Abstract
Objectives
Rheumatic diseases cause significant morbidity within American Indian populations. Clinical disease presentations, as well as historically associated autoantibodies, are not always useful in making a rapid diagnosis or assessing prognosis. The purpose of this study is to identify autoantibody associations among Oklahoma tribal populations with rheumatic disease.
Methods
Oklahoma tribal members (110 rheumatic disease patients and 110 controls) were enrolled at tribal-based clinics. Rheumatic disease patients (suspected or confirmed diagnosis) were assessed by a rheumatologist for clinical features, disease criteria, and activity measures. Blood samples were collected and tested for common rheumatic disease autoantibodies (ANA, anti-CCP, anti-RF, anti-Ro, anti-La, anti-Sm, anti-nRNP, anti-Ribosomal P, anti-dsDNA, and anti-cardiolipins).
Results
In patients with suspected systemic rheumatic diseases, 72% satisfied ACR classification: 40 (36%) rheumatoid arthritis, 16 (15%) systemic lupus erythematosus, 8 (7%) scleroderma, 8 (7%) osteoarthritis, 4 (4%) fibromyalgia, 2 (2%) seronegative spondyloarthropathy, 1 Sjogrens syndrome, and 1 sarcoidosis. When compared to controls, RA patient sera were more likely to contain anti-CCP (55% vs 2%, p<0.001) or anti-RF IgM antibodies (57% vs 10%, p<0.001); however, the difference was greater for anti-CCP. Anti-CCP positivity conferred higher disease activity scores (DAS28 5.6 vs 4.45, p=0.021) while anti-RF positivity did not (DAS28 5.36 vs 4.64, p=0.15). Anticardiolipin antibodies (25% or rheumatic disease paitents vs 10% of contros,; p=0.0022) and ANA (63% vs 21%, p<0.0001) were more common in rheumatic disease patients.
Conclusion
Anti-CCP may serve as a better RA biomarker in AI patients, while the clinical significance of increased frequency of aCLs needs further evaluation.
Keywords: Autoimmune diseases, autoantibodies, American Indian, rheumatic disease
Rheumatic diseases among American Indian (AI) populations are highly prevalent and oftentimes atypical in clinical presentation and disease course (1-4). Disease tends to be more aggressive and confers higher morbidity and mortality among AI populations (4, 5). Although reasons for this have not been entirely elucidated, variations in genetic expression, overlapping symptoms, and unique serological features obscure diagnosis and subsequent approaches to treatment (5, 6).
The relocation of AIs to present day Oklahoma in the 1830’s made for a heterogeneous amalgamation of indigenous people and is an ideal environment to better understand the pathology of rheumatic disease in AI populations. Tribal members comprise nearly 10% of the Oklahoma population and represent a diverse group of people with AI heritages (7). Previous studies report a greater incidence of systemic lupus erythematosus (SLE) in AI compared with the general European-American (EA) population (1, 3). Oklahoma Choctaw Indians have a 40 fold increase in the incidence of systemic sclerosis (SSc) with primarily diffuse involvement and anti-topoisomerase 1 autoantibodies over non-AI populations (8-11). Additionally, a greater overlap of rheumatoid arthritis (RA) with Sjögren’s syndrome (SS) and SLE is reported in AI from Oklahoma to which autoantibodies did not appear to correlate well with clinical outcomes (6). These findings support the idea that rheumatic diseases manifest uniquely among Oklahoma tribal members and necessitates a need to explore potential explanations for this diversity.
The aim of this study is to characterize serologic biomarkers in Oklahoma tribal patients with rheumatic diseases to help improve clinical care, as well as to develop new diagnostic and prognostic tools. Results from these studies will provide valuable strategies in the healthcare of AI in Oklahoma and may be applicable to other indigenous populations.
METHODS
Study Participants and Clinical Evaluation
From March 2007 to January 2010, 110 AI patients in Oklahoma (rheumatic disease patients and individuals with suspected rheumatic disease) and 110 AI controls were enrolled. Two rheumatic disease clinics were established for Oklahoma tribal patients with rheumatic disease complaints. Rheumatic disease patients were referred by primary care providers (physicians, physician assistants, or nurse practitioners) or by a tribal healthcare representative. Patients were referred to the tribal health clinic for several reasons, including presenting symptoms of systemic rheumatic disease without a clear diagnosis; abnormal blood test with rheumatic disease symptoms; systemic rheumatic disease with continued disease activity; questions regarding therapy; patient request for evaluation; or interest in being involved in a study. Healthy controls were recruited through Institutional Review Board (IRB) approved health fair flyers and email advertisements. All patients involved in this study are members of a federally recognized AI tribe, band, or nation.
At the initial visit, history, physical exam, physician global assessment, American College of Rheumatology (ACR) criteria, disease activity, disease damage and treatment histories were collected by an ABIM board-certified rheumatologist. Individuals referred to the rheumatic disease clinics were assessed for ACR criteria for classification of SLE, RA, SSc, SS, Fibromyalgia (FM), and Osteoarthritis (OA). Additionally, medical chart review was conducted for all of the participating patients referred for rheumatic evaluation according to previously published methods (12). Classification of SLE required 4 of 11 1997 ACR criteria to be met (13, 14). RA classification criteria required 4 of 7 for the 1987 ACR criteria (15). FM diagnosis required 2 of 2 criteria to be met with widespread pain present for at least 3 months (16). SSc classification required either proximal diffuse sclerosis or 2 of the following: sclerodactyly, digital pitting scars or loss of substance of the digital finger pads, and bilateral basilar pulmonary fibrosis (17). SS classification required 4 of 6 criteria as long as histopathology or serology was positive (18). Patients were diagnosed with OA with 3 of the 4 criteria for OA of the hand (19); or 2 of 3 criteria for OA of the hip (20); or knee pain and osteophytes on a radiograph with 1 of 3 of the following: age over 50 years old, stiffness lasting 30 minutes or less, or crepitus on motion (21).
