Abstract
Background:
Zoonotic cutaneous leishmaniasis (ZCL) is an expanding disease and public health problem in Iran. In the current study, natural Leishmania infection rate and seasonal fluctuation of the infection in Rhombomys opimus population of a hyperendemic focus of ZCL in Iran was investigated.
Methods:
The study was conducted from October 2006 to October 2008 in Esfahan Province, central part of Iran. An extensive sampling of rodents using Sherman traps was done in different seasons. Nested PCR assay was used for detection and identification of Leishmania species and the results were confirmed using PCR-RFLP.
Results:
Leishmania infection rate was 58.6% (34 of 58) using nested PCR. 44.8% of the gerbils were infected only with L. turanica and 1.7% with L. gerbilli alone. A mixed natural infection with L. major and L. turanica was seen in 12.1% of the rodents. L. major infection alone was not seen in R. opimus population in the study area. The highest and lowest Leishmania infection rates were observed in fall and spring respectively. L. turanica infection was observed throughout the year whereas mixed infections with L. major and L. turanica was not seen in spring.
Conclusion:
It is concluded that in the study area, L. major, L. gerbilli and L. turanica circulate in the population of R. opimus. Leishmania major infection usually accompanied by L. turanica in naturally infected gerbils with the highest rate in fall. It is recommended that the role of L. turanica in the epidemiology and transmission of ZCL be revisited.
Keywords: Rhombomya opimus, Leishmania mixed infection, Cutaneous leishmaniasis, Molecular epizootiology, Iran
Introduction
Cutaneous leishmaniasis due to Leishmania major, a neglected tropical disease, is a major public health problem in some areas of the old world (1). L. major is widely distributed in various populations of rodents in arid and savannah regions (1). The disease is endemic in many rural districts of Iran, in 17 out of the 30 provinces. Rodents belong to Gerbillinae subfamily are the main reservoir hosts for ZCL in Iran and other countries where ZCL due to L. major is endemic (2–4). Gerbils are the most abundant mammals reported from natural ecosystems of old world deserts (2). Many rodent species act as reservoir host of ZCL. Rhombomys opimus (great gerbil) in central Asia, northern Afghanistan and Iran; Meriones libycus (Libyan jird) in the Arabian Peninsula, central Asia and Iran; M. hurrianae (Indian desert jird) in India and Iran; Psammomys obesus (fat sand rat) and M. crassus in northern Africa and Middle East; and Tatera spp. in subsaharan Africa and Iran (1). R. opimus (Cricetidae: Gerbillinae) is the main L. major reservoir host in the vast territory of the Turan lowland (west and south Kazakhstan and central Asia with adjacent parts of Afghanistan and Iran), Mongolia, and apparently, in some provinces of China. In the Turan lowland, naturally infected R. opimus are reported from more than 200 regions. The number of naturally infected great gerbils showed to be greater than any other mammals (other rodents, insectivores, carnivores) (2).
All the proven vectors of ZCL belong to the subgenus Phlebotomus (Phlebotomus), i.e. P. papatasi, the main vector, and related species P. salehi and P. dubosqi. Well-described stable ZCL systems are associated with L. major and P. obesus/P. papatasi in North Africa and Middle East, and R. opimus/P. papatasi in central Asia, Afghanistan and Iran (1, 5). The distribution and the role of rodents as ZCL reservoir hosts are geographically specific in Iran. R. opimus is the main reservoir of ZCL in Central and North East Iran followed by M. libycus (Cricetidae; Gerbillinae), which is the primary reservoir of ZCL in some areas of the central and southern Iran. In the south and south west of the country including the Iran-Iraq border, the reservoir is T. indica, the Indian jird (Cricetidae: Gerbillinae). In Baluchistan of Iran (border of Pakistan), M. hurrianae (Cricetidae: Gerbillinae) acts as a reservoir host (4, 6–10). One of the major problems for control of this neglected disease is lack of information about the dynamics of Leishmania parasites infection rates in rodent populations as the reservoir hosts.
In the present study, an investigation was carried out on natural infection rates of Leishmania parasites and seasonal fluctuations of the infection in Rhombomys opimus (Rodentia: Gerbillinae) population in a hyperendemic focus of ZCL in Iran.
