor-free for at least 8 weeks were rechallenged with 2–3×105 MC32a cells on the opposite flank. Age-matched naïve CEA.Tg mice were used as controls. Tumor volumes were measured twice/week. Partial protection was defined as a ≥ 50% reduction in tumor volume vs. naïve controls 2 weeks after rechallenge. Complete protection was defined as no measurable tumor mass 4 weeks after rechallenge
Cytotoxicity Assay
Spleens from CEA.Tg mice that were cured of primary tumors following i.t. chitosan/IL-12 immunotherapy and also rejected tumor rechallenge were harvested, mechanically disrupted with a syringe plunger, and passed through a 70-µm nylon mesh strainer (BD Biosciences; Bedford, MA). Erythrocytes were lysed with ACK lysing buffer (Cambrex Bio Science; Walkersville, MD). Unfractionated splenocytes from each mouse were divided into 4 upright T-25 flasks containing 10 µg/mL CEA526-533 (EAQNTTYL; H-2Db-restricted), 1 µg/mL p15E604-611 (KSPWFTTL; H-2Kb-restricted) or 5×105 irradiated (20,000 rad) MC32a cells for in vitro stimulation. After one week, lymphocytes were collected on a Histopaque (Sigma-Aldrich; St. Louis, MO) density gradient and quantified. Cytotoxic activity of recovered lymphocytes was assayed as previously described.13 Briefly, lymphocytes were coincubated with 51Cr-labeled MC32a or EL-4 target cells (5×103/well) pulsed with 1 µg/mL p15E604-611 or CEA526-533 for 4 hours in 96-well round bottom plates. HIV gag390-398 (SQVTNPANI; H-2Db) and β-gal96-103 (DAPIYTNV; H-2Kb) were used as control peptides. Radioactivity of supernatants was measured using a Cobra II gamma counter (Packard Instruments; Downers Grove, IL). The percentage of specific lysis was calculated as follows: % specific lysis = [(experimental cpm – spontaneous cpm)/(maximal cpm – spontaneous cpm)] × 100.
In Vivo Depletion of Immune Cells
For depletion of CD4+ and CD8+ cells, anti-mouse L3T4 (clone: GK1.5) and anti-mouse Lyt 2.2 (clone: 2.43), respectively, were administered i.p. 100 µg/day for 4 consecutive days, followed by 100 µg once/week. For depletion of NK cells and granulocytes, rabbit anti-mouse/rat asialo GM1 polyclonal antibody and anti-mouse Gr-1 monoclonal antibody (Cedarlane Laboratories; Hornby, ONT, Canada) were reconstituted with 2 mL and 1 mL of distilled water, respectively, and administered i.p. 25 µL once/week. Flow cytometry analysis of splenocytes revealed depletion of > 98% of CD4+, CD8+, NK1.1+, and Gr-1+ cells following injection of the appropriate depleting antibodies. A short-term (4-h) 51Cr-release cytotoxicity assay using YAC-1 cells as targets was used to verify splenic NK cell depletion following administrations of anti-asialo-GM1.
Statistical Analysis
Differences in average tumor volumes, IL-12 residence and CTL lysis between treatment groups were analyzed via the Mann-Whitney test. Analysis of overall survival following i.t. therapy was performed using Kaplan-Meier plots and the log-rank test. Analyses were conducted using the GraphPad Prism software package (Prism 4 for Windows, version 4.03, GraphPad Software, Inc.). Statistical significance was accepted if P < 0.05.
RESULTS
Chitosan Enhances Retention of IL-12 Following I.T. Administration
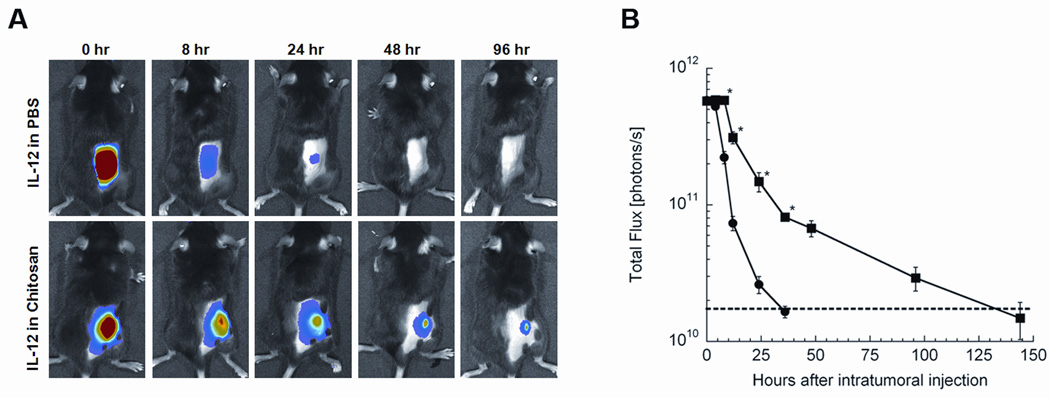
Recombinant IL-12 was labeled with AF660 to monitor via noninvasive imaging the effect of chitosan coformulation on local IL-12 retention. CEA.Tg mice bearing 7-day-old MC32a tumors were injected i.t. with 2 µg AF660-IL-12 in either DPBS or chitosan solution. When IL-12 was administered in DPBS, it quickly dissipated and became undetectable between 24 and 48 h (Fig. 1). In contrast, IL-12 formulated in a viscous chitosan solution was detectable for up to 6 days. Furthermore, because the limit of detection for AF660-IL-12 was determined to be between 16 and 31 ng (see Materials and Methods), it is likely that chitosan was able to maintain a biologically relevant concentration of IL-12 for more than one week.