Disease activity and outcome measurements were performed as appropriate: the safety of estrogens in Lupus Erythematosus national assessment - SLE disease activity index (SELENA-SLEDAI) (22, 23), the physician global assessment (PGA) and SLE international collaborating clinics damage index (SLICC)(24) for SLE patients, disease activity score (DAS)28 for RA patients (25), and the Rodman skin score for SSc disease assessment measurement (26, 27). Control subjects provided serum for comparison.
All participants provided written informed consent and the study was approved by the IRB of all four participating organizations: University of Oklahoma Health Sciences Center, Oklahoma Medical Research Foundation (OMRF), Chickasaw Nation and Cherokee Nation.
Historical non-AI Control Cohort
A historical non-AI control cohort was included for comparison of autoantibody positivity with AI control subjects. This cohort was comprised of European-American (EA, n=62) and African-American (AA, n=38) healthy unaffected individuals. IRB approval and written informed consent was given at time of enrollment. Serum samples were obtained from the control subjects and tested for autoantibodies.
Autoantibody Testing
Samples were tested in a College of American Pathologist/Clinical Laboratory Improvement Amendments (CAP/CLIA) approved lab for the presence of antinuclear antibodies (ANA) by immunoflorescence for titer and pattern, anti-dsDNA, and anti-ENA. The presence of ANAs was detected using Hep-2 cells (Inova Diagnostics, San Diego, CA) with reactivity detectable at a 1:120 dilution considered positive. In rheumatic patients, testing for anti-deoxyribonucleic acid (dsDNA) antibodies was performed by Crithidia assay (Innova Diagnostics, San Diego, CA). Additionally, precipitating antibodies to Sm, nRNP, Ro, La, PM-Scl, Mi-2, and Jo-1 were examined by double immunodiffusion (ID) using previously published protocols (28). Testing for anticardiolipins (aCLs) (IgG, IgM, IgA) used ELISA techniques with the following ranges considered positive: low titer (11-19 units), moderate titer (20-89 units) and high titer (>90 units) (Sigma, Saint Louis, MO).
In addition, all samples were evaluated by enzyme-linked immunosorbent assays (ELISA) for extractable nuclear antigen antibodies to Sm, nuclear RNP (nRNP), Ro, La (Immunovision, Springdale, AR) and Ribosomal P (Ribo P) (Molecular Biology Proteomics, OKC, OK) (29). ELISA detection was performed for rheumatoid factor (RF) IgM and IgG (Jackson Immunoresearch Laboratories, West Grove, PA) with positive results as an OD greater than 0.35. Patients were considered RF positive if a positive ELISA result was obtained for RF IgM and/or IgG. These methods have been previously described in detail (30). Serum antibodies directed to cyclic citrullinated protein IgG (anti-CCP) were assessed by a commercial ELISA kit according to the manufacturer’s recommendations (QUANTA Lite™ CCP2 IgG ELISA, Inova, San Diego, CA). Samples were considered positive when the index value was greater than 20.
Statistical Analysis
Statistical analysis for this study was largely focused on chi-square methods. Contingency data tables with cells representing less than five subjects were identified and Fisher’s Exact Test was utilized. Pearson correlation coefficients were used to test for an association between two independently measured continuous variables. Analysis of Variance (ANOVA) methods were used in making comparisons on continuous data among subsets of patients. Levene’s test was utilized to test for homogeneity of variance of a given parameter and the Shapiro-Wilk test was used to investigate the assumption of normality. When either the homogeneity of variance or normality assumptions required under ANOVA was not satisfied, nonparametric methods were employed. P values less than 0.05 were considered significant. All analyses were performed using SAS version 9.1 or GraphPad Prism 5 for Windows. Logistic regression model-building techniques utilized the purposeful selection method of identifying covariates of interest (31). Models were compared using the likelihood ratio test.
