Abstract
Background:
Recent circumstantial evidences are suggesting that an increasing number of Iranian patients with cutaneous leishmaniasis are unresponsive to meglumine antimoniate (Glucantime®). Pistacia atlantica is native plant in Iran (central, western, and eastern regions). Gum obtained Pistacia atlantica has been reported to possess considerable in vitro antimicrobial activity. In this study, we aimed to investigate antileishmanial activity of P. atlantica.
Methods
Male BALB/c mice were inoculated subcutaneously 2×106 L. major Promastigotes (MHROM/IR/75/ER) at the base of tail in 2007. Mice were randomly divided into 3 groups. in group 1 Glucantime® was administered to the BALB/c mice in regimen of 60 mg per kg of body weight for 28 days by intraperitoneal injections per day, in group 2 the gum of P. atlantica var. Kurdica were tested by rubbing of local lesions for 28 days, group 3 infected but non-treated. Comparisons of treated groups and untreated group were done by two-way analysis of variance (ANOVA)
Results
Topically rubbing administration of gum obtained P. atlantica var. kurdica daily for 28 days like Glucantime® decreased skin lesion size in the BALB/c mice infected with L. major compared with that in the control (P< 0.01). Treatment BALB/c mice with gum obtained P. atlantica var. kurdica and Glucantime® causes decrease number of parasitologicaly positive mice (P< 0.05).
Conclusion
Our results show that gum obtained P. atlantica var. kurdica can be used for controlling cutaneous leishmaniasis caused by L. major and inhibiting development of cutaneous leishmaniasis lesions.
Keywords: Cutaneous leishmaniasis, Pistacia atlantica, L. major, Balb/c
Introduction
Cutaneous leishmanisis (CL) is a disease resulting from infection with species of the genus Leishmania including L. major and L. tropica in different parts of Iran (1, 2). L. major causes zoonotic cutaneous leishmaniasis (ZCL) in many rural areas of Iran (3). CL can be associated with significant morbidity and occasional deforming scars. Pentavalent antimonial compounds (sodium stibogluconate and meglumine antimoniate) have been the first drugs of choice in last decades for the treatment of this disorder. These drugs are parenteral and associated with significant side effects (4). These compounds, particularly meglumine antimoniate, are the first line drugs for the treatment of all forms of leishmaniasis in Iran too (5, 6). Based on a few studies that have been carried out in recent years, about 10 to 15% of CL has not desirable response to meglumine antimoniate in Iran (7). Recent circumstantial evidences are suggesting that an increasing number of Iranian patients with cutaneous leishmaniasis are unresponsive to meglumine antimoniate (Glucantime®) (7). Based on a recent studies miltefosine as an oral drug is apparently at least as good as meglumine antimoniate for the treatment of cutaneous leishmaniasis caused by L. major in Iran (6, 8).
The genus Pistacia belongs to the family Anacardiaceae. Among 15 known species of pistachios, only 3 species grow in Iran, including P. vera, P. Khinjuk and P. atlantica. They are the most important species of pistachio and for this reason, Iran is known as the origin of pistachios (9). P. atlantica is native to a number of countries of temperate Asia, e.g. Armenia, Azerbaijan, Iran, Iraq, Turkey, etc (10). In Iran, this plant grows in the central, western, and eastern areas (11). The resin of this plant, mastic gum, is obtained as an exudate after hurting the trunk and branches. Mastic gum has been used in traditional Greek medicine for various gastrointestinal disorders like gastralgia, dyspepsia and peptic ulcer for more than 2500 years. Ancient Greek physicians, such as Hippocrates, Dioscorides, Theophrastos and Galenos mentioned its properties and recommended its use (12). Mastic gum has been reported to possess considerable in vitro antibacterial and antifungal activity (13). Total mastic extract without polymer might be effective in reducing Helicobacter pylori colonization (12).
Because of existing high percentage of an insoluble and sticky polymer (poly-B-myrcene) in gum that hinders its oral administration and reduces bioavailability and that L. major causes topical lesion, in this study we aimed to determine cure rate of topical administration of P. atlantica gum on BALB/c mice infected by Iranian strain of L. major (MRHO/IR/75/ER).
Materials and Methods
Animal infection
Male BALB/c mice, 6–8 wk old with a body weight of approximately 20 g, used in this study. The animals were obtained from the Animal Breeding Stock Facility of Razi Institute of Iran,Hesarak, Karaj, Iran. Male BALB/c mice were inoculated subcutaneously about 2×106 L. major Promastigotes (MHROM/IR/75/ER) at the base of tail. Disease progression was monitored by parasitological examination of lesions after 4 to 8 wk post inoculation.