FIGURE 1.
Chitosan solution enhances local retention of IL-12. CEA.Tg mice bearing 7-day-old MC32a tumors on their flanks were shaved and given a single i.t. injection of 2 µg AF660-IL-12 in either DPBS or 1.5% (w/v) chitosan solution. Photographic and fluorescence images were acquired over an 8-day period. (A) representative photographic and fluorescence overlay images as a function of time. (B) average fluorescence signal in photon/s from mice receiving AF660-IL-12 in either DPBS (●) or chitosan solution (■) as a function of time after administration. Data are represented as mean ± SEM for 5 mice. Dashed line represents average background fluorescence from mice prior to AF660-IL-12 administration. * indicates P < 0.05 vs. DPBS.
Chitosan/IL-12 Eradicates Established, Poorly Immunogenic Tumors
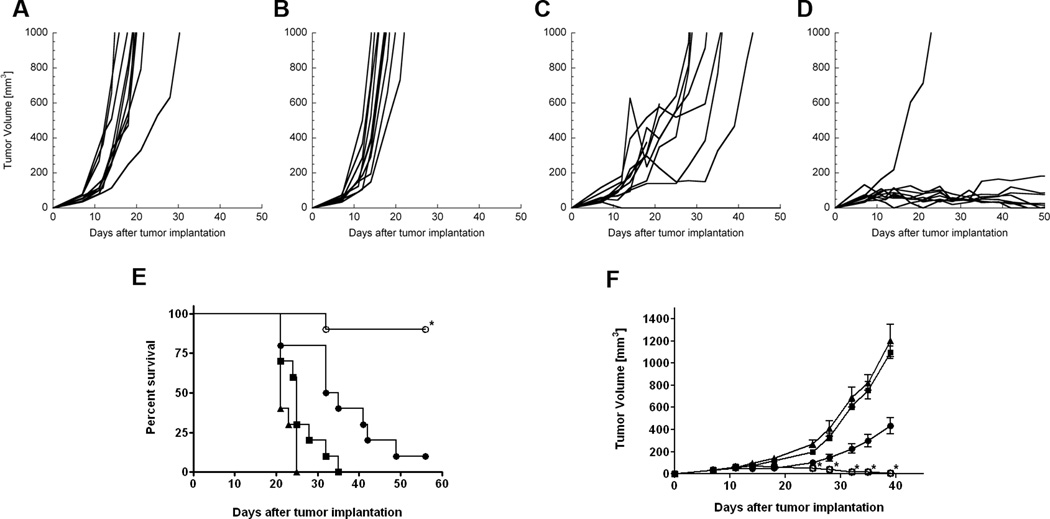
CEA.Tg mice bearing 7-day-old MC32a tumors were given 3 weekly i.t. injections of DPBS (control), IL-12 (1 µg), chitosan solution, or chitosan/IL-12 (1 µg). Treatment with chitosan alone had no impact on tumor growth (Figs. 2A, B). Intratumoral injections of IL-12 alone delayed tumor growth; however, 9 of 10 mice developed progressive disease (Fig. 2C). In contrast, i.t. injections of chitosan/IL-12 led to complete tumor regression in 9 of 10 mice (Fig. 2D). Measurable masses in the chitosan/IL-12 group after 40 days were attributed to residual chitosan deposits.
FIGURE 2.
Antitumor efficacy of chitosan/IL-12. CEA.Tg mice were inoculated with MC32a tumors on day 0 and treated i.t. on days 7, 14, and 21. Individual tumor growth curves for mice treated with (A) PBS, (B) chitosan solution alone, (C) 1 µg IL-12 alone, or (D) 1 µg chitosan/IL-12. Chitosan/IL-12-treated tumors were significantly smaller than IL-12 treated tumors (P<0.05 from day 14 to 32); (E) overall survival of mice receiving i.t. immunotherapy with DPBS (■), chitosan (▲), 1 µg IL-12 (●), or 1 µg chitosan/IL-12 (○). The median survival of chitosan/IL-12-treated mice is significantly greater than IL-12-treated mice (P=0.0004; log-rank test). Figures represent a compilation of 2 separate experiments. (F) C57BL/6 mice were inoculated with Panc02 tumors on day 0 and treated i.t. on days 10 and 17 with DPBS (■), chitosan (▲), 1 µg IL-12 (●), or 1 µg chitosan/IL-12 (○). Each data point represents mean tumor volume ± SEM from 5 mice. * indicates P < 0.05 vs. IL-12.
Overall survival curves revealed that treatment with IL-12 alone significantly improved median survival vs. controls (P < 0.05; log-rank test) and led to one long-term tumor regression (Fig. 2E). Chitosan/IL-12 immunotherapy further enhanced survival compared to IL-12 alone (P < 0.05; log-rank test) and led to long-term tumor regression in 90% of treated mice (Fig. 2E).
The potent antitumor effects of chitosan/IL-12 were not limited to MC32a tumors, as 100% of s.c.-implanted pancreatic tumors (Panc02) were eradicated following i.t. chitosan/IL-12 immunotherapy (Fig. 2F). IL-12 alone was found to delay the growth of Panc02 tumors, but could not completely eradicate them. Notably, in the slower-growing Panc02 model, only 2 i.t. injections were required to effect tumor regression.