RESULTS
Rheumatic Disease Presentations to Tribal-Based Referral Clinics
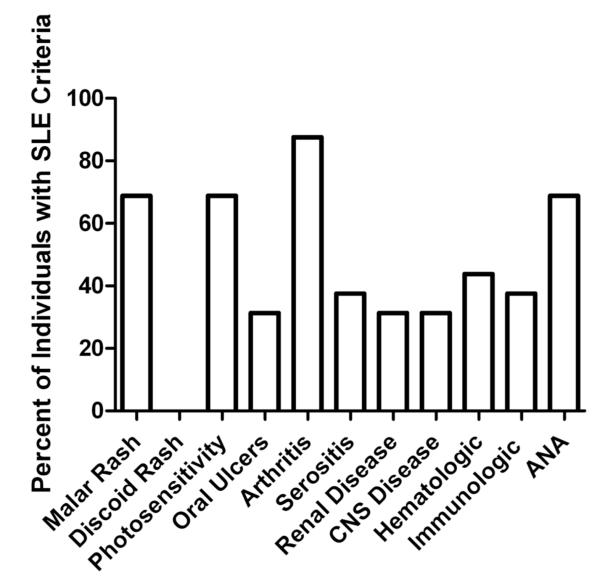
Of the 110 patients referred for systemic rheumatic disease evaluation or therapeutic revision, 72% met ACR criteria for classification: 40 (36%) have RA (including those patients with secondary SS), 16 (15%) SLE, and 8 (7%) SSc. Additional rheumatic diagnoses were comprised of 8 osteoarthritis [OA] (7%), 4 fibromyalgia [FM] (4%), 2 seronegative spondyloarthropathy [SpA] (2%) (1 ankylosing spondylitis and 1 reactive arthritis), 1 SS and 1 sarcoidosis. A large number of patients had a previous rheumatic disease diagnosis (including 23 of 25 RA and 13 of 16 SLE patients). The distribution of cumulative SLE ACR criteria is shown in Figure 1. Briefly, malar rash, photosensitivity, arthritis, and ANA are the most prevalent SLE ACR criteria met by these AI SLE patients. The average number of SLE ACR classification criteria was 5.3 ± 1.2 (range = 4 to 7). On average the SLE patients have had a SLE diagnosis for 7.8 ± 7.5 years (median= 6 years, range = 1 to 28 years). The AI RA patients exhibited morning stiffness, arthritis of three or more joints, arthritis of the hand, erosions on x-rays, and had detectable serum RF. On average the RA patients have had a disease diagnosis for 11.3 ± 8.8 years (median = 10 years, range = 0 to 37 years).
Figure 1.
Distribution of cumulative SLE classification criteria in AI participants.
At enrollment, patients were examined for rheumatic diseases by a rheumatologist and assessed for ACR classification criteria. Medical chart review for the presence of ACR classification criteria was also performed for all AI participants referred for rheumatic evaluation. Cumulative SLE ACR classification criteria as percent of patients positive are shown.
A group of patients referred for rheumatic disease evaluation or treatment revision did not meet ACR classification criteria for disease and accounted for 28% (33/110) of the patient population in this study. These include 11 patients with polyarthralgia [PA], 11 patients with polyarthritis [pa], 4 with undifferentiated connective tissue disease (UCTD), 3 with anterior uveitis, and 1 with sclerodactyly. Patients with suspected systemic rheumatic disease were slightly older than controls (27% vs 13% ≥ 60 years of age) and 76% of all participants were female (Table 1).
Table 1.
Baseline characteristics of Oklahoma tribal members referred for rheumatic disease evaluation and healthy controls.
| Characteristic | Rheumatic Disease Clinic Patients (n=110) |
Controls (n=110) |
P Value |
|---|---|---|---|
| Mean age in years (+/−SD) | 49.2 (+/−13) | 40.7 (+/− 14.4) | p < 0.001 |
| Range | 17 to 83 | 16 to 75 | |
| Women, n (%) | 88 (80%) | 80 (73%) |
AI Rheumatic Disease Patient Sera contains Autoantibodies Frequently detected in Other Disease States
Atypical disease associations were found in patients exhibiting disease specific antibodies within this study. Table 2 illustrates autoantibody patterns in Oklahoma tribal patients referred to the rheumatic disease clinic. Only 11 of the 16 (69%) SLE patients had detectable ANAs at the time of enrollment. Of these five ANA negative SLE patients, four exhibited ANA positivity historically at some point prior to study enrollment. No significant differences in age, ACR criteria, length from disease diagnosis, or medication use between the patients that lost ANA positivity and those that remained ANA positive were observed.
Table 2.
Autoantibody specificities detected in sera from Oklahoma tribal patients referred to the rheumatic disease clinic.
| RA n=40 |
SLE n=16 |
SSc n=8 |
SS n=1 |
PA n=11 |
pa n=11 |
FM n= 4 |
OA n=8 |
Other n= 11 |
|
|---|---|---|---|---|---|---|---|---|---|
| ANA | 25* | 11 | 7 | 1 | 8 | 5 | 2 | 5† | 5‡ |
| Anti-dsDNA | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| aCLs IgG | 10 | 2 | 3 | 0 | 5 | 1 | 2 | 1 | 3§ |
| aCLs IgM | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1†† |
| Anti-Ro | 2* | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Anti-La | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Anti-Sm | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1†† |
| Anti-nRNP | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1** |
| Anti-Ribo P | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anti-Jo 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Unidentified | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anti-CCP | 22 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1†† |
| Anti-RF IgM | 23 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 |
| Anti-RF IgG | 12 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
PA = Polyarthritis; pa = polyarthralgia; FM = Fibromyalgia; OA = Osteoarthritis
Other =UCTD (4), Anterior Uveitis (3), Sarcoidosis (1), Sclerodactyly (1), Seronegative SpA (2)
= Patient with both RA and Sjogren’s (1)
= Patient with both FM and OA (2)
= UCTD (3) and Anterior Uveitis (1)
= UCTD (2) and Sclerodactly (1)
= UCTD (1)
= Inflammatory Eye Disease
Unidentified = antibody binding lines detected during immunodiffusion that do not match known rheumatic disease antigens
ANA measurements showed that Oklahoma tribal patients referred for rheumatic disease evaluation were more likely to be ANA positive when compared to controls (63% vs 18.2%, p<0.0001) (Tables 2 and 3). Alternatively, 13/22 PA and pa (59%), 25/40 RA (63%), and 5/8 OA (63%) exhibited ANA positivity. Eight patients have more than one ANA pattern (3 RA, 2 SSc, 1 SLE, 1 OA and 1 PA patient). The ANA positivity of the individuals referred for rheumatic disease evaluation is significantly higher (p<0.0001) when compared to ANA positivity in control individuals. Anti-dsDNA positivity tended to be found among AI patients referred for rheumatic evaluation than in controls, although this did not reach statistical significance (p=0.06).