Materials and Methods
Study area
The investigation was conducted over a period of 24 mo from October 2006 to October 2008 in Borkhar and Sejzi rural districts, 15–35 km, northeast and east of Esfahan City (32° 39′ 35″ N/51° 40′ 17″ E), Esfahan Province, central Iran, respectively where ZCL is hyperendemic. The study areas are located at an altitude of around 1,550 m, with a desert climate, hot summer and cold winter. In 2007, the maximum and minimum mean monthly temperatures were 37.8 ºC and −6.3 ºC in July and January, respectively. The total annual rainfall in this year was 109.7 mm. The maximum mean monthly relative humidity was 83.3% in January and the minimum was 13.9% in September (Esfahan Metrological Organization). Wheat, barley, cotton, vines, beetroot, pistachio, alfalfa, Indian corn, clover and summer crops are cultivated in these areas (10).
Collection of rodents
In the first year of the study, active colonies of gerbils in the district were identified and the rodents were caught using 20–45 Sherman traps baited with cucumber for detection and identification of Leishmania parasites. In the second year, the rodents were collected using around 40 Sherman traps baited with cucumber each season to determine the seasonal fluctuations of the parasites in the populations of R. opimus. In spring to fall, the traps were placed at the gerbil holes in the afternoon and collected in the morning of the following day. In winter, Sherman traps were placed after sunrise and collected at the same day afternoon. The trapped gerbils were transferred to the animal house facility at the Esfahan Training and Health Research Center, National Institute of Health, Esfahan, Iran, and maintained until use for parasitological and molecular testing.
Identification of the rodents was done using morphological characters (11) and only great gerbils, R. opimus, were included in the study.
Direct examination test
In the laboratory, the rodents were anaesthetized using intramuscular Ketamine hydrochloride (60 mg/kg) and Xylazine (5 mg/kg). Regardless of having any obvious lesions, impression smears were prepared from the ear lobes of the animals (12), and stained using Giemsa and directly examined under a light microscope at high magnification (1000x). After preparing direct smears, ear lobe samples were removed while the rodents were anaesthetized. The ear lobes were transferred to cold phosphate-buffered saline (pH=7.4) and thoroughly disrupted by grinding with a pestle and kept at −20 °C until use. The animals were nursed to complete recovery.
Ethical consideration
Animal experiments were approved by the Ethical Committee of Tehran University of Medical Sciences, Tehran, Iran.
Nested PCR assay
Genomic DNA was extracted and purified using a conventional phenol-chloroform protocol. Briefly, 200 μl of lysis buffer (100 mM Tris-HCl, pH= 8; 10 mM EDTA, pH=8; 1% SDS; 100mM NaCl; 2% Triton X-100) with proteinase K (100 mg/ml) were added to 100 μl homogenized suspension of disrupted tissues and cells. The sample was incubated at 56 °C for one hour and subjected to a phenol-chloroform extraction (phenol-chloroform followed by chloroform). Extracted DNA was precipitated with an equal volume of isopropanol and 1/10 volume of 3M sodium acetate (pH= 5.2). The pellet was washed with 70% ethanol, air dried at room temperature and resuspended in 20 ul of sterile distilled water.
Fragment length polymorphism of the second internal transcribed spacer (ITS2) in the ribosomal RNA gene (rDNA) based on a nested PCR system was used for detection and species-identification of Leishmania. The sequence of the designed primers were as follows: Leish out F (5′AAA CTC CTC TCT GGT GCT TGC-3′) and Leish out R (5′-AAA CAA AGG TTG TCG GGG G-3′) as the outer primers, and Leish in F (5′-AAT TCA ACT TCG CGT TGG CC-3′) and Leish in R (5′-CCT CTC TTT TTT CTC TGT GC-3′) as the inner primers. The PCR products were visualized by agarose gel electrophoresis and ethidium bromide staining. The results of nested PCR were confirmed based on species-specific pattern of PCR-RFLP using the restriction digestion with MnlI. Digestion was performed by adding 5U (0.5 μl of the enzyme) and 1.5 μl of the relevant buffer to a 13 μl aliquot of the nested PCR product in a final volume of 15 μl. The mixture was incubated at 37 °C for 3 h and the products were separated using 2.5% agarose gel electrophoresis and visualized using ethidium bromide staining.
Statistical analysis
The Fisher s exact test using SPSS 11.5 software was used and P< 0.05 was considered as significant.
Results
A total of 58 R. opimus (32.1% male and 67.9% female), were captured and examined by two diagnostic techniques, direct examination and nested PCR. Fourteen out of 58 specimens (24.1%) were positive by microscopic examination and 34 (58.6%) by the nested PCR. In 29 samples which the amastigote was not seen by through direct examination, the nested PCR showed positive results, and every positive smear was also found positive by nested PCR. Out of 34 nested PCR positive samples, 26 (76.5%) were identified as L. turanica, 1 (2.9%) was L. gerbilli, and 7 (20.6%) were mixed infection of L. major and L. turanica. Leishmania infection rate of male and female gerbils were 52.9% and 61.1% respectively which was not statistically different. Based on direct smear examination, none of the Leishmania positive gerbils showed cutaneous lesion.