Tumor Regression from I.T. Chitosan/Cytokine Immunotherapy Is Specific to IL-12
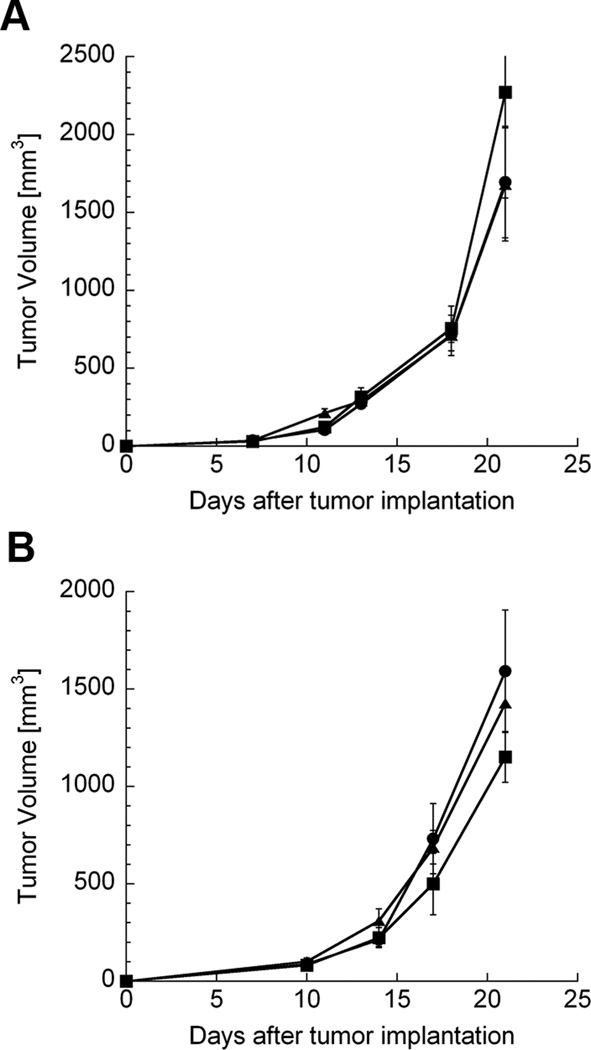
Both GM-CSF and IFN-γ have demonstrated significant potential for use in cancer vaccines and immunotherapies. In addition, our previous data demonstrate that chitosan coformulation can enhance the immunomodulatory activity of GM-CSF.13 Chitosan/GM-CSF and chitosan/IFN-γ treatments were administered to MC32a-bearing mice at the schedule described above. Doses of GM-CSF and IFN-γ which elicited maximum biological activity in vivo according to our previous studies13,26 were selected. Neither GM-CSF (40 µg) nor chitosan/GM-CSF (40 µg) was able to delay the growth of MC32a tumors (Fig. 3A). Similarly, neither IFN-γ (25,000 IU) nor chitosan/IFN-γ (25,000 IU) had any appreciable impact on tumor growth (Fig. 3B). The median survival times of chitosan/GM-CSF-treated or chitosan/IFN-γ-treated mice were not different from controls (p>0.1) (data not shown).
FIGURE 3.
Lack of antitumor activity following i.t. immunotherapy with other cytokines. CEA.Tg mice were inoculated with MC32a tumors on day 0. (A) mice were treated on days 7, 14, and 21 with PBS (●), 40 µg GM-CSF alone (■), or 40 µg chitosan/GM-CSF (▲). (B) mice were treated on days 7, 14, and 21 with PBS (●), 25,000 IU IFN-γ alone (■), or 25,000 IU chitosan/IFN-γ (▲). Each data point represents mean tumor volume ± SEM from 5 mice. P > 0.05 for all treatments vs. controls (PBS).
Intratumoral Chitosan/IL-12 Immunotherapy Confers Protection from Distal Tumor Rechallenge
Mice that had undergone complete tumor regression following i.t. immunotherapy with chitosan/IL-12 were rechallenged with tumor on the opposite flank. Tumor-naïve mice were used as controls. Of the 17 mice rechallenged in 3 separate experiments, 8 (47%) were completely protected and 6 (35%) were partially protected (Table 1). A closer look at the results from these studies suggests that increasing the dose of tumor cells in the rechallenge from 200,000 to 300,000 reduced the murine immune system’s ability to control tumor growth, as did increasing the time from immunotherapy to rechallenge. Nevertheless, 14 of 17 mice initially cured with i.t. chitosan/IL-12 demonstrated some level of protection from tumor rechallenge.
TABLE 1.
Protection from tumor rechallenge following complete tumor eradication with i.t. chitosan/IL-12 immunotherapy
| Experiment | Number of mice |
Day of rechallenge |
Dose of rechallenge |
Partial protectiona | Complete protectionb |
|---|---|---|---|---|---|
| 1 | 8 | 56 | 200,000 | 3/8 (37.5%) | 5/8 (62.5%) |
| 2 | 5 | 56 | 300,000 | 2/5 (40%) | 2/5 (40%) |
| 3 | 4 | 126 | 300,000 | 1/4 (25%) | 1/4 (25%) |
| TOTAL | 6/17 (35%) | 8/17 (47%) | |||
NOTE: Mice experiencing complete regression of s.c. MC32a tumors following i.t. chitosan/IL-12 immunotherapy were rechallenged with either 200,000 or 300,000 MC32a cells in the opposite flank either 56 or 126 days after original tumor inoculation. Age-matched naïve mice (n = 5) challenged with the same number of cells served as controls.