Table 3.
ELISA Autoantibody profiles of American Indian, European-American, and African-American control subjects
| AI (n=110) | EA (n=62) | AA (n=38) | |
|---|---|---|---|
| ANA | 18.2% | 33.9% | 47.4% |
| Anti-dsDNA | 0% | 4.8% | 7.9% |
| aCL IgG | 11.8% | 6.5% | 5.3% |
| Anti-Ro | 0.9% | 1.6% | 5.3% |
| Anti-La | 0% | 1.6% | 2.6% |
| Anti-Sm | 0.9% | 4.8% | 5.3% |
| Anti-nRNP | 1.8% | 1.6% | 0% |
| Anti-Ribo P | 0% | 6.5% | 2.6% |
AI= American Indian, EA= European-American, AA= African-American
Of the patients whose sera tested positive for anti-dsDNA, one had polyarticular inflammatory arthritis, while only 2/16 (13%) SLE patients had anti-dsDNA antibodies (Table 2). Among RNA-binding protein antibodies, 2/16 patients with SLE had detectable anti-nRNP while one had anti-Sm. Anti-Ro antibodies were detected in 7 of the 110 referred patients, including 3 patients with RA and one each with SSc, SS and inflammatory eye disease.
Of the 33 patients that did not meet ACR classification criteria, 24 had detectable ANA (5 PA, 8 pa, 2 FM, 8 OA, 4 other disorders) (Table 2). Additionally, sera from 2 PA patients and 1 pa patient were positive for anti-RF IgM; and 1 PA and 2 pa patients were positive for anti-RF IgG (Table 2) although these individuals have not yet met ACR RA classification criteria.
Anticardolipin IgG Antibodies are Enriched in Individuals Referred for Rheumatic Disease Evaluation
In patients with rheumatic disease symptoms or diagnoses, 27/110 (25%) had detectable IgG aCLs with 3/27 (11%) also exhibiting IgM aCLs (Table 2). Of these rheumatic disease patients with detectable aCLs, 18/27 (67%) met ACR criteria for disease as follows: 10 RA, 3 SSC, 2 SLE, 1 OA and 2 FM. Of those patients not meeting criteria for disease associated conditions (9/27, 33%): 6 had PA, 2 UCTD, and 1 sclerodactly. When compared to controls, Oklahoma tribal patients with rheumatic disease were more likely to be positive for IgG aCLs (27 vs 13, p=0.022). Of these rheumatic disease patients, 16/27 (59%) were low titer (11-19 units) and 11/27 (41%) moderate titer (20-89 units). In the three rheumatic patients with detectable IgM aCLs: one RA patient exhibited low titer IgM aCLs and also had detectable low titer IgG aCLs, another patient with RA and one with UCTD exhibited both moderate titer IgM and IgG aCLs. In contrast, 13/110 controls had detectable aCLs with 8/11 positive for low titer IgG aCLs, the remaining 5 had moderate titer. No controls tested positive for IgM aCLs autoantibodies. None of the aCL positive individuals had a history of clots, pulmonary embolism, or deep vein thrombosis to date as determined through medical chart review.
The Combination of Anti-CCP and RF antibodies is a Sensitive and Specific Biomarker of Rheumatoid Arthritis in AI Rheumatic Disease Patients
Among Oklahoma tribal patients with rheumatic disease, a comparison was made between RF and anti-CCP in all patients to determine the strongest association with RA. Anti-CCP antibodies appeared to be found almost exclusively in patients with RA while several patients without RA had detectable RF IgM and/or IgG (Table 2). The diagnostic accuracy of anti-CCP in our AI study population has 55% sensitivity, 98.6% specificity, a positive predictive value (ppv) of 0.96 (95% Confidence Interval, CI, 0.78-0.99) and a negative predictive value (npv) of 0.79 (95% CI 0.69-0.87). The diagnostic accuracy of RF (IgM and IgG) in our AI study population has 57.5% sensitivity, 87.1% specificity, a ppv of 0.72 (95% CI 0.53-0.86) and a npv of 0.78 (95% CI 0.67-0.86). In patients with RA, 18/40 patients were both anti-CCP antibody and RF positive, 13/40 were anti-CCP and RF negative, 5/40 were anti-CCP antibody negative RF positive, and 4/40 were anti-CCP positive and RF negative. When used together anti-RF and anti-CCP antibodies are better biomarkers of RA than by either alone (p=0.007). Anti-CCP antibody positivity (DAS28: 5.6 vs 4.45, p=0.021) or having both anti-CCP and RF IgM and/or IgG antibodies (DAS28 5.7 vs 4.64, p=0.039) was associated with higher disease activity scores. Among RA patients, those ANA positive (62%, 25/40) had higher DAS28 scores (5.46 vs 4.42, p=0.031) but only anti-CCP antibodies were independently associated with higher disease activity when evaluating both anti-CCP antibody and ANA positivity in these patients (p=0.025 vs p=0.067).
