Abstract
Background:
GB virus C (GBV-C) is a flavivirus that was characterized in 1995. The prevalence of GBV-C RNA in HIV-infected subjects has not been previously studied in Iran and was therefore determined.
Methods:
We have investigated serum samples of 80 patients from Imam Khomeini Hospital, Tehran, Iran, whose HIV infection was confirmed in our laboratory by Western-blotting. We used nested-PCR to detect GBV-C/HGV RNA in their sera.
Results:
GBV-C/HGV RNA was detected in 15 (18.8%) of 80 patients. There was no significant difference in GBV-C/HGV RNA prevalence between males and females, in different age groups and HIV viral loads groups.
Conclusion:
The prevalence of GBV-C RNA was high in HIV-infected patients. There was no association between GBV-C RNA prevalence and specific gender, age, and HIV viral loads groups.
Keywords: GBV-C RNA, HIV, Co-infection, Iran
Introduction
GB virus C (GBV-C) is a blood borne flavivirus that was first characterized in 1995 (1, 2). Based on comparison of genome organization and sequence homologies, GBV-C is the most closely related human virus to HCV, another member of the flaviviridae (2–4). GBV-C infection has association with neither specific disease nor substantial health risk (5).
The presence of serum GBV-C indicates viremia, whereas the HGV envelope E2 glycoprotein-specific antibody (E2Ab) is associated with recovery (6–16).
About 1–4% of healthy blood donors have GBV-C viremia in their sera (7–9). GBV-C is primarily parenterally or sexually (9). Male to male sex has been proposed to be an effective mode of transmission of GBV-C (10). However, a vertical transmission route is also likely (9). Considering that GBV-C is a blood borne virus, its prevalence is high in haemodialysis patients (6.8%), multiple blood transfusion recipients (24.4%), and hemophilia patients (35.2%) (11). Because of common routes of transmission, GBV-C infection is also high in both, HIV and HCV positive patients (12). Its prevalence is about 14%–45% in HIV positive patients and about 24.4% in HCV infected individuals (13, 14).
Actual prevalence of GBV-C varies according to the particular population studied. In addition, little is known about prevalence of active GBV-C infection in HIV-infected individuals in Iran. The aim of the present study was to evaluate the prevalence of HGV RNA in serum samples of HIV positive patients using RT-PCR. We compared the presence of GBV-C viremia with sex, age, HIV viral load. For this purpose, we collected sera from patients referred to Imam Khomeini Hospital in Tehran, Iran.
Materials and Methods
Patients
Eighty patients from Imam Khomeini Hospital, Tehran, Iran who had been identified positive for HIV by ELISA and we confirmed their HIV infection by Western blotting involved in this study. The patients’ ages were between 5 and 54 yr (meanly 34.7 yr), 87.5% male and 12.5% female. Serum samples were collected and stored at −70 °C.
Information such as age, sex, transmission routs were extracted from patient’ files. We were unable to follow up eight patients in further analysis.
RNA GBV-C/HGV extraction and cDNA synthesis
RNA was extracted from 100μl of serum or plasma using the Acid Guanidium Isothiocyanate Phenol Chloroform method. 10 μl of extracted RNA was used for c-DNA synthesis in a final volume of 20 μl. cDNA was synthesized by incubation at 42 °C with MMLV reverse transcriptase and random hexamers for 30 min. The cDNA was stored at −20 °C for using in nested-PCR method. Positive and negative controls were included in each run. Positive controls were provided from Keyvan laboratory and their GBV-C genome was sequenced at Seq. Lab Co. Germany.