Partial protection = ≥ 50% reduction in tumor volume vs. naïve controls after 2 weeks.
Complete protection = no measurable tumor mass 4 weeks after rechallenge.
Chitosan/IL-12 Immunotherapy Elicits Tumor-Specific Immune Responses
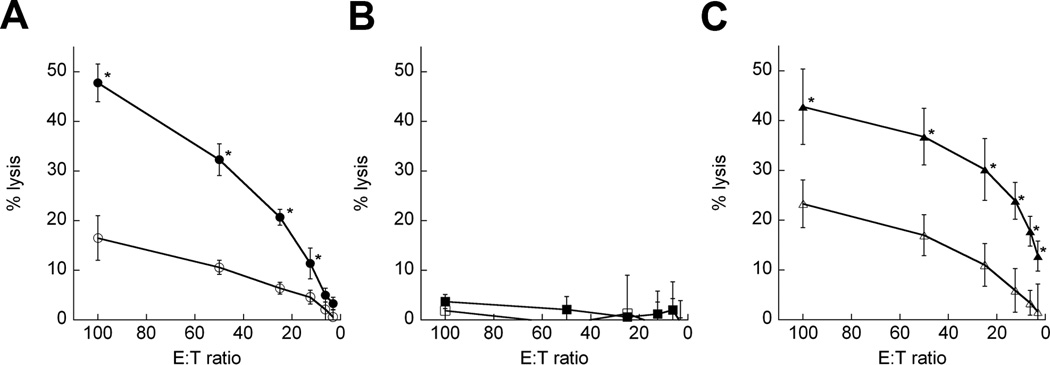
To determine the specificity of the adaptive immune response generated by chitosan/IL-12 immunotherapy, we assessed CTL activity of splenocytes from mice that resisted tumor challenge. CTLs were found to recognize and lyse MC32a targets at levels of up to 50% (Fig. 4A). Although CEA is overexpressed in numerous human tumors as well as the MC32a model, CEA peptide-pulsed targets were not lysed (Fig. 4B). Substantial CTL activity (approximately 45% lysis) was found against targets pulsed with an endogenous retroviral epitope of gp70 (Fig. 4C). Others have found that gp70 is a CTL-immunodominant epitope that is commonly overexpressed on mouse tumors such as MC32a.27
FIGURE 4.
Tumor-specific CTL activity following i.t. immunotherapy with chitosan/IL-12. Splenocytes from mice that were cured of primary tumors following i.t. chitosan/IL-12 immunotherapy and also resisted tumor rechallenge were stimulated in vitro for one week and tested for CTL activity. (A) splenocytes were stimulated with irradiated MC32a cells and measured for CTL activity against MC32a targets (●) or EL-4 targets (○). (B) splenocytes were stimulated with 10 µg/mL CEA526-533 and measured for CTL activity against CEA526-533-pulsed EL-4 targets (■) or HIV gag390-398-pulsed EL-4 targets (□). (C) splenocytes were stimulated with 1 µg/mL p15E604-611 (gp70 peptide) and measured for CTL activity against p15E604-611-pulsed EL-4 targets (▲) or β-gal96-103-pulsed EL-4 targets (Δ). Each data point represents mean percent lysis ± SEM from 4 mice. * indicates P < 0.05 vs. controls.
CD8+ T Cells and NK Cells Are Responsible for Chitosan/IL-12-Mediated Tumor Regression
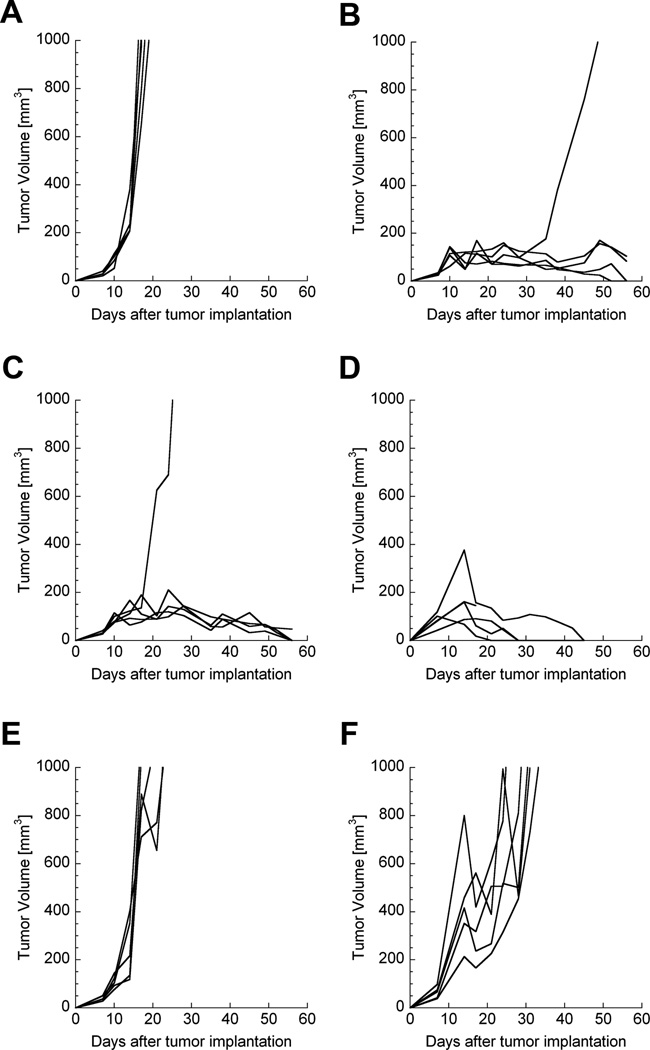
In order to understand which immune cells were essential effectors during chitosan/IL-12-mediated MC32a tumor regression, groups of CEA.Tg mice were individually depleted of immune cell subsets prior to tumor implantation and during i.t. immunotherapy. Similar to previous results, 4 of 5 immunologically intact mice experienced complete tumor regression following i.t. chitosan/IL-12 immunotherapy (Fig. 5B). Mice depleted of CD4+ or Gr-1+ cells also demonstrated complete tumor regression in 4 of 5 and 4 of 4 mice, respectively (Figs. 5C, D). In contrast, CD8+ cell depletion completely abrogated the antitumor activity of chitosan/IL-12 (Fig. 5E). Removal of NK cells allowed for a modest delay in tumor growth, but 5 of 5 mice developed progressive disease (Fig. 5F).