Of the 40 RA patients, 35 (88%) were on a disease modifying anti-rheumatic drug (DMARD) therapy, 17 (43%) were maintained on prednisone (average dose 8.8mg), and 11 (28%) were on or had been on biological therapy. No relationship was observed between current medications and DAS28 scores.
Autoantibody Prevalence in American Indian Control Subjects is Not Enriched
In order to fully define the differences between AI autoantibody profiles, we compared the ELISA serology of our AI controls (n=110) to a historic cohort of healthy unaffected European-American (EA) and African-American (AA) control subjects (n=100). The EA controls were 44.74 ± 14.81 years old and were comprised of 89% females; while the AA controls were 37.45 ± 10.60 and were comprised of 87% females. The age of the non-AI controls was not significantly different than that of the AI controls (p>0.05 for both AA and EA). Differences in autoantibody positivity were observed between AI controls and the EA and AA controls (Table 3). ANA positivity (39% vs 18.2%, p=0.0012), antibody positivity toward dsDNA (6% vs 0%, p=0.011) and Ribo P (5% vs 0%, p=0.023) were more likely to be found in EA and AA controls than in AI. No statistically significant differences in aCL, anti-Ro, La, Sm, or nRNP positivity were observed.
DISCUSSION
Several different studies involving specific rheumatic diseases in Native Americans have indicated a higher prevalence of RA (122 cases in 100,000 vs 48 cases in 100,000 in the non-Native American population) (32) and SLE (42.3 cases in 100,000 vs 20.6 cases in 100,000 in the non-Native American population) (4). Our study was not designed to serve as a population-based study to evaluate the incidence and prevalence of each systemic autoimmune rheumatic disease, but focuses on the clinical and serologic presentations of these patients which impair diagnosis. At the start of our study, Oklahoma was home to 395,500 American Indians. However, only a subset of these individuals is cared for in the Chickasaw and Cherokee health systems (and therefore would be available for referral to our clinics). To date, over 150 patients have been provided care at the Chickasaw and Cherokee tribal health clinics. With the prevalence rates outlined above (4, 32) rates we should expect 27 cases of RA and 9 cases of SLE. Over the course of this study (2007-2010), 110 rheumatic patients were recruited. Of these patients, 40/110 were diagnosed with RA and 16/110 were diagnosed with SLE. While these numbers are slightly higher than the 2007 population estimate, we believe this population is representative of the Native American rheumatic disease patients.
Most of the initial data regarding correlation of specific autoantibodies and rheumatic diseases have been generated from cohorts that were predominantly patients with European or African heritage (33-36). In our study, the majority of the Oklahoma tribal patients evaluated for rheumatic disease tested positive for ANA with varying titers and patterns, while only 69% of SLE patients had a positive ANA at the time of evaluation. No differences in age, length from disease diagnosis, ACR criteria, or treatment was observed between SLE patients that remained ANA positive compared to those whose sera lost ANA positivity. A numerical difference in SLEDAI scores was observed (5.45 ± 4.80 ANA positive SLE patients vs. 2.67 ± 1.15 ANA negative). While this difference was not statistically significant (p=0.46, Mann Whitney test), ANA positive SLE patients demonstrating higher disease activity is consistent with previous work. Additionally, other studies have identified a subset of SLE patients that are ANA negative (37-39).
SLE specific antibodies were detected in 3% of the AI patients with rheumatic disease; however, the majority of these patients lacked clinical features of SLE. Furthermore, anti-Ro antibodies are historically found in sera from almost 50% of SLE patients and can also be detected in the vast majority of patients with SS (40). In our cohort, anti-Ro antibodies were present in patients with SLE and SS, as well as in patients with RA, SSc and anterior uveitis. Interestingly, the myositis specific antibody, anti-Jo-1, was detected in a patient with SSc without clinical features of inflammatory myositis. In this study, 5% of the patients referred for rheumatic disease evaluation had antibodies detected by immunodiffusion which were unidentifiable. These findings support evidence of overlapping antibodies and rheumatic diseases and highlight the lack of prognostic knowledge of autoantibody specificities in this AI population.
Anticardiolipin antibodies (aCLs) encompass a heterogenous group of antibodies and are associated with SLE as well as risk of clinical complications such as arterial and venous thrombosis (41). aCLs are associated with vascular impairment in certain connective tissue diseases (42). However, aCL have also been observed in rheumatic diseases without the presence of antiphospholipid syndrome (APS) (43, 44). Detectable aCLs were found in a number of patients with systemic rheumatic disease (RA, SSc), along with other alternative diagnoses (FM, OA, PA, and pa). The clinical significance of the role of these aCLs in AI patients and pathologic risk for thrombosis remains to be determined.
Historically RF has been the serologic criterion used in the diagnosis of RA. More recently, anti-CCP along with RF antibodies appears to be more sensitive and specific for RA diagnosis and a better predictor of joint destruction (45-47). This finding was reinforced among the RA Oklahoma tribal patients, suggesting that anti-CCP antibodies may be more strongly associated with RA and a biomarker of disease in this population. In addition, nearly 60% of Oklahoma RA tribal patient sera contained ANA, much higher than previously reported in this geographic population (48), but in agreement with findings from other AI tribes (3, 32, 49). Interestingly, our study cohort has a high percentage (32.5%) of individuals seronegative for anti-CCP and anti-RF antibodies as compared to other AI studies (3, 5, 49). Taken together this emphasizes ethnic, and potentially tribal differences in autoantibody expression, specifically in AI populations, and has potential to elicit changes in current evaluation and treatment practices.