Ethical consideration
This study was conducted in adherence to ethical standards required for animal subjects protection.
Parasites
Iranian reference strain L. major promastigotes (MHROM/IR/75/ER) (prepared in the Protozoology Unit of the School of Public Health, Tehran University of Medical Sciences, Iran) were grown in RPMI-1640 supplemented with 15% inactivated fetal calf serum (FCS), 100 mg/ml streptomycin and 100 IU/ml penicillin G at 23–25 °C. Promastigotes from stationary-growth phase cultures were used for infection of mice.
Gum and Glucantime® treatment and evaluation
The gum of P. atlantica var. kurdica was collected from Kerend of Kermanshah (western part of Iran) summer 2007. The gum was obtained as an exudate of the trunk and branches. Glucantime® (Rorer Rhone-Poulenc Specia, Paris, France) kindly received from the Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences. Treatment for both substances was initiated after inoculation, when the infection was well preformed and local lesions were obvious. Before treatment diameter of lesions were measured and the mice were randomly divided into 3 groups of 15. in group 1 Glucantime® was administered to the BALB/c mice in regimen of 60 mg per kg of body weight for 28 d by intraperitoneal injections per day, in group 2 the gum of P. atlantica var. Kurdica were tested by daily rubbing of local lesions for 28 d, group 3 infected but non-treated.
Before treatment, four and 8 wk after beginning treatment, diameter of skin lesions were measured and impression smears were prepared from them lesions, the slide were fixed with absolute methanol, stained with Geimsa stain10% and examined by light microscopy with high magnification (X1000). Treatment effects of gum were determined by compared to Glucantime® and non-treatment groups by measuring the size of the skin lesions and the number of parasitologicaly positive and negative mice in treated with gum and Glucantime® and control.
Statistical analysis
The mean and standard deviation were calculated by using Microsoft EXCEL soft ware. Comparisons of treated groups and untreated group were done by two-way analysis of variance (ANOVA). Data were considered statistically significant at P< 0.05.
Results
The antileishmanial activity of the gum obtained P. atlantica var. kurdica was first tested in vivo on L. major. Gum of P. atlantica exhibited growth-inhibitory activity on L. major comparison control.
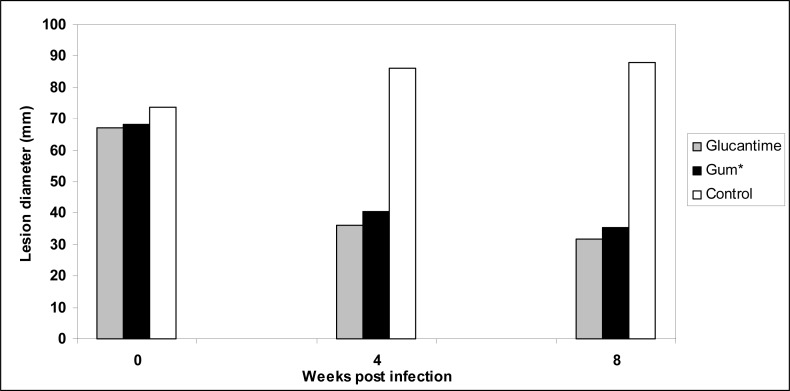
The lesions diameter in different groups of mice was measured before treatment, four and 8 wk after treatment. Table 1 shows that topically rubbing administration of gum obtained P. atlantica var. kurdica daily for 28 d prevented lesion development in the BALB/c mice infected with L. major compared with that in the control.
Table 1:
Inhibitory effects of treatments Lesion size reduction (mm) with Glucantime® and gum obtained Pistacia atlantica var. kurdica on leishmanial skin lesion sizes in BALB/c mice infected with L.major
| Week after treatment | |||
|---|---|---|---|
|
| |||
| Groups | 0 | 4 | 8 |
| Glucantime® | 67.01±12.46 | 36.14±5.01 | 31.9±8.02 |
| gum* | 68.4±6.92 | 40.36±4.98 | 35.3±5.01 |
| Control | 73.8±8.01 | 86.08±7.87 | 88.01±7.68 |
gum of pistacia atlantica var. kurdica
Effect of treatment of BALB/c mice infected with L. major with Glucatime at dose of 60 mg/kg daily for 28 d by the intraperitoneal route is presented in Table 1 data in this table show decreasing size of lesion comparison control (P< 0.01).