FIGURE 5.
Immune cell depletion during i.t. chitosan/IL-12 immunotherapy. One week prior to tumor inoculation and throughout the course of the experiment, groups of mice were administered specific antibodies to deplete immune cell subsets. CEA.Tg mice (n = 5) were inoculated with 3 × 105 cells in the flank on day 0. On days 7, 14, and 21, mice were treated i.t. with 1 µg chitosan/IL-12, as before. Figure shows tumor growth curves of individual mice: (A) no immune cell depletion (untreated); (B) no immune cell depletion; (C) depletion of CD4+ cells; (D) depletion of Gr-1+ cells; (E) depletion of CD8+ cells; (F) depletion of NK cells. CD4+ cell depletion and Gr-1+ depletion had no significant impact on chitosan/IL-12-mediated tumor regression (P > 0.05 vs. tumors from treated, intact mice at day 28). CD8+ cell and NK cell depletion abrogated the antitumor effects of chitosan/IL-12 (P < 0.05 vs. tumors from treated, intact mice at day 28).
DISCUSSION
The lack of an effective, translatable strategy for the paracrine delivery of cytokines, including IL-12, has limited the clinical potential of cytokine-based immunotherapies. Cell-based, virus-based, and plasmid-based IL-12 delivery strategies have all shown promise preclinically, but each faces unique obstacles. Recombinant IL-12 protein-based delivery strategies are the most direct and quantifiable approaches in terms of ensuring the accuracy and reproducibility of locally delivered IL-12. Several protein-based delivery strategies, such as IL-12 encapsulation in polymeric microspheres and IL-12 incorporation into gels9,10 and liposomes,11 are designed to maximize IL-12 levels in the tumor microenvironment while limiting systemic exposure. Most notably, several studies by Egilmez et al have demonstrated that encapsulation of IL-12 in polylactic acid (PLA) microspheres and polycaprolactone:PLA microspheres can control murine28 and human tumors29,30 following i.t. immunotherapy. Specifically, IL-12-loaded PLA microspheres were found to eradicate 70% of Line-1 tumors and 80% of CT26 tumors.30 However, others have found that the same immunotherapy regimen could not prevent the growth of B16 melanoma31 or MT-901 mammary carcinomas.32 It is not known whether these differences in efficacy are due to methodological variations, differences in tumor models, or inherent limitations of IL-12-loaded microspheres. Another drawback of IL-12-loaded microspheres is the need to use organic solvents during formulation, which can denature IL-12 immediately or adversely affect long-term storage if the solvents are not completely removed. In fact, over 80% of the bioactivity of IL-12 was lost when PLA/IL-12 microspheres were stored for 3 weeks.33 When loaded into liposomes, IL-12 has shown a sustained release and antitumor activity against tumor xenografts.11 In addition, previous studies have shown that IL-12 can be encapsulated in liposomes for potential use as a vaccine adjuvant.12,34 IL-12 has also been incorporated into biocompatible gels for either slow systemic release10 or paracrine enhancement of cancer vaccines.9 To our knowledge, none of these protein-based delivery platforms has been evaluated in clinical studies.
Here, we utilize a straightforward technology whereby recombinant IL-12 is admixed with chitosan under mild, aqueous conditions. Chitosan/IL-12 coformulations offer several advantages. Chitosan is inexpensive and can be reproducibly manufactured under cGMP conditions. Preparation of chitosan/IL-12 requires neither harsh organic solvents nor sonication that could denature IL-12. Chitosan and IL-12 can be admixed at bedside immediately prior to administration, eliminating the need for long-term storage. Finally, chitosan has an excellent safety record in humans and is readily digested by lysozyme, released by polymorphonuclear neutrophils and macrophages, to yield glucosamine.
This is the first study to demonstrate enhanced retention of IL-12 in the tumor microenvironment following i.t. administration with a delivery system. Chitosan increases the retention of IL-12 in the tumor from 1 to 2 days to 5 to 6 days (Fig. 1B). This is probably an underestimate of IL-12 retention since the lower limit of detection—between 16 and 31 ng (see Materials and Methods)—is far greater than the limit of IL-12 bioactivity. Furthermore, chitosan increases total IL-12 exposure in the tumor microenvironment by approximately 3-fold, as determined by integrating the area under the curve (Fig. 1B). Once again, this is likely an underestimate, as the imaging system becomes saturated at high levels (≥ 1 µg).