Of the Oklahoma AI patients referred for rheumatic disease evaluation, 28% were unclassifiable by ACR criteria. This percentage is significantly smaller than the 48% seen with the Nuu-Chah-Nulth tribe (50). However, these populations of individuals with unclassifiable rheumatic disease highlight differences in disease presentation between AI and other demographic groups. Interestingly, a subset of our study patients with polyarthritis and polyarthralgia exhibited detectable antibody production. It would be of considerable interest to follow those patients which did not currently fulfill ACR criteria to assess evolving disease. This also illustrates the concept that application of current ACR criteria may not hold distinct applicability to the AI population.
Limitations exist in this study. Rheumatic disease patients on average were older than our AI healthy controls, potentially confounding the autoantibody comparisons. However, the percent of antibody positive individuals in the control population was 18% compared to 39% of controls from either EA or AA descent with a similar age at participation suggesting this difference would not significantly change the results. Medication reporting in this study has a potential limitation. While no statistical significances was observed between medication, disease activity scores, and antibody positivity, the medication list at time of evaluation may not fully represent what the study participant has taken in the past. This limitation can be minimized in the future with the recent implementation of electronic medical records. Patients were also referred to this clinical for a variety of reasons, many of which may bias the severity or presentation of rheumatic disease clinical manifestations. Additional population-based studies are warranted to confirm and/or expand these observations. Historical serologies obtained by medical record review may be variable due to difference in laboratory used, types of testing, sample shipping, and other such confounding issues. These limitations may explain in part some of the difference between SLE ACR ANA criteria and ANA at time of evaluation.
Another potential limitation is that the results might not be fully representative of those seen in rheumatology clinics as the patients referred for rheumatic evaluation may have included a subset of patients who presented with more atypical disease features, were difficult to treat, or had increased disease severity. However, the majority of the rheumatic disease presentations did match the clinical characteristics of disease classification with the exception of the serology. Additionally, our study subjects included a number of different AI tribes evaluated in the Chickasaw and Cherokee catchment areas and may exhibit differences in rheumatic disease presentation unique to the Oklahoma area. Thus, while our subjects might not represent the typical rheumatic disease patient, they are representative of the rheumatic disease patients seen in Oklahoma. A future direction that would elevate some of the study limitations is the development of a cohort of newly diagnosed AI and non-AI rheumatic disease patients and evaluating differences in clinical symptoms, serology, and disease activity. Additionally, the development of a longitudinal cohort which would follow AI and non-AI rheumatic disease individuals could give valuable insights into the differences in disease progression.
In summary, Oklahoma tribal members affected with rheumatic disease fail to exhibit all of the typical disease-specific autoantibody markers. ACR criteria for classification of disease do not appear to be as inclusive in this unique population as in previously studied ethnic groups. Further studies are needed to better define more reliable diagnostic biomarkers to help guide treatment and improve outcomes in AI patients with rheumatic disease.
Acknowledgements
We express appreciation to all Oklahoma tribal members who provided informed consent for participation and all tribal providers in this study. We would also like to thank Scott Stewart, Dorothy Abbott, Cassidy Varnell and the OMRF Clinical Immunology laboratory personnel for their technical and analytical assistance.
Supported by grants from: This publication was made possible by Native American Research Centers for Health (U26 IHS300125), the Center of Biomedical Research Excellence (P20RR015577 and P30RR031152/P30GM103510), the Oklahoma Rheumatic Disease Research Core Center (P30AR053483) and the Oklahoma Autoimmunity Center of Excellence (U19 AI082714) from the National Institutes of Health (NIH). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or its relevant institutes. J.R. Gaddy, MD, Division of Rheumatology/Allergy/Immunology, University of Oklahoma Health Science Center and Arthritis and Clinical Immunology, Oklahoma Medical Research Foundation; E.S. Vista, MD, Divisions of Rheumatology, University of Santo Tomas Hospital; J.M. Robertson, PhD, Arthritis and Clinical Immunology, Oklahoma Medical Research Foundation; A.B. Dedeke, MD, Arthritis and Clinical Immunology, Oklahoma Medical Research Foundation; V.C. Roberts, LPN, CCPN, Arthritis and Clinical Immunology, Oklahoma Medical Research Foundation; W. S. Klein, B.S., Arthritis and Clinical Immunology, Oklahoma Medical Research Foundation; J. H. Levin, B.S., Arthritis and Clinical Immunology, Oklahoma Medical Research Foundation; F.H. Mota, MD, Chickasaw Nation Medical Center; T.M. Cooper, MD, Chickasaw Nation Medical Center; G.A. Grim, MD, Cherokee Nation Health Services; S. Khan, MBBS, MPH, Cherokee Nation Health Services; and J.A. James, MD, PhD, Arthritis and Clinical Immunology, Oklahoma Medical Research Foundation and Departments of Medicine, Pathology, and Microbiology/Immunology, University of Oklahoma Health Sciences Center.
Footnotes
Disclosures: J.R. Gaddy, None; E.S. Vista, None; J.M. Robertson, None; A.B. Dedeke, None; V.C. Roberts, None; W.S. Klein, None; J.H. Levin, None; F.H. Mota, None; T. A. Cooper, None; G.A. Grim, None; S. Khan, None; J.A. James, None.