The parasite existence in leishmanial lesions were examined in three groups of mice before treatment and four and 8 wk after treatment.
Table 2 presents the results of the effect of gum obtained P. atlantica var. kurdica and Glucantime® on parasite existence in the BALB/c mice infected with L. major before treatment, four and 8 wk after treatment comparison that in the control animals.
Table 2:
Effect of treatment with Glucantime® and gum obtained Pistacia atlantica var. kurdica on parasite existence in BALB/c mice infected with L.major
| Week after treatment | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 4 | 8 | ||||
|
| ||||||
| substance | Negative | Positive | Negative | Positive | Negative | Positive |
| Glucantime® | 0 | 15 | 8 | 7 | 8 | 7 |
| gum* | 0 | 15 | 5 | 10 | 5 | 10 |
| Control | 0 | 15 | 0 | 15 | 0 | 15 |
gum of pistacia atlantica var. kurdica
As shown in Fig. 1, a decrease in the lesions size is observed in mice treated with gum obtained P. atlantica var. kurdica and Glucantime® comparison control animals (P< 0.01).
Fig. 1:
Treatment effects of Glucantime® and gum obtained Pistacia atlantica var. kurdica on leishmanial lesions sizes (mm) in BALB/c mice infected with L.major
Statistical analysis of Table 1 data shows treatment with gum obtained P. atlantica var. kurdica and Glucantime® causes decrease number parasitologicaly positive mice (P< 0.05).
Discussion
Despite the tremendous progress made in the understanding of the molecular biology of Leishmania and the clinical possibilities presented by some experimental chemotherapeutic agents, a few drugs have been developed for treating leishmaniasis (14). Also development of a new drug for the treatment of human leishmaniasis has been impeded by the lack of a simple, rapid drug-evaluation system that is universally applicable to the various Leishmania species (15).
A range of treatment options exists in leishmaniasis, which include two pentavalent antimonails, amphoterisin B, paromomycin, pentamidine, and the new oral agent miltefosine (16, 17).
Most of the commonly used antileishmanial drugs, such as pentavalent antimonail agents, exhibit considerable toxicity, and there are reports of large-scale clinical drug resistance among the organisms visceral leishmaniasis (18). The second-line drugs, such as amphoterisin B and pentamidine, do not have a therapeutic index as well as that of SbV, long-term therapy is often required, and they have toxic effects (19). About 75% of reported CL cases from Iran are zoonotic cutaneous leishmaniasis caused by L. major (3).
The crude gum of Pistacia contains an insoluble polymer (poly-B-myrcene), monoterpenes (such as α-pinene, limonene, α-phellandrene, β-pinene, β-myrcene, 3-carene, aldehyde citral, epoxypinene, limonene oxide) and triterpenes (oleanonic acid, moronic acid, 24Z-masticadienonic acid, 24Z-isomasticadienonic acid, 24Z-masticadienolic acid, and 24Z-isomasticadienolic acid) (11, 12).
Some studies have shown that terpenic compounds such as labdone diterpene (isolated from Polyalthia macropoda), the phorbol ester, iridoid glycosides (found in Picrorhiza kurroa and Nyctanthes arbortritis) and Picroliv (isolated from Picroliv kurroa) have antileishmanial effects (20–22).
Our results show that gum obtained P. atlantica var. kurdica can used for controlling cutaneous leishmaniasis caused by L. major, to inhibit development of cutaneous leishmaniasis lesions(P< 0.01) and to be active against L. major in 30% of mice (P< 0.05).
With regard to the sticky polymer in gum reduces bioavailability of the contained active compounds, our results suggest if full adsorption of active compounds the effect of gum will increase.
Recently, the resistance against antimonial drugs, toxicity and side effects of systemically administrative drugs have been reported (6, 7, 18), therefore there is a great for the development of effective, safe and topically administrative drugs for the different forms of leishmaniasis.
Acknowledgments
This study financially was supported by Tehran University of Medical Sciences, Iran. Authors are thankful to Dr SM Massoumi for identification of plant. The authors declare that they have no conflicts of interest.