Interestingly, despite the enhanced local retention of IL-12, our recently published paper demonstrated that s.c. injections of IL-12 and chitosan/IL-12 resulted in similar serum levels of IL-12 and IFN-γ.21 Taken together, these findings imply that the majority of IL-12 is released from chitosan in < 24 h, while a significant and biologically relevant concentration of IL-12 remains at the injection site for at least 6 days. IL-12 in circulation may complement local IL-12 by expanding CD8+ T-cell and NK-cell populations35,36 and by enhancing the trafficking and migration of NK37 and TH1 cells.38 In preliminary studies, we found that i.t. administration of chitosan/IL-12 may activate splenic NK and CD8+ cells, as demonstrated by increases in size (forward scatter) and granularity (side scatter) during flow cytometry analyses (unpublished data).
Although a significant amount of IL-12 reaches the blood stream following i.t. chitosan/IL-12 administration, it is unlikely that the severe IL-12-related toxicities seen in early trials would be duplicated at the schedule reported here. In humans, IL-12-related toxicities have been associated more with daily administration than with a specific dose level. In fact, weekly or twice-weekly systemic administrations of IL-12 at the maximum tolerated dose or higher have been well tolerated.39,40 Therefore, it is reasonable to infer that weekly i.t. immunotherapy with chitosan/IL-12 would be well tolerated. It is important to note that no evidence of toxicity, such as ruffled fur, hunched habitus, or lethargy, was noted in any mouse receiving i.t. chitosan/IL-12. Additional toxicology studies to quantify organ weights, serum chemistry, and hematological parameters are planned.
Regarding antitumor activity, chitosan/IL-12 is at least as effective as any IL-12-based local immunotherapy published to date. When given alone, IL-12 had marginal impact against established, poorly immunogenic MC32a and Panc02 tumors (Fig. 2). However, coformulations of chitosan/IL-12 cured 80% to 100% of mice with established MC32a and Panc02 tumors. In fact, only 2 i.t. treatments with chitosan/IL-12 were required to achieve 100% eradication of the Panc02 line, which is less aggressive than the MC32a line. This complete tumor regression appears to be unique to chitosan/IL-12, as coformulations of chitosan with other cytokines such as GM-CSF and IFN-γ were totally ineffective (Fig. 3).
Perhaps more clinically significant than eradication of primary tumors is the finding that chitosan/IL-12 immunotherapy appears to protect cured mice from tumor recurrence, as determined in tumor rechallenge studies (Table 1). These findings agree with previous reports which show that IL-12-based therapies can generate long-term protection in numerous animal models.41,42 It should be noted that tumor rechallenge is only an approximation of the ability of an immunotherapy to protect from tumor recurrence or metastasis. Table 1 demonstrates that manipulation of the timing and dose of tumor rechallenge can alter interpretations of the level of protection. Furthermore, an assault with hundreds of thousands of tumor cells is not representative of tumor recurrence in humans. Therefore, future studies of the ability of i.t. chitosan/IL-12 to confer protection from metastasis in the neoadjuvant setting must employ a more clinically relevant model.
The high frequency of tumor rejection in our rechallenge studies correlated with the robust CTL activity measured in cured mice (Fig. 4). Together, these data show that i.t. chitosan/IL-12 is capable of generating systemic, tumor-specific immunity in the absence of traditional vaccination. In vitro-stimulated splenocytes from cured mice reacted strongly to an epitope of gp70, which is an endogenous murine retroviral envelope protein overexpressed on numerous murine tumors.27 This demonstrates that i.t. chitosan/IL-12 immunotherapy can make use of antigen from host tumor cells to create an adaptive immune response and that targeting specific tumor antigens may not be a prerequisite for effective immunotherapy.
CD8+ cells and NK cells were revealed as critical immune cell subsets during chitosan/IL-12-mediated tumor regression (Fig. 5). The importance of these particular immune cell subsets in IL-12-based immunotherapies has also been shown by others.43 Our finding on the nonessential role of CD4+ cells is in agreement with some reports44 but not others.42,45 Given the ability of IL-12 to induce a strong TH1 cytokine cascade, including the production of massive amounts of IFN-γ by NK and CD8+ cells, additional assistance from CD4+ helper cells does not appear to be necessary.
Finally, many cancer vaccines have been shown to induce robust tumor-specific T-cell responses. However, these T-cell responses are not always successful in controlling tumor growth because T cells have difficulty infiltrating the tumor or are inactivated by the immunosuppressive tumor microenvironment.46 I.t. injections of chitosan/IL-12 may disrupt the restrictive tumor architecture and encourage infiltration by both innate and adaptive immune cells. Furthermore, a high local concentration of IL-12 may reverse the action of, or eliminate, immunosuppressive cells such as tumor-associated macrophages47 and regulatory T cells.48
In sum, we have demonstrated that i.t. chitosan/IL-12 immunotherapy can (a) increase local retention of IL-12 in the tumor microenvironment, (b) eradicate aggressive murine tumors, and (c) generate systemic tumor-specific immunity capable of inhibiting tumor recurrence. These results, together with a favorable weekly administration schedule, as well as its simplicity of formulation, form the rationale for clinical investigation of i.t. chitosan/IL-12 immunotherapy for the management of solid tumors.
Acknowledgements
The authors thank Alexander Ng, Garland Davis, Bertina Gibbs, LaJuan Chase, and Curtis Randolph for their superior technical assistance, and Bonnie L. Casey for editorial assistance in the production of this manuscript.