Nested-PCR for GBV-C RNA detection:
Two sets of primers located in 5′ non-coding region of GBV-C genome were used in nested-PCR. External primers:
HG1: 5′CACTATAGGTGGGTCTTAAG3′
HG2: 5′GCCTATTGGTCAAGAGAGAC3′
Internal primers:
HG3: 5′GCGCACGGTCCACAGGTGTT3′
HG4: 5′GGGCGACGTGGACCGTACGT3′
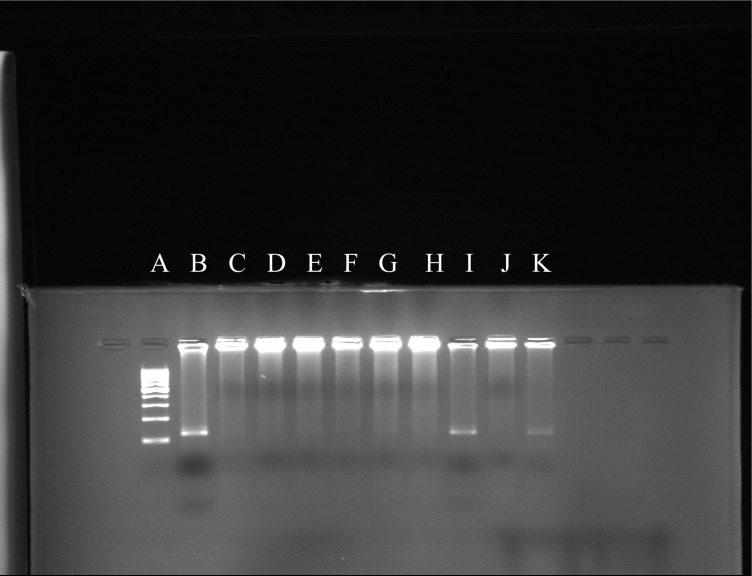
For the first PCR round, 10 μl c-DNA was mixed with PCR master mixture containing 10 pmol Primer (HG1, HG2) 0.6 mM dNTP, 1.5 unit Taq DNA polymerase, 5 μl 10x Buffer and 1.5 mM Mgcl2 in a final volume of 50 μl for 30 cycles (94 °C 0.5 min, 55 °C 1.5 min, 72 °C 1.5 min). For the second PCR round 10μl product from the first PCR round was used as template the other reagents (except primers: HG3, HG4) were the same as that in the first PCR round. The second PCR round was 35 cycles (94 °C 0.5 min, 55 °C 1.5 min, 72 °C 1.5 min). Amplified products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining (Fig. 1).
Fig. 1:
Nested-PCR products. A: Ladder, B: Positive control, C: Negative control, I & K: GBV-C Positive samples, D, E, F, G, H & J: GBV-C Negative samples
Statistical analysis
For comparing prevalence data between different subgroups in the studied population Chi square test and Man Whitney test were used. A P< 0.05 was considered significant. All P values were calculated as two-tailed.
Ethical considerations
Informed consent was given from all patients involved in the study.
Results
In 15 of 80 patients, GBV-C RNA was detectable therefore; the prevalence of GBV-C carriers was 18.8%. In our study, there was no significant difference between prevalence of GBV-C RNA in different age groups among GBV-C positive patients and between mean age in GBV-C negative and positive groups of HIV positive population.
There was no significant difference in GBVC RNA prevalence between male and female patients. The prevalence was slightly higher in male group but it can be because of involving more men in our study than women.
We compared GBV-C prevalence in different HIV viral loads and there was no significant difference. Transmission routs were compared in GBV-C positive group. In 16.7% patients transmission rout was sexual and in the other were intravenous drug using. We did not have patients with other transmission routs such as blood transmission or mother to infant in our GBV-C positive group (Table 1).