References
- 1.Nadim A, Seyedi-Rashti MA. A brief review of the epidemiology of various types of leishmaniasis in Iran. Acta Med Iranica. 1971;XIV:99–106. [Google Scholar]
- 2.Yaghoobi MR, Hanafi AA, Javadian E, Jafari R, Mohebali M. A new focus of cutaneous leishmaniasis caused by Leishmania tropica. Saudi Med J. 2002;24:98–101. [PubMed] [Google Scholar]
- 3.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of the Islamic Republic of Iran. East Mediterr Health J. 2004;10:591–99. [PubMed] [Google Scholar]
- 4.Van-voorhis WC. Therapy and prophylaxis of systemic protozoan infections. Drugs. 1990;40:176–202. doi: 10.2165/00003495-199040020-00002. [DOI] [PubMed] [Google Scholar]
- 5.Momeni AZ, Aminjavaheri M. Treatment of non-healing cases of cutaneous leishmaniasis. Successful treatment using a combination of meglumine antimoniate plus allopurinol. EJD. 2003;13:40–43. [PubMed] [Google Scholar]
- 6.Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhoundi B, Rahnema A, Razaghian AR, Kabir MJ, Nadim A. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103(1):33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. Unresponsiveness to Glucantime® treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med. 2006;3(5):e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmaeili J, Mohebali M, Edrissian GH, Rezayat SM, Ghazi-Khansari M, Charehdar S. Evaluation of miltefosine against Leishmania major (MRHO/IR/75/ER): in vitro and in vivo studies. Acta Med Iranica. 2008;46:191–96. [Google Scholar]
- 9.Behboodi B.Sh. Ecological distribution study of wild pistachios for selection of rootstock. Options Mediterran. 2003;63:61–66. [Google Scholar]
- 10.http://www.ars-grin.gov/cgi-bin/html/taxon.pl/101771.
- 11.Delazar A, Reid RG, sarker SD. GC-MS analysis og the essential oil from the oleoresin of Pistacia atlantica var mutica. Chem Nat Comp. 2004;40(1):2004. [Google Scholar]
- 12.Paraschos S, Magiatis P, Mitakou S, Petraki K, Kalliaropoulos A, Maragkoudakis P, Mentis A, Sgouras D, Skaltsounis A. In Vitro and In Vivo Activities of Chios Mastic gum Extracts and Constituents against Helicobacter pylori. Antimicrob Agents Chemother. 2007;51(2):551–59. doi: 10.1128/AAC.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassou CC, Nychas GJE. Antimicrobial activity of the essential oil of mastic gum (Pistacia lentiscus var. chia) on gram positive and gram negative bacteria in broth and model food system. Int Biodeter Biodegradation. 1995;36:411–20. [Google Scholar]
- 14.Croft SL. In vitro screens in the experimental chemotherapy of leishmaniasis and trypanosomiasis. Parasitol Today. 1986;2:64–69. doi: 10.1016/0169-4758(86)90157-2. [DOI] [PubMed] [Google Scholar]
- 15.Iwu MM, Jacksona JE, Schuster BG. Medicinal plants in the fight against leishmaniasisa. Parasitol Today. 1994;10:65–68. doi: 10.1016/0169-4758(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 16.Davidson RN. Practical guide for the treatment of leishmaniasis. Drugs. 1998;56:1009–18. doi: 10.2165/00003495-199856060-00005. [DOI] [PubMed] [Google Scholar]
- 17.Jha Tk, Sundar S, Thakur CP, Bachmann P, Karbwang J, Fisher C. Miltefosine an oral agent, fro the treatment of Indian leishmaniasis. N Engl J Med. 1999;341:1795–800. doi: 10.1056/NEJM199912093412403. [DOI] [PubMed] [Google Scholar]
- 18.United Nations development program/World Health Organization Special program for research and training in leishmaniasis” alarming”. TDR news. 1990;34:1–7. [Google Scholar]
- 19.Berman JD, Lee LS, Robins RK, Revankar GR. Avtivity of purine analogs against Leishmania tropica within human macrophage in vitro. Antimicrob Agents Chemother. 1983;24:233–36. doi: 10.1128/aac.24.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson RA, Manian A, Hall D, Harcus JL, Hewlett L. Antileishmanial activity of chlorpromazine. Antimicrob Agents Chemother. 1984;25:571–74. doi: 10.1128/aac.25.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tandon JS, Srivastava V, Guru PY. Iridoids: a new class of. leishmanicidal agents from Nyctanthes arbortristis. J Nat Prod. 1991;54:1102–1104. doi: 10.1021/np50076a030. [DOI] [PubMed] [Google Scholar]
- 22.Puri A, Saxena RP, Sumati PY, Kulshreshtha DK, Saxena KC, Dhawan BN. Immunostimulant Activity of Picroliv, the Iridoid Glycoside Fraction of Picrorhiza kurroa, and its Protective Action against Leishmania donovani Infection in Hamsters. Planta Med. 1992;06:517–32. [PubMed] [Google Scholar]