REFERENCES
- 1.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 2.Del Vecchio M, Bajetta E, Canova S, et al. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 3.Leonard JP, Sherman ML, Fisher GL, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood. 1997;90:2541–2548. [PubMed] [Google Scholar]
- 4.Cohen J. IL-12 deaths: explanation and a puzzle. Science. 1995;270:908. doi: 10.1126/science.270.5238.908a. [DOI] [PubMed] [Google Scholar]
- 5.Hurteau JA, Blessing JA, DeCesare SL, et al. Evaluation of recombinant human interleukin-12 in patients with recurrent or refractory ovarian cancer: a gynecologic oncology group study. Gynecol Oncol. 2001;82:7–10. doi: 10.1006/gyno.2001.6255. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Rakhit A, Thompson JA, et al. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha 2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2001;21:257–263. doi: 10.1089/107999001750169934. [DOI] [PubMed] [Google Scholar]
- 7.Salem ML, Gillanders WE, Kadima AN, et al. Review: novel nonviral delivery approaches for interleukin-12 protein and gene systems: curbing toxicity and enhancing adjuvant activity. J Interferon Cytokine Res. 2006;26:593–608. doi: 10.1089/jir.2006.26.593. [DOI] [PubMed] [Google Scholar]
- 8.Sangro B, Melero I, Qian C, et al. Gene therapy of cancer based on interleukin 12. Curr Gene Ther. 2005;5:573–581. doi: 10.2174/156652305774964712. [DOI] [PubMed] [Google Scholar]
- 9.Salem ML, Kadima AN, Zhou Y, et al. Paracrine release of IL-12 stimulates IFN-gamma production and dramatically enhances the antigen-specific T cell response after vaccination with a novel peptide-based cancer vaccine. J Immunol. 2004;172:5159–5167. doi: 10.4049/jimmunol.172.9.5159. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Sakaguchi T, Kanda T, et al. Delivery of interleukin-12 in gelatin hydrogels effectively suppresses development of transplanted colonal carcinoma in mice. Cancer Chemother Pharmacol. 2003;51:53–57. doi: 10.1007/s00280-002-0547-y. [DOI] [PubMed] [Google Scholar]
- 11.Simpson-Abelson MR, Purohit VS, Pang WM, et al. IL-12 delivered intratumorally by multilamellar liposomes reactivates memory T cells in human tumor microenvironments. Clin Immunol. 2009;132:71–82. doi: 10.1016/j.clim.2009.03.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baca-Estrada ME, Foldvari M, Ewen C, et al. Effects of IL-12 on immune responses induced by transcutaneous immunization with antigens formulated in a novel lipid-based biphasic delivery system. Vaccine. 2000;18:1847–1854. doi: 10.1016/s0264-410x(99)00379-5. [DOI] [PubMed] [Google Scholar]
- 13.Zaharoff DA, Rogers CJ, Hance KW, et al. Chitosan solution enhances the immunoadjuvant properties of GM-CSF. Vaccine. 2007;25:8673–8686. doi: 10.1016/j.vaccine.2007.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai K, Kinumaki T, Fujita T. Toxicity of chitosan. Bull Tokai Reg Fish Lab. 1968;43:89–94. [Google Scholar]
- 15.Singla AK, Chawla M. Chitosan: some pharmaceutical and biological aspects--an update. J Pharm Pharmacol. 2001;53:1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- 16.Mhurchu CN, Dunshea-Mooij C, Bennett D, et al. Effect of chitosan on weight loss in overweight and obese individuals: a systematic review of randomized controlled trials. Obes Rev. 2005;6:35–42. doi: 10.1111/j.1467-789X.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 17.McNeela EA, Jabbal-Gill I, Illum L, et al. Intranasal immunization with genetically detoxified diphtheria toxin induces T cell responses in humans: enhancement of Th2 responses and toxin-neutralizing antibodies by formulation with chitosan. Vaccine. 2004;22:909–914. doi: 10.1016/j.vaccine.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Wedmore I, McManus JG, Pusateri AE, et al. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Trauma. 2006;60:655–658. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 19.Zaharoff DA, Rogers CJ, Hance KW, et al. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25:2085–2094. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKenzie IZ, Burnet FR, Embrey MP. In vitro studies of the characteristics of the release of prostaglandins from viscous solutions. Br J Obstet Gynaecol. 1980;87:292–295. doi: 10.1111/j.1471-0528.1980.tb04542.x. [DOI] [PubMed] [Google Scholar]
- 21.Zaharoff DA, Hoffman BS, Hooper HB, et al. Intravesical Immunotherapy of Superficial Bladder Cancer with Chitosan/Interleukin-12. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orange JS, Salazar-Mather TP, Opal SM, et al. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman HL, Swartout BG, Horig H, et al. Combination interleukin-2 and interleukin-12 induces severe gastrointestinal toxicity and epithelial cell apoptosis in mice. Cytokine. 2002;17:43–52. doi: 10.1006/cyto.2001.0986. [DOI] [PubMed] [Google Scholar]
- 24.Eades-Perner AM, van der Putten H, Hirth A, et al. Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res. 1994;54:4169–4176. [PubMed] [Google Scholar]
- 25.Corbett TH, Roberts BJ, Leopold WR, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer Res. 1984;44:717–726. [PubMed] [Google Scholar]