Table 1:
Prevalence of GBV-C RNA according to sex, age groups, HIV transmission routes, and HIV viral load
| Groups | % (No.) with GBV-C RNA (Total 15) | % (No.) without GBV-C RNA (Total 65) | P value |
|---|---|---|---|
| sex | |||
| male | 20.6 (13) | 79.4 (50) | 0.67 |
| female | 11.1 (1) | 88.9 (8) | |
| age (years) | |||
| <10 | 0 (0) | 100 (1) | 0.95 |
| 10_20 | 0 (0) | 0 (0) | |
| 20_30 | 26.7 (4) | 73.3 (11) | |
| 30_40 | 18.2 (6) | 81.8 (27) | |
| 40_50 | 22.2 (4) | 77.8 (14) | |
| >50 | 0 (0) | 100 (4) | |
| HIV transmission routes | |||
| intravenous drug use | 83.3 | 35.3 | not evaluated |
| heterosexual contact | 16.7 | 35.3 | |
| blood transmission | 0 | 16.7 | |
| mother to infant | 0 | 5.9 | |
| addict + sexual | 0 | 5.9 | |
| HIV viral load groups | |||
| <1000 | 9.1 (1) | 90.9 (1) | 0.43 |
| 1000–9999 | 17.4 (4) | 82.6 (19) | |
| 10000–99999 | 23.1 (6) | 76.9 (20) | |
| >100000 | 20 (4) | 80 (16) |
Discussion
The aim of this study was to evaluate the prevalence of GBV-C RNA in serum samples of HIV-1 positive patients: 15 of 80 patients were viremic (18.8% GBV-C seroprevalence of GBV-C virus genome). This finding confirms with the previous reports of virus genome prevalence of 14% to 45% (13, 14). Similar prevalence of GBVC viremia among HIV infected patients (19.5% and 18.2%) was reported (11, 15). Higher GBV-C RNA prevalence was reported by other groups: Aster et al. reported 33.3% prevalence of GBVC RNA in HIV-infected patients (16). Similar prevalence was reported separately as 37% (13, 17). There is a higher GBV-C prevalence among HIV-infected persons in Scotland (51%) (18). Such controversy may be a result of different number of patients involving in these studies and the fact that in the study which was conducted in Scotland, the most patient’s transmission route was homosexuality (male to male transmission) which has been demonstrated that GBV-C transmission trough it, is more frequent. In addition, it was shown that male to male transmission is a very effective rout in HIV transmission (1 in 10 to 1 in 1,600) and this rate is really noticeable compared with other HIV transmission routs such as heterosexuality (female to male transmission: 1 in 700 male to female transmission: 1 in 200), needle sharing (1 in 150), needle stick (1 in 200) (19). In Iran prevalence of 12.6%, GBV-C RNA was reported in haemodialysis patients (20). In addition, a low prevalence of HGV exposure was found in the dialysis staff (0%) (21). These reported results are lower than HGV prevalence in HIV-infected individuals in our study. This difference is expected because the routes of transmission of HIV and GBV-C are identical.
The prevalence of GBV-C RNA did not vary according to the age groups and sex in our study. Although the prevalence was slightly higher in male, the difference was not significant and it can be a result of involving more men than women in our study (63 out of 72). Our results were similar to those reported by Rey et al., which showed no difference in GBV-C RNA prevalence between male and female and their mean ages were similar in GBV-C positive and GBV-C negative groups. However, in the GBV-C genome prevalence was lower in patients over 50 yr compared to other age groups (13). In our study, there were only 4 persons over 50 yr and none of them were GBV-C RNA positive.
There are some studies, which had demonstrated that HIV-infected persons who were co-infected with GBV-C had lower HIV viral loads in comparison with HIV-infected individuals without GBV-C viremia (6, 22, 23) although there are some other studies which showed conflicting results (24–26).
We suggest that if there is a relation between GBV-C infection and HIV viral loads it could affect prevalence of GBV-C RNA in different HIV viral loads, hence we compared GBV-C prevalence in different HIV viral loads groups and there was no significant difference. It can be explained by the fact that those studies which reported lower HIV viral loads in HIV-infected individuals with GBV-C viremia, had followed up their patients for a period of time, whereas we considered test-time HIV viral loads only. However, our main goal was to evaluate the prevalence of GBV-C RNA in HIV infected patients, and HIVGBV-C co-infection and their possible interactions were not concerned.
In our GBV-C positive group, the transmission routes were intravenous drug use (83.3%), and sexual relationship (16.7%). Rey et al. (13) reported similar findings. Despite this result, it is not valid to report these routes as the main GBV-C transmission routes in IRAN, because we did not have other possible transmission routes such as parenterally and mother to infant in our GBV-C positive patients, so we were unable to compare different transmission routes.