References
- 1.Acers TE, Acers-Warm A. Incidence patterns of immunogenetic diseases in the North American Indians. J Okla State Med Assoc. 1994;7:3091–4. [PubMed] [Google Scholar]
- 2.Mauldin J, Cameron HD, Jeanotte D, Solomon G, Jarvis JN. Chronic arthritis in children and adolescents in two Indian health service user populations. BMC Musculoskelet Disord. 2004;5:30–36. doi: 10.1186/1471-2474-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peschken CA, Esdaile JM. Rheumatic diseases in North America’s indigenous peoples. Semin Arthritis Rheum. 1999;28:368–91. doi: 10.1016/s0049-0172(99)80003-1. [DOI] [PubMed] [Google Scholar]
- 4.Peschken CA, Esdaile JM. Systemic lupus erythematosus in North American Indians: a population based study. J Rheumatol. 2000;27:1884–91. [PubMed] [Google Scholar]
- 5.Ferrucci ED, Templin DW, Lanier AP. Rheumatoid arthritis in American Indians and Alaska Natives: a review of the literature. Semin Arthritis Rheum. 2005;34:662–67. doi: 10.1016/j.semarthrit.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Scofield RH, Fogle M, Rhoades ER, Harley JB. Rheumatoid arthritis in a United States Public Health Service Hospital in Oklahoma: serologic manifestations in rheumatoid arthritis vary among tribal groups. Arthritis Rheum. 1996;39:283–86. doi: 10.1002/art.1780390216. [DOI] [PubMed] [Google Scholar]
- 7.State and County QuickFacts [webpage on the Internet] U.S. Census Bureau; [updated 2011 June 3;cited 2010 November 15]. Available from: http://quickfacts.census.gov/qfd/states/40000.html. [Google Scholar]
- 8.Kuwana M, Kaburaki J, Arnett FC, Howard RF, Medsger TA, Jr, Wright TM. Influence of ethnic background on clinical and serologic features in patients with systemic sclerosis and anti-DNA topoisomerase I antibody. Arthritis Rheum. 1999;42:465–74. doi: 10.1002/1529-0131(199904)42:3<465::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Arnett FC, Howard RF, Tan F, Moulds JM, Bias WB, Durban E, et al. Increased prevalence of systemic sclerosis in a Native American tribe in Oklahoma. Associated with an Amerindian HLA haplotype. Arthritis Rheum. 1996;38:1362–70. doi: 10.1002/art.1780390814. [DOI] [PubMed] [Google Scholar]
- 10.Reveille JD. Ethnicity and race and systemic sclerosis: how it affects susceptibility, severity, antibody genetics, and clinical manifestations. Curr Rheumatol Rep. 2003;5:160–67. doi: 10.1007/s11926-003-0045-1. [DOI] [PubMed] [Google Scholar]
- 11.Tan FK, Arnett FC, Reveille JD, Ahn C, Antohi S, Sasake T, et al. Autoantibodies to fibrillin 1 in systemic sclerosis: Ethnic differences in antigen recognition and lack of correlation with specific clinical features or HLA alleles. Arthritis Rheum. 2000;43:2464–71. doi: 10.1002/1529-0131(200011)43:11<2464::AID-ANR13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen A, Sevier S, Kelly JA, Glenn SB, Aberle T, Cooney CM, et al. The lupus family registry and repository. Rheumatology (Oxford) 2011;50:47–59. doi: 10.1093/rheumatology/keq302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 14.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–77. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy S, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 17.Committee SfSCotARADaTC Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 18.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman R, Alarcon G, Appelrouth D, Bloch DA, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthrits of the hand. Arthritis Rheum. 1990;33:1601–10. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 20.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 21.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of the criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 22.Petri M. Disease activity assessment in SLE: do we have the right instruments? Ann Rheum Dis. 2007;66:iii61–iii64. doi: 10.1136/ard.2007.078477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petri M, Kim M, Kalunian K, Grossman J, Hahn B, Sammaritano L, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353:2550–58. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 24.Gladman DD, Urowitz MB. The SLICC/ACR damage index: progress report and experience in the field. Lupus. 1999;8:632–37. doi: 10.1191/096120399680411335. [DOI] [PubMed] [Google Scholar]
- 25.Prevoo MLL, van’T Hof MA, Kuper HH, van Leeuwen M, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 26.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 27.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: High-dose versus low-dose penicillamine trial. Arthritis & Rheumatism. 2000;43:2445–54. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.McClain M, Scofield R, Kurien B, Gross T, James J. Selective Small Antigenic Structures are Capable of Inducing Widespread Autoimmunity Which Closely Mimics the Humoral Fine Specificity of Human SLE. Scandinavian Journal of Imunology. 2002;56:399–407. doi: 10.1046/j.1365-3083.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 29.Crowe SR, Merrill JT, Vista ES, Dedeke AB, Thompson DM, Stewart S, et al. Influenza vaccination responses in human systemic lupus erythematosus: impact of clinical and demographic features. Arthritis Rheum. 2011;63:2396–406. doi: 10.1002/art.30388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinlen LD, McClain MT, Merrill J, Akbarali YW, Edgerton CC, Harley JB, et al. Clinical criteria for systemic lupus erythematosus precede diagnosis, and associated autoantibodies are present before clinical symptoms. Arthritis Rheum. 2007;56:2344–51. doi: 10.1002/art.22665. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer DW, Lemeshow S. Applied logistic regression. In: Cressie NA, Fisher NI, et al., editors. Wiley Series in probability and statistics. John Wiley & Sons; New York: 2000. pp. 1–392. [Google Scholar]