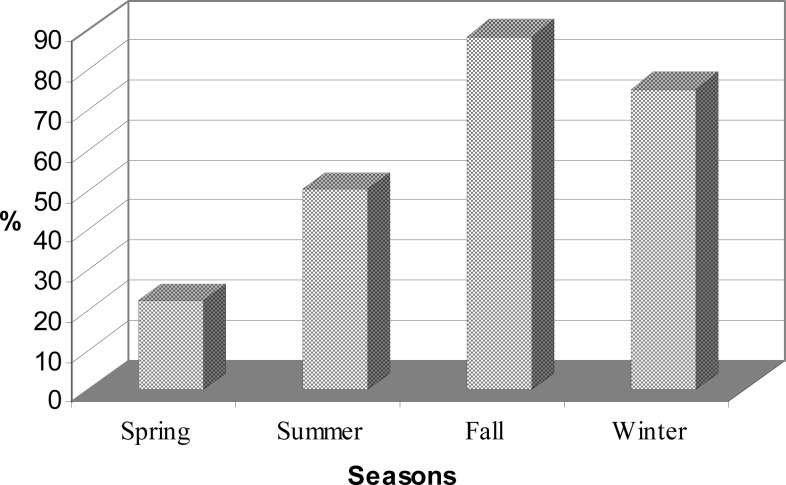
Twenty six out of 58 (44.8%) of the gerbils were identified to be infected only with L. turanica, and 1 (1.7%) with L. gerbilli. Interestingly no gerbil was found to be infected with L. major alone. A mixed natural infection with L. major and L. turanica was seen in 12.1% of the rodents. Mixed infection of L. major and L. gerbilli and also L. gerbilli and L. turanica, were not seen in this study. The results of the seasonal variation of Leishmania infection which was performed only in the second year showed that pure L. turanica infection was seen throughout the year whereas mixed infection of L. major and L. turanica was seen in all seasons except spring. The highest Leishmania infection rate was observed with L. turanica (70.6%) in fall (Table 1). The highest (88.2%) and lowest (22.2%) Leishmania infection rate was observed in fall and spring respectively (Fig. 1). Statistically significant difference was observed in Leishmania infection rate in different seasons (P= 0.005).
Table 1:
Leishmania species infection rate of Rhombomys opimus population in different seasons, Sejzi and Borkhar rural districts, Esfahan Province, Iran, Oct 2007–Oct 2008
| Species | L. turanica | L. major and L. turanica | |||
|---|---|---|---|---|---|
|
|
|||||
| Season | No. of positive samples/no. of examined samples | % Positive | No. of positive samples/no. of examined samples | % Positive | |
| Spring | 2/9 | 22.2 | 0/9 | 0 | |
| Summer | 3/8 | 37.5 | 1/8 | 12.5 | |
| Fall | 12/17 | 70.6 | 3/17 | 17.7 | |
| Winter | 4/8 | 50 | 2/8 | 25 | |
| Total | 21/42 | 50 | 6/42 | 14.3 | |
Fig. 1:
Seasonal fluctuations of Leishmania infection rate of great gerbil population, Sejzi and Borkhar rural districts, Esfahan Province, Iran, Oct 2007–Oct. 2008
Discussion
The results of the current study showed that L. major, L. gerbilli and L. turanica circulate in R. opimus populations in the study region. L. turanica was the dominant species detected in R. opimus population in the study areas in all seasons. Infection with L. major alone was not seen in R. opimus and L. gerbilli alone was rare. L. major infection was mostly accompanied by L. turanica. In spring, at the beginning of active season of sand flies (13), the only Leishmania infection in R. opimus population was due to L. turanica. (Table 1). In Turkmenia and Uzbekistan, epizootics among R. opimus population always developed with L. turanica at the beginning of transmission season. L. major infection rate was extremely low in June and increased in late August and September (14). In the district of Borkhar, an hyperendemic focus of the disease in Esfahan Province, the highest Leishmania infection rate in great gerbils was previously reported from August to December (10).