In conclusion, the prevalence of GBV-C RNA was high in HIV infected patients and there was no significant difference in GBV-C RNA prevalence between age groups, different genders, and HIV viral load groups.
Acknowledgments
This work was financially supported by the Department of Virology, Iran University of Medical Sciences. We are grateful to AIDS Research Center, Tehran University of Medical Sciences for helping in specimen provision. The authors declare that they have no conflicts of interest.
References
- 1.Simson JN, Leary TP, Dawson JG, Pilot-Matias TJ, Muehoff AS, Schlauder GG, et al. Isolation of novel virus-like sequences associated with human hepatitis. Nature Med. 1995;6:564–69. doi: 10.1038/nm0695-564. [DOI] [PubMed] [Google Scholar]
- 2.Linnen J, Wages J, Zhang-Keck Z, Fry KE, Krawczynski KZ, Alter H, et al. Molecular cloning and disease association of hepatitis G virus: a transfusion transmissible agent. Science. 1996;271:505–8. doi: 10.1126/science.271.5248.505. [DOI] [PubMed] [Google Scholar]
- 3.Leary TP, Muerhoff AS, Simons JN, Pilot-Matias TJ, Erker JC, Chalmers ML, et al. Sequence and genomic organization of GBV-C: a novel member of the Flaviviridae associated with human non A–E hepatitis. J Med Virol. 1996;48:60–67. doi: 10.1002/(SICI)1096-9071(199601)48:1<60::AID-JMV10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Reed KD, Rice CM. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr Top Microbiol Immunol. 2000;242:55–84. doi: 10.1007/978-3-642-59605-6_4. [DOI] [PubMed] [Google Scholar]
- 5.Polgreen PM, Xiang J, Chang Q, Stapleton JT. GB virus type C/hepatitis G virus: a non-pathogenic flavivirus associated with prolonged survival in HIV-infected individuals. Microbes Infect. 2003;5:1255–61. doi: 10.1016/j.micinf.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Stapleton JT. GB virus type C/Hepatitis G virus. Semin Liver Dis. 2003;23(2):7088–95. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 7.Moaven LD, Hyland CA, Young IF, Bowden DS, McCaw R, et al. Prevalence of hepatitis G virus in Queensland blood donors. Med J Aust. 1996;165:369–71. doi: 10.5694/j.1326-5377.1996.tb125019.x. [DOI] [PubMed] [Google Scholar]
- 8.Alter HJ. A reassessment of the literature on the hepatitis G virus. Transfusion. 1997;37:569. doi: 10.1046/j.1537-2995.1997.37697335149.x. [DOI] [PubMed] [Google Scholar]
- 9.Stapleton JT. GB virus type C / hepatitis G virus. Semin Liver Dis. 2003;23:137–48. doi: 10.1055/s-2003-39943. [DOI] [PubMed] [Google Scholar]
- 10.Berzsenyi MD, Bowden DS, Bailey MJ, White C, Coghlan P, Dudley FJ, et al. Male to male sex is associated with high prevalence of exposure to GB virus C. J Clin Virol. 2005;33(3):243–6. doi: 10.1016/j.jcv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Feucht H-H, Zollner B, Polywka S, Knodler B, Scrother M, Nolte H, et al. Prevalence of hepatitis G viremia among healthy subjects, individuals with liver disease, and persons at risk for parenteral transmission. J Clin Microbiol. 1997;35:767–8. doi: 10.1128/jcm.35.3.767-768.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefrere J, Roudot-Thoraval F, Morand-Joubert L, Brossard Y, Parnet-Mathieu F, Mariotti M, et al. Prevalence of GB virus C RNA and of anti-E2 in individuals at high or low risk for blood-borne or sexually transmitted viruses: evidence of sexual and parenteral transmission. Transfusion. 1999;39:83–94. doi: 10.1046/j.1537-2995.1999.39199116899.x. [DOI] [PubMed] [Google Scholar]