- 32.Ferrucci ED, Templin DW, Lanier AP. Rheumatoid arthritis in American Indians and Alaska Natives: a review of the literature. Semin Arthritis Rheum. 2004;34:662–67. doi: 10.1016/j.semarthrit.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Dubois EL. Serologic abnormalities in spontaneous and drug-induced systemic lupus erythematosus. J Rheumatol. 1975;2:204–14. [PubMed] [Google Scholar]
- 34.Swaak AJ, Huysen V, Nossent JC, Smeenk RJ. Antinuclear antibody profiles in relation to specific disease manifestations of systemic lupus erythematosus. Clin Rheumatol. 1990;9:82–94. doi: 10.1007/BF02205555. [DOI] [PubMed] [Google Scholar]
- 35.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Cohen PL, et al. Differences by race, sex, and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus. 2002;11:161–67. doi: 10.1191/0961203302lu161oa. [DOI] [PubMed] [Google Scholar]
- 36.Vila LM, Alarcon GS, McGwin G, Jr, Bastain HM, Fessler BJ, Reveille JD. Systemic lupus erythematosus in a multiethnic US Cohort, XXXVII: association of lymphopenia with clinical manifestations, serologic abnormalilites, disease activity, and damage accrual. Arthritis Rheum. 2006;55:799–806. doi: 10.1002/art.22224. [DOI] [PubMed] [Google Scholar]
- 37.Alessandri C, Conti F, Conigliaro P, Mancini R, Massaro L, Valesini G. Seronegative autoimmune diseases. Ann N Y Acad Sci. 2009;1173:52–9. doi: 10.1111/j.1749-6632.2009.04806.x. [DOI] [PubMed] [Google Scholar]
- 38.Ippolito A, Wallace DJ, Gladman D, Fortin PR, Urowitz M, Werth V, et al. Autoantibodies in systemic lupus erythematosus: comparison of historical and current assessment of seropositivity. Lupus. 2011;20:250–5. doi: 10.1177/0961203310385738. [DOI] [PubMed] [Google Scholar]
- 39.Reichlin M. ANA negative systemic lupus erythematosus sera revisited serologically. Lupus. 2000;9:116–9. doi: 10.1191/096120300678828091. [DOI] [PubMed] [Google Scholar]
- 40.Harley JB, Gaither KK. Autoantibodies. Rheum Dis Clin North Am. 1988;14:43–56. [PubMed] [Google Scholar]
- 41.Lockshin MD. Antiphospholipid antibody syndrome. Rheum Dis Clin North Am. 1994;20:45–59. [PubMed] [Google Scholar]
- 42.Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders. Prevalence and clinical significance. Ann Intern Med. 1990;112:682–98. doi: 10.7326/0003-4819-112-9-682. [DOI] [PubMed] [Google Scholar]
- 43.Marie I, Jouen F, Hellot MF, Leyesque H. Anticardiolipin and anti-beta2 glycoprotein I antibodies and lupus-like anticoagulant: prevalence and signifcance in systemic sclerosis. Br J Dermatol. 2008;158:141–44. doi: 10.1111/j.1365-2133.2007.08309.x. [DOI] [PubMed] [Google Scholar]
- 44.Olech E, Merrill JT. The prevalence and clinical significance of antiphospholipid antibodies in rheumatoid arthritis. Curr Rheumatol Rep. 2006;8:100–08. doi: 10.1007/s11926-006-0049-8. [DOI] [PubMed] [Google Scholar]
- 45.van der Heijde DM, van Riel PL, van Rijswik MH, van de Putte LB. Influence of prognostic features on the final outcome in rheumatoid arthritis: a review of the literature. Semin Arthritis Rheum. 1988;17:284–92. doi: 10.1016/0049-0172(88)90013-3. [DOI] [PubMed] [Google Scholar]
- 46.van der Linden MP, van der Woude D, Ioan-Facsinay A, Levarht EW, Stoeken-Rijsbergen G, Huizinga TW, et al. Value of anti-modified citrullinated vimentin and third-generation anti-cyclic citrullinated peptide compared with second-generation anti-cyclic citrullinated peptide and rheumatoid factor in prediciting disease outcome in undifferentiated arthritis and rheumatoid arthritis. Arthritis Rheum. 2009;60:2232–41. doi: 10.1002/art.24716. [DOI] [PubMed] [Google Scholar]
- 47.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, III, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 48.Nishimura S, Nishiya K, Hisakawa N, Chikazawa H, Ookubo S, Nakatani K, et al. Positivity for antinuclear antibody in patients with advanced rheumaotid arthritis. Acta Med Okayama. 1996;50:261–65. doi: 10.18926/AMO/30501. [DOI] [PubMed] [Google Scholar]
- 49.Peschken CA, Hitchon CA, Robinson DB, Smolik I, Barnabe CR, Prematilake S, et al. Rheumatoid Arthritis in a north american native population: longitudinal followup and comparison with a white population. J Rheumatol. 2010;37:1589–95. doi: 10.3899/jrheum.091452. [DOI] [PubMed] [Google Scholar]
- 50.Atkins C, Reuffel L, Roddy J, Platts M, Robinson H, Ward R. Rheumatic disease in the Nuu-Chah-Nulth native indians of the pacific northwest. J Rheumatol. 1988;15:684–90. [PubMed] [Google Scholar]