- 26.Zeytin H, Reali E, Zaharoff DA, et al. Targeted delivery of murine IFN-gamma using a recombinant fowlpox virus: NK cell recruitment to regional lymph nodes and priming of tumor-specific host immunity. J Interferon Cytokine Res. 2008;28:73–87. doi: 10.1089/jir.2007.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang JC, Perry-Lalley D. The envelope protein of an endogenous murine retrovirus is a tumor-associated T-cell antigen for multiple murine tumors. J Immunother. 2000;23:177–183. doi: 10.1097/00002371-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Egilmez NK, Jong YS, Sabel MS, et al. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60:3832–3837. [PubMed] [Google Scholar]
- 29.Egilmez NK, Jong YS, Hess SD, et al. Cytokines delivered by biodegradable microspheres promote effective suppression of human tumors by human peripheral blood lymphocytes in the SCID-Winn model. J Immunother. 2000;23:190–195. doi: 10.1097/00002371-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Kuriakose MA, Chen FA, Egilmez NK, et al. Interleukin-12 delivered by biodegradable microspheres promotes the antitumor activity of human peripheral blood lymphocytes in a human head and neck tumor xenograft/SCID mouse model. Head Neck. 2000;22:57–63. doi: 10.1002/(sici)1097-0347(200001)22:1<57::aid-hed9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 31.Arora A, Su G, Mathiowitz E, et al. Neoadjuvant intratumoral cytokine-loaded microspheres are superior to postoperative autologous cellular vaccines in generating systemic anti-tumor immunity. J Surg Oncol. 2006;94:403–412. doi: 10.1002/jso.20572. [DOI] [PubMed] [Google Scholar]
- 32.Sabel MS, Skitzki J, Stoolman L, et al. Intratumoral IL-12 and TNF-alpha-loaded microspheres lead to regression of breast cancer and systemic antitumor immunity. Ann Surg Oncol. 2004;11:147–156. doi: 10.1245/aso.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Sharma A, Harper CM, Hammer L, et al. Characterization of cytokine-encapsulated controlled-release microsphere adjuvants. Cancer Biother Radiopharm. 2004;19:764–769. doi: 10.1089/cbr.2004.19.764. [DOI] [PubMed] [Google Scholar]
- 34.Baca-Estrada ME, Foldvari M, Snider M. Induction of mucosal immune responses by administration of liposome-antigen formulations and interleukin-12. J Interferon Cytokine Res. 1999;19:455–462. doi: 10.1089/107999099313893. [DOI] [PubMed] [Google Scholar]
- 35.Mortarini R, Borri A, Tragni G, et al. Peripheral burst of tumor-specific cytotoxic T lymphocytes and infiltration of metastatic lesions by memory CD8+ T cells in melanoma patients receiving interleukin 12. Cancer Res. 2000;60:3559–3568. [PubMed] [Google Scholar]
- 36.Kieper WC, Prlic M, Schmidt CS, et al. Il-12 enhances CD8 T cell homeostatic expansion. J Immunol. 2001;166:5515–5521. doi: 10.4049/jimmunol.166.9.5515. [DOI] [PubMed] [Google Scholar]
- 37.Fogler WE, Volker K, Watanabe M, et al. Recruitment of hepatic NK cells by IL-12 is dependent on IFN-gamma and VCAM-1 and is rapidly down-regulated by a mechanism involving T cells and expression of Fas. J Immunol. 1998;161:6014–6021. [PubMed] [Google Scholar]
- 38.Colantonio L, Iellem A, Clissi B, et al. Upregulation of integrin alpha6/beta1 and chemokine receptor CCR1 by interleukin-12 promotes the migration of human type 1 helper T cells. Blood. 1999;94:2981–2989. [PubMed] [Google Scholar]
- 39.Bajetta E, Del Vecchio M, Mortarini R, et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res. 1998;4:75–85. [PubMed] [Google Scholar]
- 40.Gollob JA, Mier JW, Veenstra K, et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-gamma induction is associated with clinical response. Clin Cancer Res. 2000;6:1678–1692. [PubMed] [Google Scholar]
- 41.Zitvogel L, Tahara H, Robbins PD, et al. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J Immunol. 1995;155:1393–1403. [PubMed] [Google Scholar]
- 42.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-gamma production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 43.Colombo MP, Vagliani M, Spreafico F, et al. Amount of interleukin 12 available at the tumor site is critical for tumor regression. Cancer Res. 1996;56:2531–2534. [PubMed] [Google Scholar]
- 44.Martinotti A, Stoppacciaro A, Vagliani M, et al. CD4 T cells inhibit in vivo the CD8-mediated immune response against murine colon carcinoma cells transduced with interleukin-12 genes. Eur J Immunol. 1995;25:137–146. doi: 10.1002/eji.1830250124. [DOI] [PubMed] [Google Scholar]
- 45.Zilocchi C, Stoppacciaro A, Chiodoni C, et al. Interferon gamma-independent rejection of interleukin 12-transduced carcinoma cells requires CD4+ T cells and Granulocyte/Macrophage colony-stimulating factor. J Exp Med. 1998;188:133–143. doi: 10.1084/jem.188.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 47.Watkins SK, Egilmez NK, Suttles J, et al. IL-12 rapidly alters the functional profile of tumor-associated and tumor-infiltrating macrophages in vitro and in vivo. J Immunol. 2007;178:1357–1362. doi: 10.4049/jimmunol.178.3.1357. [DOI] [PubMed] [Google Scholar]
- 48.Kilinc MO, Aulakh KS, Nair RE, et al. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–6973. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]