- 13.Rey D, Fraize S, Vidinic J, Meyer P, Fritsch S, Labouret N, Schmitt C, et al. High prevalence of GB virus C/hepatitis G virus RNA in patients infected with human immunodeficiency virus. J Med Virol. 1999;57:75–9. doi: 10.1002/(sici)1096-9071(199901)57:1<75::aid-jmv11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Puig-Basagoiti F, Cabana M, Gimenez-Barcons M, Sirera G, Tural C, Clotet B, et al. Prevalence and rout of transmission of infection with a novel DNA virus (TTV), hepatitis C virus, and hepatitis G virus in patients infected with HIV. J Acquir Immune Defic Syndr. 2003;23:89–94. doi: 10.1097/00126334-200001010-00012. [DOI] [PubMed] [Google Scholar]
- 15.Canducci F, Uberti Foppa C, Boeri E, Racca S, Gallotta G, Grasso MA, et al. Characterization of GBV-C infection in HIV-1 infected patients. J Biol Regul Homeost Agents. 2003;17:191–4. [PubMed] [Google Scholar]
- 16.Aster V, Konig J, Stankova M, Rozsypal H, Prochazka B. Prevalence of GBV-C/HGV in HIV-infected and potential influence of co-infection on the course of the disease. Klin Mikrobiol Infekc Lek. 2005;11(6):199–203. [PubMed] [Google Scholar]
- 17.Lau DT, Miller KD, Detmer J, Kolberg J, Herpin B, Metcalf J, et al. Hepatitis G virus and human immunodeficiency virus co infection: response to interferon-alpha therapy. J Infect Dis. 1999;180:1334–37. doi: 10.1086/315031. [DOI] [PubMed] [Google Scholar]
- 18.Scallan MF, Clutterbuck D, Jarvis LM, Scott G, Simmonds P. Sexual transmission of GB virus C/hepatitis G virus. J Med Virol. 1998;55:203–208. doi: 10.1002/(sici)1096-9071(199807)55:3<203::aid-jmv4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Kuritzkes DR, Walker BD. HIV-1: Pathogenesis, clinical manifestations, and treatment. In: Knipe, Howley, editors. Fields Virology. 5th ed. lippincott Williams & Wilkins; USA: 2007. p. 2189. [Google Scholar]
- 20.Samadi M, Keyvani H, Hosseini-Moghaddam SM. Prevalence and risk factors of the hepatitis G (HGV) infection in hemodialysis patients. Iran J Clin Infect Dis. 2008;3(1):7–11. [Google Scholar]
- 21.Eslamifar A, Hamkar R, Ramezani A, Ahmadi F, Gachkar L, Jalilvand S, et al. Hepatitis G virus exposure in dialysis staff. Ther Apher Dial. 2007;11(5):370–74. doi: 10.1111/j.1744-9987.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 22.Lefrere JJ, Roudot Thoraval F, Morand Joubert L, Morand-Joubert L, Petit JC, Lerable J, et al. Carriage of GB virus C RNA is associated with slower immunologic, virologic, and clinical progression of human immunodeficiency virus disease in co-infected persons. J Infect Dis. 1999;179:783–89. doi: 10.1086/314671. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda H, Fukuda Y, Hayakawa T, Takamatsu J, Saito H. Effect of GB virus C co-infection on the course of HIV infection in hemophilia patients in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:209–13. doi: 10.1097/00042560-199803010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Bjorkman P, Flamholc L, Naucler A, Molnegren V, Wallmark E, Widell A. GB virus C during the natural course of HIV-1 infection: Viremia at diagnosis does not predict mortality. AIDS. 2003;18:877–86. doi: 10.1097/00002030-200404090-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kaye S, Howard M, Alabi A, Hansmann A, Whittle H, Schim van der Loeff M. No observed effect of GB virus C co-infection in disease progression in a cohort of African women infected with HIV-1 and HIV-2. Clin Infect Dis. 2005;40:876–8. doi: 10.1086/428123. [DOI] [PubMed] [Google Scholar]
- 26.Williams C, Allen J, Benning L, Gange S, Anastos K, Greenblatt R, et al. Prevalence acquisition, and clearance of GB virus type C in the Women’s Interagency HIV study. Abstract 163, 12th CROI; Boston. 2005. [Google Scholar]