For vast territories of central Asia, mixed infections of wild rodents with L. major (pathogenic to human) and L. turanica (non-pathogenic to human) are typical. Each parasite has own range of pathogenicity and virulence (3). This animal proved to be susceptible to L. major, L. turanica and L. gerbilli. Infection with L. major alone rarely occurred in R. opimus. L. turanica promotes the persistence of L. major infection in the great gerbil (14). In an experimental Leishmania infection, the duration of L. major infection was 7 months, in L. turanica infection was 15 months, and L. gerbilli infection was 18 months. However, co-infection of L. major and L. turanica the duration of infection was extended up to 39 mo. Ulceration and visceralization never reported in great gerbils (15). Substantial part of great gerbils population live for more than one year (2) and remained as ZCL reservoir host for entire life and seem to be potential sources of Leishmania transmission until death (3). L. turanica proved to be the dominant species in R. opimus population located in hypoendemic, as well as meso- and hyperendemic foci of ZCL in Turkmenistan and Uzbekistan (14).
Rhombomys opimus is the main reservoir host of ZCL in Iran as well as some other countries (1, 10, 14, 15). In the previous studies, only L. major was isolated from great gerbils and characterized using isoenzyme or DNA-based molecular techniques in Iran (5, 10, 16, 17). However, there are rare reports of L. turanica infection in R. opimus (18). In most of the studies, identification of Leishmania species was done after isolation of Leishmania parasites from the culture media, which usually resulted in growth of only one species of Leishmania (4, 5, 16). Regarding the sand fly vectors, L. major is also isolated and characterized from Phlebotomus papatasi, P. caucasicus, as well as human lesions, in Iran (5, 19–21). Recently naturally infection of sand flies with L. major, L. turanica and L. gerbilli are reported from Iran (22). The three species were identified in naturally infected gerbils from Turkmenistan, Uzbekistan and Kazakhstan (3, 14). L. major infection of gerbils is crucial in the transmission cycle of ZCL (1, 3, 5). The distribution of L. major as the causative agent of ZCL in central Asia has been found to coincide with R. opimus (3).
It is concluded that L. major, L. gerbilli and L. turanica circulate in the population of R. opimus in central part of Iran. L. turanica was the dominant species in the population of great gerbils. Infection with L. major alone was not seen in the population of the gerbil. Leishmania major infection usually accompanied with L. turanica in naturally infected gerbils with the highest rate in fall. It is recommended that the role of L. turanica in the epidemiology and transmission of ZCL be revisited carefully.
Acknowledgments
The authors would like to thank director and staff of Esfahan Training and Health Research Center, National Institute of Health Research, especially Mr F Mohaghegh for their kind administrative assistance. This work was funded by Deputy of Research of Tehran University of Medical Sciences, through project no 3025. The authors declare that they have no conflicts of interests.
References
- 1.Gramiccia M, Gradoni L. The current status of zoonotic leishmaniasis and approaches to disease control. Int J Parasitol. 2005;35:1169–180. doi: 10.1016/j.ijpara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Dubrovsky YA. 1979. Biology of great gerbil-the principal carrier of zoonotic cutaneous leishmaniasis. WHO Traveling Seminar on leishmaniasis control. ex-USSR Ministry of Health.
- 3.Strelkova MV. Progress in studies on Central Asian foci of zoonotic cutaneous leishmaniasis-a review. Folia Parasitol. 1996;43:1–6. [PubMed] [Google Scholar]
- 4.Yaghoobi-Ershadi MR, Akhavan AA, Mohebali M. Meriones libycus and Rhombomys opimus (Rodentia: gerbillidae) are the main reservoir hosts in a new focus of zoonotic cutaneous leishmaniasis in Iran. Trans R Soc Trop Med Hyg. 1996;90:503–4. doi: 10.1016/s0035-9203(96)90295-3. [DOI] [PubMed] [Google Scholar]
- 5.Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AR, Abaei MR, Ebrahimi B, Vafaei-Nezhad R, Hanafi-Bojd AA, Jafari R. Epidemiological study in a new focus of cutaneous leishmaniasis in the Islamic Republic of Iran. EMHJ. 2003;9:816–26. [PubMed] [Google Scholar]
- 6.Javadian E. Reservoir host of cutaneouse leishmaniasis in Iran. Abstracts of XII International Congress for Tropical Medicine and Malaria; 18–23 September 1988; Amsterdam, The Netherlands. 1988. [Google Scholar]
- 7.Javadian E, Dehestani M, Nadim A, Rassi Y, Tahvildare-Bidruni GH, Seyedi-Rashti MA, Shadmehr A. Confirmation of Tatera indica (Rodentia:Gerbillidae) as the main reservoir host of zoonotic cutaneous leishmaniasis in the west of Iran. Iranian J Publ Health. 1988;27:55–60. [Google Scholar]
- 8.Rassi Y, Jalali M, Javadian E, Moatazedian MH. Confirmation of Meriones libycus (Rodentia; Gerbillidae) as the Main Reservoir Host of Zoonotic Cutaneous Leishmaniasis in Arsanjan, Fars Province, South of Iran (1999–2000) Iranian J Publ Health. 2001;30:143–44. [Google Scholar]
- 9.Rassi Y, javadian E, Amin M, Rafizadeh S, vatandoost H, Motazedian H. Meriones libycus is the main reservoir zoonotic cutaneous leishmaniasis in south Islamic Republic of Iran. EMHJ. 2006;12:474–77. [PubMed] [Google Scholar]
- 10.Yaghoobi-Ershadi MR, Javadian E. Epidemiological study of reservoir hosts in an endemic area of zoonotic cutaneous leishmaniasis in Iran. WHO Bull. 1996;74:587–90. [PMC free article] [PubMed] [Google Scholar]
- 11.Etemad E. 1978. Mammals of Iran, Vol. 1. Rodents and their Identification Keys. Tehran, National Society of Guardianship of Natural Resources and Human Environment.
- 12.Edrissian GH, Zovein Z, Nadim A. A simple technique for presentation of smears from the ear of Rhombomys opimus for the detection of leishmanial infection. Trans R Soc Trop Med Hyg. 1982;76:706–707. doi: 10.1016/0035-9203(82)90255-3. [DOI] [PubMed] [Google Scholar]
- 13.Yaghoobi-Ershadi MR, Javadian E. Studies on sandflies in an hyperendemic area of zoonotic cutaneous leishmaniasis in Iran. Indian J Med Res. 1997;105:61–6. [PubMed] [Google Scholar]
- 14.Strelkova MV, Elseev LN, Ponirovsky EN, Dergacheva TL, Annacharyeva Dk, Erokhin PI, Evans DA. Mixed leishmanial infection in Rhombomys opimus: a key to the persistence of Leishmania major from one transmission season to the next. Ann Trop Med Parasitol. 2001;95:811–819. doi: 10.1080/00034980120111154. [DOI] [PubMed] [Google Scholar]
- 15.Strelkova MV. Susceptibility to and the characteristics of the course of experimental leishmaniasis in different species of mammals infected with Leishmania major, L. turanica and L. gerbilli. Med Parizitol. 1991;1:35–39. [PubMed] [Google Scholar]
- 16.Mohebali M, Javadian E, Yaghoobi-Ershadi MR, Akhavan AA, Hajjaran H, Abaei MR. Characterization of Leishmania infection in rodents from endemic areas of Islamic Republic of Iran. EMHJ. 2004;10:591–99. [PubMed] [Google Scholar]
- 17.Parvizi P, Moradi Gh, Akbari Gh, Farahmand M, Ready PD, Piazak N, Assmar M, Amirkhani PCR detection and sequencing of parasite ITS-rDNA gene from reservoir host of zoonotic cutaneous leishmaniasis in central Iran. Parasitol Res. 2008;103:1289–95. doi: 10.1007/s00436-008-1124-z. [DOI] [PubMed] [Google Scholar]
- 18.Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AR, Abaei MR, Ebrahimi B, Vafaei-Nezhad R, Hanafi-Bojd AA, Jafari R. Letter to editor. EMHJ. 2004;10:1. [PubMed] [Google Scholar]
- 19.Akhavan AA, Yaghoobi-Ershadi MR, Hasibi F, Jafari R, Abdoli H, Arandian MH, Soleimani H, Zahraei-Ramazani AR, Mohebali M, Hajjaran H. Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of Southern Iran. Iranian J Arthropod-Borne Dis. 2007;1(1):1–8. [Google Scholar]
- 20.Yaghoobi-Ershadi MR, Javadian E, Tahvildare-Bidruni GH. The isolation of Leishmania major from Phlebotomus (Paraphlebotomus) caucasicus in Isfahan Province, Islamic Republic of Iran. Trans R Soc Trop Med Hyg. 1994;88:518–19. doi: 10.1016/0035-9203(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 21.Yaghoobi-Ershadi MR, Akhavan AA, Zahraei-Ramazani AR, jalali-zand AR, Piazak N. Bionomics of Phlebotomus papatasi (Diptera: Psychodidae) in an endemic focus of zoonotic cutaneous leishmaniasis in Iran. JVector Ecol. 2004;30:115–18. [PubMed] [Google Scholar]
- 22.Parvizi P, Ready PD. Sequencing of nuclear ITS-rDNA fragments detects three Leishmania species of gerbils in sand-flies from Iranian foci of zoonotic cutaneous leishmaniasis. Trop Med Int Health. 2008;13:1159–71. doi: 10.1111/j.1365-3156.2008.02121.x. [DOI] [PubMed] [Google Scholar]